Abstract
Objectives
To evaluate the feasibility of a proof-of-concept multidomain dementia risk reduction intervention.
Method
An 8-week, parallel-group RCT, focused on increasing adherence to lifestyle domains of Mediterranean diet (MeDi), Physical Activity (PA), and Cognitive Engagement (CE). Feasibility was evaluated against the Bowen Feasibility Framework objectives of: Acceptability of the intervention, compliance with the protocol, and efficacy of the intervention to change behaviour in the three domains of interest.
Results
High acceptability of the intervention was demonstrated through a participant retention rate of 80.7% (Intervention: 84.2%; Control: 77.4%). Compliance to the protocol was strong with 100% of participants completing all educational modules and all MeDi and PA components, with 20% compliance for CE. Linear mixed models demonstrated efficacy to change behaviour through significant effects of adherence to MeDi (χ2 = 16.75, df = 3, p < .001) and CE (χ2 = 9.83, df = 3, p =.020), but not PA (χ2 = 4.48, df = 3, p =.211).
Conclusion
Overall the intervention was shown to be feasible. Recommendations for future trials in this area are: The implementation of practical, one-on-one sessions as they are more effective than passive education at eliciting behaviour change; use of booster sessions to increase likelihood of lifestyle changes being sustained; and collection of qualitative data to identify barriers to change.
Introduction
At present there are approximately 50 million people worldwide living with dementia; by 2030 this number is projected to exceed 80 million and by 2050 more than 150 million (World Health Organisation, Citation2019). It is estimated that together lifestyle risk factors (such as physical inactivity, obesity, and lack of cognitive engagement) are responsible for between a third to half of all cases of Alzheimer’s disease (AD) (Barnes & Yaffe, Citation2011; Gill Livingston et al., Citation2020; G. Livingston et al., Citation2017). Given the expected increase in the number of people developing dementia, there is an urgent need for interventions to reduce dementia risk (World Health Organisation, Citation2019). Several large-scale trials are planned and underway internationally (Heffernan et al., Citation2019; Rosenberg et al., Citation2020).
An important part of maximising the research effort is investigating the feasibility of these interventions (Rosenberg et al., Citation2020). The 2016 CONSORT Statement Extension to Randomised Pilot and Feasibility Trials defines feasibility as “whether a trial can be done, should be done, and if so, how” it ought to be done (Eldridge et al., Citation2016a). Feasibility studies answer questions such as “Will this protocol work, if not, why not and how should it be changed?” (Eldridge et al., Citation2016b).
There are theoretical frameworks to guide the conduct of feasibility studies. The Bowen Feasibility Framework (Bowen et al., Citation2009) proposes eight potential areas of focus for feasibility studies including: acceptability, demand, implementation, practicality, adaptation, integration, expansion, and limited efficacy testing. The focus may be on one or some combination of these; it is recommended that the focus be tailored to the developmental stage and objectives of the research. While feasibility studies typically occur in preparation for randomised controlled trials (RCT) (Eldridge et al., Citation2016b), aspects of feasibility can be investigated following a trial to determine the methodological aspects that may be improved upon in future iterations of studies, or in similar research. Such post-hoc investigations were conducted to assess adherence in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) and Multidomain Alzheimer Preventive Trial (MAPT) studies (Coley et al., Citation2019). Both interventions were large multidomain dementia risk reduction studies including over 1,000 participants addressing cardiovascular risk factors, nutritional counselling, physical activity, and cognitive activity. Outcomes included identifying participant characteristics that predicted adherence, identifying research questions remaining to be explored, and offering recommendations to maximise adherence in similar trials.
Another example of feasibility research is a lifestyle intervention focused on diet and exercise for participants with metabolic syndrome, conducted by Jeejeebhoy et al. (Citation2017). The study investigated compliance (percentage of intervention visits attended) and adherence (changes in measures of diet and exercise) to research protocols to draw conclusions about the practicality of its implementation and make preliminary recommendation for such studies.
The focus of the present study, Body, Brain, Life for Cognitive Decline (BBL-CD), is a multidomain dementia risk reduction trial for people experiencing subjective cognitive decline (SCD) and mild cognitive impairment (MCI) (McMaster et al., Citation2018). This RCT demonstrated efficacy in the primary outcomes of lifestyle risk of AD and cognition (McMaster et al., Citation2020). BBL-CD adapted a previously successful primary prevention intervention, the BBL trial, to a secondary prevention focus which incorporated methodological aspects of other previously successful trials in other participant groups (K. J. Anstey et al., Citation2020; Estruch et al., Citation2013; Rebok et al., Citation2014).
The aim of this article is to examine three areas of feasibility of the BBL-CD intervention to identify potential changes for future iterations of the intervention: Acceptability, Implementation and Efficacy. In accordance with the Bowen Feasibility Framework, the acceptability of the intervention will be examined through participant retention and attrition; implementation of the project will be explored through participant compliance with requirements of the protocol; and the efficacy of behaviour change will be examined through an analysis of the participant adherence achieved in the domains of Mediterranean diet (MeDi), cognitive engagement (CE) and physical activity (PA).
Methods
Design
The full trial methodology has been published previously (McMaster et al., Citation2018). BBL-CD was an eight-week, two-arm, parallel group RCT of a multidomain dementia risk reduction program for people experiencing cognitive decline. The study aimed to reduce dementia risk by primarily focusing on the lifestyle factors of MeDi, CE, and PA. The trial was designed and conducted in accordance with the CONSORT statement (Schulz et al., Citation2010) and the extension for non-pharmacological interventions (Boutron et al., Citation2008). This article was written in accordance with the CONSORT Extension for Randomised Pilot and Feasibility Trials (Eldridge et al., Citation2016a) (Appendix A). The study was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12617000792325) and ethical approval was granted by the Australian National University Human Research Ethics Committee (Protocol: 2016/360). All participants provided written informed consent to take part.
Participants
Participants were 119 community-dwelling individuals, living in the Canberra region of Australia, who responded to radio and newspaper calls from July-September 2017. Participants had a mean age of 73.0 (5.5) years and 61% (n = 73) were female. Three (3%) participants had an MCI diagnosis, and all remaining participants were experiencing SCD (n = 116, 97%). Participants were randomised to either intervention (n = 57) or active control (n = 62) in a 1:1 ratio, stratified by gender, high and low baseline lifestyle risk of AD (median split of ANU-ADRI) and high and low cognition (median split of ADAS-Cog 11).
Inclusion criteria were: 65 years or over; willing to make lifestyle changes to improve their health; owning a computer with internet access; having sufficient English skills; and either a diagnosis of MCI or experiencing SCD, according to the Jessen criteria (clinically normal on objective assessment, self/informant-reported cognitive decline, and decline not better accounted for by major medical, neurological, or psychiatric diagnosis). The exclusion criteria were any major neurological, psychiatric, or chronic condition which would prevent participation in a lifestyle behaviour change program, and current participation in any other lifestyle change interventions.
Interventions
Control group
The active control group undertook an online, four-module educational course on dementia risk reduction. The 1-hour modules covered: General information on dementia and lifestyle risk factors for AD (week 1); Mediterranean diet (week 2); cognitive engagement (week 4); and physical activity (week 6). These modules explained forms of cognitive decline and that these conditions were associated with additional risk of AD and dementia and outlined the evidence to support these lifestyle factors in general health and dementia risk reduction. In the week following each of the modules on the three lifestyle factors, participants were given a week with no additional education and instructed to implement the changes they learnt about into their own lifestyle.
Intervention group
The intervention group completed the same online educational modules accompanied by additional active components. These active components were completed in the weeks in which active control participants were requested to implement the information they had learnt into their lifestyle.
Diet
Participants had an initial one-hour appointment (week 3) and two, 30-minute follow-up face-to-face appointments (weeks 10 and 21) with the study dietitian. The session involved the dietitian reviewing the participant’s previous diet assessments and discussing ways to increase adherence to the MeDi.
Cognitive engagement
Participants were provided with a BrainHQ (Posit Science, Citation2022) brain training account (week 5). Each week participants were asked to complete two executive function tasks and two memory tasks for 30 minutes each (i.e. a total of two hours). The four tasks were: Double Decision (divided and selective attention, speed of processing, dual task, and useful field of view); Freeze Frame (visual phasic and tonic attention, inhibitory control, and motor response inhibition); Syllable Stacks (auditory working memory); and Memory Grid (auditory spatial memory).
Physical activity
An exercise physiologist had a one-hour appointment with intervention participants to create a PA plan (week 7). This plan took account of the participant’s current level of PA, any medical conditions and PA preferences. The eventual aim was to increase PA levels to 150 minutes of moderate exercise per week. The initial design of the protocol also included two, 30-minute follow-up appointments with the exercise physiologist to monitor progress and make alterations to the PA plan, as required. The exercise physiologist was unexpectedly hospitalised during the study and a suitable replacement could not be located. The initial face-to-face appointment was carried out as per the protocol, but no follow-up appointments took place.
Outcomes
The outcomes of interest are retention, compliance and adherence, three variables which are identified as important objectives in feasibility research, by the UK’s National Institute for Health Research (Eldridge et al., Citation2016b). These outcomes were selected for evaluation with a view to maximise the benefits of a longer future trial.
Retention outcomes
Retention was the number and percentage of participants in each group who remained in the intervention until the final follow-up. For those that did not remain in the intervention, the reasons for withdrawal were examined. Participant retention falls under the Bowen Feasibility Framework criterion of Acceptability (Bowen et al., Citation2009).
Compliance outcomes
To evaluate the feasibility of implementing the intervention, four aspects of compliance were reviewed (Bowen et al., Citation2009): percentage of participants who completed all four educational modules (both groups); percentage of participants who attended the initial one-hour MeDi appointment and two, 30-minute follow-up appointments with the dietitian (intervention group only); percentage of participants who completed two hours of online brain training on BrainHQ weekly (intervention group only); and percentage of participants who attended the one-hour appointment with the exercise physiologist (intervention group only).
Adherence outcomes
The efficacy of the intervention to bring about behaviour change was determined by the degree to which participants were able to adhere to the domains of MeDi, CE, and PA (Bowen et al., Citation2009).
Diet: Adherence to the MeDi was evaluated via the Mediterranean Diet Adherence Screener (MEDAS) (Schröder et al., Citation2011). The MEDAS is a 14-point checklist, with one point awarded for each aspect of the diet adhered to. The MEDAS includes intake of vegetables, fruit, fish, legumes, nuts, white meat, red meat, primary source of dietary fat, olive oil, butter/cream, wine, sofrito, sweet/carbonated beverages, and sweets. The MEDAS was administered by researchers in a discussion format to ensure accuracy of scoring.
CE and PA were both measured as components of the Australian National University- Alzheimer’s Disease Risk Index (ANU-ADRI) (K. J. Anstey et al., Citation2013). The ANU-ADRI assesses 11 AD risk and four protective factors to provide an overall risk score. Risk factors are positively scored, and protective factors are negatively scored, determined by the relative risk score of the levels of each factor.
Cognitive engagement: Cognitive risk scores in the ANU-ADRI are scored on the basis of frequency of cognitively stimulating activities such as reading books and magazines, writing letters, playing cognitively stimulating games, participating in brain training, visiting museums or libraries, and attending concerts, plays or musicals. ANU-ADRI scores are assigned to low (0), medium (-6), and highly (-7) cognitively stimulating lifestyles.
Physical activity: In the ANU-ADRI, PA is measured by the International Physical Activity Questionnaire (IPAQ) (Hagströmer et al., Citation2006). The IPAQ covers frequency and duration of vigorous, moderate, and light activity for work/volunteering, transportation, housework/yardwork, and recreation/leisure. The IPAQ uses an algorithm to combine these data to determine low, medium, and high PA lifestyles. The ANU-ADRI scores for these levels are low = 0, moderate = −2 and high activity= −3.
The protocol specified levels of activity for the intervention group such that if participants had high compliance with the intervention (e.g. dietitian sessions, exercise physiologist sessions, and brain training), they would achieve high adherence scores.
Testing timepoints: All outcomes were assessed at baseline (week 0, T1), immediate follow-up (week 9, T2), 3-month follow-up (week 20, T3); and 6 month follow-up (week 32, T4). All researchers involved in data collection were blind to group allocation. Participants were asked not to discuss the intervention with the researchers and to direct any questions to the unblinded project manager (MM).
Statistical methods
Sample size calculation
Based on previous interventions a difference of 0.70 SDs in the primary outcome measures (lifestyle risk and cognition) was determined to be feasible and corresponded to clinically relevant changes in these outcomes (K. J. Anstey et al., Citation2014; Skinner et al., Citation2012). To detect a difference of 0.70 SDs required a minimum of 36 participants per arm (N = 72) at the final follow-up period. Accounting for a potential 10% attrition rate per testing period, led to a target sample of 60 participants per arm (N = 120) (McMaster et al., Citation2018).
Randomisation and stratification
Participants were randomised 1:1 into intervention and control, in permuted blocks of eight, stratified by gender, baseline cognition (above or below median ADAS-Cog), and baseline lifestyle risk of AD (above or below median ANU-ADRI). An independent researcher (RB) generated the permuted block sequence from www.sealedenvelope.com and the templates with a randomised group allocation sequence were emailed back to the unblinded project manager (MM). The project manager then allocated participants to the first available slot to which they fit, based on strata. The project manager emailed each participant regarding their allocation and first steps to commence the trial.
Statistical analyses
Retention was evaluated by the number and percentages of participants who remained in the study until the final follow-up, formally withdrew or were lost to follow-up. Compliance was expressed as a percentage of participants who completed the intervention as specified in the protocol (McMaster et al., Citation2018).
Adherence data was analysed using linear mixed models including group, timepoint, group x timepoint and stratification variables (gender, baseline lifestyle risk of AD strata, and baseline cognition strata). Significance of the fixed effects was determined using the likelihood ratio test (LRT) method described by Winter (Citation2013). A statistically significant interaction term indicates that between group differences changed over time showing that the intervention was efficacious. Least square means adjusted for strata variables are reported with between group t-tests performed to determine significant differences at specific timepoints.
Preliminary analyses were conducted in SPSS 26.0 (IBM Corp. Released, Citation2019). Linear mixed modelling and LRT analyses were conducted in R 3.6.0 (R Core Team, Citation2017) using the lme4, (Bates et al., Citation2015) lmerTest (Kuznetsova & Christensen, Citation2017), and emmeans (Lenth et al., Citation2017. https://mran.microsoft.com/snapshot/2017-12-11/web/packages/emmeans/emmeans.pdf) packages, with graphs constructed using ggplot2 (Wickham, Citation2016).
Results
The baseline characteristics and adherence to the lifestyle behaviours of MeDi, PA and CE are shown in .
Table 1. Baseline characteristics and lifestyle adherence behaviours.
Retention
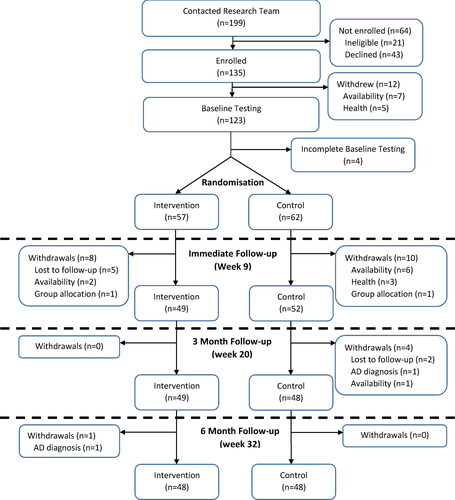
From the initial sample of 119 (Control:62; Intervention:57) participants randomised at baseline, 101 (Control:52 (83.9%); Intervention:49 (86.0%)) participants remained at immediate follow-up data collection (week 9). By the end of the intervention (week 32) the control group had a further four withdrawals for a final sample of 48 (77.4% of initial sample). The intervention group had a further one withdrawal due to an AD diagnosis, which occurred between T3 and T4, following all intervention components for a final sample size of 48 (84.2% of initial sample). The main reasons for loss and withdrawal of participants were: availability (n = 9, 7.6%), loss to follow-up (n = 7, 5.9%), and health problems (n = 3, 2.5%). The participant flowchart for the study can be found in .
Figure 1. Participant flowchart for BBL-CD study.
Lost to follow-up: These participants could not be contacted/did not respond. Availability: These participants formally withdrew due to other commitments. Group allocation: These participants formally withdrew due to the group they were randomised to. Adapted from “Lifestyle Risk Factors and Cognitive Outcomes from the Multidomain Dementia Risk Reduction Randomized Controlled Trial, Body Brain Life for Cognitive Decline (BBL-CD)” by M. McMaster et al., Citation2020, Journal of the American Geriatrics Society, 68,(11), 2629-2637.
Compliance
Participants in both groups were able to achieve a high degree of compliance for three of the four components of the prescribed protocol. The compliance rates for both groups can be found in .
Table 2. Percentage of Participants completing components of the intervention.
A high level of compliance was achieved by both groups for all four educational modules with 100% completion rates by participants that remained in the study. All scheduled dietitian and exercise physiologist appointments were attended by participants in the intervention group. The only aspect of the protocol where participants did not achieve a high level of compliance was the active component of CE.
Adherence
Mediterranean diet adherence
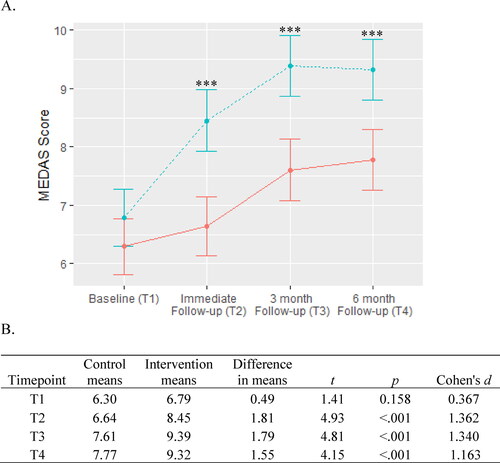
The linear mixed models showed that there was a significant group x timepoint interaction for MeDi (χ2= 16.75, df = 3, p < .001). shows that while both groups increased their adherence to the diet over the course of the intervention, the intervention group showed significantly greater adherence than the control group at every follow-up period.
Figure 2. Mediterranean diet adherence.
A. Adherence to MeDi is scored from 0 to 14 with higher scores indicating greater adherence to the diet. The intervention group is represented by the dashed blue line and the control group is represented by the solid red line. Between-group significance denoted by ***p < .001.
B. Means, differences, and t-tests for MEDAS scores.
Abbreviation: MEDAS, Mediterranean Diet Adherence Screener.
Cognitive engagement adherence
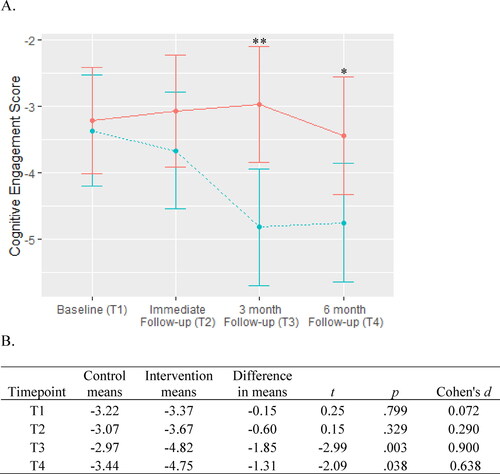
Adherence to CE for both groups is shown in . There was a significant group x timepoint interaction (χ2 = 9.83, df = 3, p = .020). There was no significant difference between groups at the immediate follow-up, but by the 3 and 6 month follow-ups the intervention group showed significantly greater levels of adherence than the control group.
Figure 3. Cognitive engagement adherence.
A. CE is scored between 0 and -7 with lower scores indicating higher adherence to CE requirements (i.e., lower lifestyle risk). The intervention group is represented by the dashed blue line and the control group is represented by the solid red line. Between-group significance denoted by *p < .05 and **p < .01.
B. Means, differences, and t-tests for CE scores.
Abbreviation: CE, cognitive engagement.
Physical activity adherence
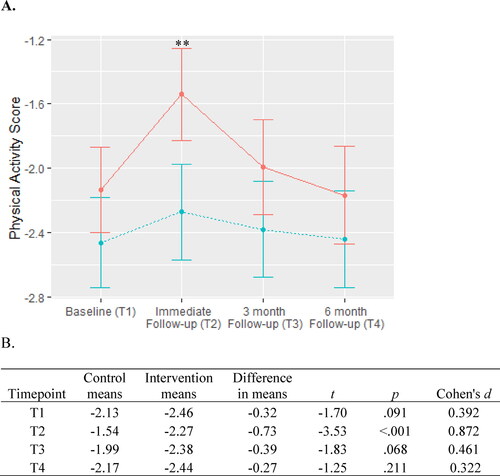
For PA there were significant effects for group (χ2 = 8.26, df = 3, p = .004) and timepoint (χ2 = 14.69, df = 3, p = .002), but the group x timepoint interaction was not significant (χ2 = 4.48, df = 3, p = .211). At the immediate follow-up there was significantly greater adherence for the intervention group, than the control group, but this difference was not retained at the final two follow-up periods as shown in .
Figure 4. Physical activity adherence.
A. PA adherence is scored between 0 and -3 with lower scores indicating higher adherence (i.e., lower lifestyle risk. The intervention group is represented by the dashed blue line and the control group are represented by the solid red line. Between-group significance denoted by *** indicates p < .001.
B. Means, differences, and t-tests for PA scores.
Abbreviation: PA, physical activity.
Discussion
The BBL-CD intervention demonstrated proof-of-concept through significant reductions in lifestyle risk and an increase in cognitive performance (McMaster et al., Citation2020), in a population experiencing cognitive decline. The purpose of this feasibility article was two-fold: The identification of areas which may require modification to maximise outcomes in a larger, longer BBL trial and to provide recommendations to inform future, similar trials. The main findings were that the trial was feasible as demonstrated by high levels of participant retention (>85% for intervention group); strong compliance (100% compliance in 3 of 4 components); and efficacious to change lifestyle behaviours (in 2 of 3 domains). These results are discussed with relevance to the feasibility framework and previous literature.
Retention
There were high levels of participant retention, with more than 80% of participants remaining in the intervention until the final follow-up. Most participants who withdrew did so during the intervention period (weeks 1–8), citing reasons unrelated to the study. The attrition rates were slightly higher in the control group. The high levels of participant retention demonstrate that the intervention meets the Bowen Feasibility criteria of Acceptability, a key component of retaining participants in a longer trial.
Mediterranean diet
The MeDi aspects of the intervention were very successful in terms of compliance and adherence. All participants completed all prescribed activities. While the adherence to MeDi increased for both groups, there were significantly higher rates of adherence for the intervention group. This demonstrates that an online MeDi education module is sufficient to increase adherence to MeDi; however significantly greater levels of adherence can be achieved by combining this with individualised dietitian sessions.
Previous studies have acknowledged the challenge of implementing MeDi interventions in non-Mediterranean countries (Hoffman & Gerber, Citation2013; Martínez-González et al., Citation2017). One element that is consistently noted as having positive outcomes is one-on-one support with overcoming barriers and maximising adherence (Jeejeebhoy et al., Citation2017). In a qualitative study, following a MeDi RCT, participants expressed the need for close individualised support (Middleton et al., Citation2015). Though the FINGER study did not implement a MeDi component, nutritional counselling was found to have the highest rates of compliance among the domains covered by the intervention (Coley et al., Citation2019). Although nutritional education can improve adherence to certain dietary patterns, the inclusion of an interventionist is usually more effective (de Menezes et al., Citation2020).
Findings suggest that dietary interventions and more specifically MeDi interventions are feasible in this participant group, and the effects can be enhanced by including an interventionist. In relation to the Bowen Feasibility Framework, strong compliance indicates that it was possible to implement the intervention as per the protocol and a significant group x timepoint interaction indicates that the intervention displayed efficacy in changing dietary behaviour. Future trials could include a similar dietary component without large modifications.
Cognitive engagement
All participants from both groups were able to complete the CE educational module. For the intervention group there was a wide range in compliance for the active component; participants completed an average of only 20% (10.8 hours) of the 54 hours prescribed in the protocol (McMaster et al., Citation2018). Similar results have been seen with other multidomain dementia risk reduction studies. In the FINGER trial, the cognitive training component had the lowest compliance with 24.7% of participants completing only two-thirds of the prescribed training. One of the reasons cited was that this component was completed independently by participants with low supervision, similarly to BBL-CD (Coley et al., Citation2019).
Despite the poor compliance by the intervention group in BBL-CD, there was still a significant increase in adherence to cognitively engaged behaviours relative to the control group, which showed little change over the course of the intervention. Our interpretation of this pattern of results is that passive, online education to increase CE is largely ineffective and that the cognitive training dosage prescribed for the intervention group in the protocol may have exceeded the level required to have an effect; hence low compliance was still sufficient to show increased adherence.
Although there is limited evidence on what level of dosage of cognitive training is required for a positive effect, the amount actually undertaken by participants in this study was similar to the prescribed dose in prior trials. For example, in the ACTIVE trial, 10 hours of computerised speed of processing training resulted in improved levels of instrumental activities of daily living, speed of processing and lower rates of dementia at 10 years post-intervention, compared to the comparison conditions (Edwards et al., Citation2016; Rebok et al., Citation2014).
A systematic review of brain training in older, cognitively normal participants found that individual home-based training was far less effective than supervised group based training, citing low levels of participant compliance and adherence as potential reasons (Lampit et al., Citation2014). Some research has indicated that cognitive outcomes from combined physical and cognitive training show a dose dependent effect, so maximising compliance and adherence is of considerable importance and interest (Bamidis et al., Citation2015).
Viewed through the feasibility framework lens, low compliance is indicative of ineffective implementation for this aspect of the intervention. However, the significant interaction effect indicates that the intervention did display efficacy to change CE behaviour for the intervention group. Drawing on previous literature and these outcomes, a more directly supervised or group intervention for CE may be beneficial.
Physical activity
Strong compliance was seen for the educational components for PA for both groups, and participants in the intervention group did comply with the revised protocol. In terms of adherence, for unknown reasons the control group experienced a dramatic reduction in PA at immediate follow-up, before returning to baseline levels. The intervention group showed a small reduction over the same period, but this was much less pronounced. The linear mixed models showed that while there were significant effects of group and timepoint there was not a significant group x timepoint interaction, showing that this aspect of the intervention was ineffective at changing PA behaviours.
The conclusion considering the results from the other active components is that for PA, neither an online education module nor education combined with a single exercise physiology session were sufficient to achieve increased levels of adherence. Lifestyle interventions with as many as 20 interventionist appointments have been shown to have strong compliance (median compliance >75% of appointments), so additional PA interventionist sessions are likely to be feasible (Jeejeebhoy et al., Citation2017). Given the adherence achieved in the MeDi component, follow-up interventionist appointments were likely to have led to increased adherence. A meta-analysis by Lemstra et al. (Citation2016) looking at PA and dietary interventions found that supervision and support were major determinants of compliance and adherence. A recent RCT which included MeDi, PA, CE, and social engagement showed that weekly contact with an interventionist to assess progress, overcome barriers and adjust goals over time, resulted in significant adherence across all domains relative to controls (H. E. Schwartz et al., Citation2019). Strong compliance shows the implementation of the PA aspects of this intervention were feasible. Despite this the intervention did not display efficacy to change PA behaviour. From this we can conclude that education with a single exercise physiology appointment is insufficient to change PA behaviours in this participant group and a similar intervention model to the MeDi component would likely be more beneficial.
Limitations
One of the primary limitations of this study was that due to the hospitalisation of the exercise physiologist, the original protocol of three PA sessions could not be implemented and was revised to a single session. This may have reduced the adherence to this aspect of the intervention. Additionally, the short follow-up time did not permit the investigation of long-term adherence. No qualitative data to determine reasons for non-compliance and non-adherence were collected. These limitations allow for some improvements to be made to the protocol for any future BBL interventions and allow for some recommendations to be made for future studies in this area.
Implications and recommendations
These findings on the feasibility of BBL-CD may be helpful in maximising compliance and adherence for other studies. Key recommendations are:
Practical, one-on-one sessions are recommended for behaviour change for participants with cognitive decline. Passive education regarding dementia risk factors was less effective than more direct, intensive education.
Booster sessions are recommended to maximise and sustain lifestyle change. Further improvements in lifestyle ceased in the absence of further education.
Future research should include qualitative data collection to investigate barriers and enablers to compliance and adherence.
These recommendations are further elaborated on below.
Firstly, this trial demonstrates that participants experiencing cognitive decline can complete a set of relatively demanding educational modules. However, depending on the topic, education modules may or may not be effective to elicit significant behaviour change. For example, improved levels of adherence were seen in the control group for the MeDi component, but improvements were quite limited in the control group for CE and PA. Much greater levels of adherence and behaviour change can be elicited through one-on-one sessions with an interventionist, provided there are follow-up sessions to assist with implementation and overcoming any barriers encountered. This is consistent with research across all three of the lifestyle domains included in this study (Lampit et al., Citation2014; Lemstra et al., Citation2016; J. Schwartz et al., Citation2019).
A plateauing of adherence for the intervention group was seen across all three domains between timepoints 3 and 4; this demonstrates that even with the greater adherence of one-on-one sessions, behaviour change only continues to take place in the presence of continuing education. “Booster sessions” after the conclusion of the intervention are most likely required to elicit further change and long-term maintenance (Fleig et al., Citation2013; Lachman et al., Citation2018). A useful addition to further studies in the area would be qualitative follow-up to determine specific reasons and barriers to limited compliance and adherence in participants that remained in the study.
In summary, BBL-CD was a multidomain lifestyle intervention which was successful in its primary aims of reducing lifestyle risk of AD and improving cognition for individuals experiencing SCD and MCI (McMaster et al., Citation2020). This study builds on these findings by showing that the intervention protocol was feasible. The intervention was highly acceptable with more than 80% of participants remaining in the study. Both groups were able to achieve 100% compliance with the four educational modules and for the active components, two of the three domains achieved 100% compliance (MeDi and PA), with 20% for the third (CE). For adherence, two of the three domains (MeDi and CE) demonstrated efficacy for behaviour change, while the third did not (PA).
An important finding of this study is that despite undertaking a more demanding program, the intervention group achieved similar levels of withdrawals, similar levels of compliance (with the exception of CE) and greater levels of adherence. These results are highly suggestive that in the population of older people with cognitive decline the more intensive approach to lifestyle risk reduction is feasible and has greater efficacy.
Acknowledgments
The authors would also like to thank: Prof. Catherine D’Este for input with the conception, design, and analysis of the BBL-CD study; Dr. Richard Burns (RB) for his advice and assistance with the randomisation of the sample; the dietitian interventionist Tania Mathewson for her dedication and flexibility; and Dr. Mouna Attarha from BrainHQ for assistance with collation of BrainHQ website usage data and the provision of information related to effective dosage levels of cognitive training.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Anstey, K. J., Cherbuin, N., & Herath, P. M. (2013). Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prevention Science: The Official Journal of the Society for Prevention Research, 14(4), 411–421. https://doi.org/10.1007/s11121-012-0313-2
- Anstey, K. J., Cherbuin, N., Herath, P. M., Qiu, C., Kuller, L. H., Lopez, O. L., Wilson, R. S., & Fratiglioni, L. (2014). A self-report risk index to predict occurrence of dementia in three independent cohorts of older adults: The ANU-ADRI. PLoS One, 9(1), e86141. https://doi.org/10.1371/journal.pone.0086141
- Anstey, K. J., Cherbuin, N., Kim, S., McMaster, M., D’Este, C., Lautenschlager, N., Rebok, G., McRae, I., Torres, S. J., Cox, K. L., & Pond, C. D. (2020). An internet-based intervention augmented with a diet and physical activity consultation to decrease the risk of dementia in at-risk adults in a primary care setting: Pragmatic randomized controlled trial. Journal of Medical Internet Research, 22(9), e19431. https://doi.org/10.2196/19431
- Bamidis, P. D., Fissler, P., Papageorgiou, S. G., Zilidou, V., Konstantinidis, E. I., Billis, A. S., Romanopoulou, E., Karagianni, M., Beratis, I., Tsapanou, A., Tsilikopoulou, G., Grigoriadou, E., Ladas, A., Kyrillidou, A., Tsolaki, A., Frantzidis, C., Sidiropoulos, E., Siountas, A., Matsi, S., … Kolassa, I.-T. (2015). Gains in cognition through combined cognitive and physical training: The role of training dosage and severity of neurocognitive disorder. Frontiers in Aging Neuroscience, 7, 152. https://doi.org/10.3389/fnagi.2015.00152
- Barnes, D. E., & Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. The Lancet. Neurology, 10(9), 819–828. https://doi.org/10.1016/S1474-4422(11)70072-2
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
- Boutron, I., Moher, D., Altman, D. G., Schulz, K. F., & Ravaud, P, for the CONSORT Group. (2008). Methods and processes of the CONSORT Group: Example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine, 148(4), W-60–66. https://doi.org/10.7326/0003-4819-148-4-200802190-00008-w1
- Bowen, D. J., Kreuter, M., Spring, B., Cofta-Woerpel, L., Linnan, L., Weiner, D., Bakken, S., Kaplan, C. P., Squiers, L., Fabrizio, C., & Fernandez, M. (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36(5), 452–457. https://doi.org/10.1016/j.amepre.2009.02.002
- Coley, N., Ngandu, T., Lehtisalo, J., Soininen, H., Vellas, B., Richard, E., Kivipelto, M., Andrieu, S., van Gool, P., & van Charante, E. M, HATICE, FINGER, and MAPT/DSA groups. (2019). Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 15(6), 729–741. https://doi.org/10.1016/j.jalz.2019.03.005
- de Menezes, M. C., Duarte, C. K., Costa, D. V. D P., Lopes, M. S., de Freitas, P. P., Campos, S. F., & Lopes, A. C. (2020). A systematic review of effects, potentialities, and limitations of nutritional interventions aimed at managing obesity in primary and secondary health care. Nutrition, 75-76, 110784. https://doi.org/10.1016/j.nut.2020.110784
- Edwards, J. D., Xu, H., Clark, D., Ross, L. A., & Unverzagt, F. W. (2016). S2‐01‐02: The Active Study: What we have learned and what is next? Cognitive training reduces incident dementia across ten years. Alzheimer’s & Dementia, 12(7S_Part_4), P212–P212. https://doi.org/10.1016/j.jalz.2016.06.373
- Eldridge, S. M., Chan, C. L., Campbell, M. J., Bond, C. M., Hopewell, S., Thabane, L., & Lancaster, G. A, PAFS consensus group. (2016a). CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ (Clinical Research ed.), 355, i5239. https://doi.org/10.1136/bmj.i5239
- Eldridge, S. M., Lancaster, G. A., Campbell, M. J., Thabane, L., Hopewell, S., Coleman, C. L., & Bond, C. M. (2016b). Defining feasibility and pilot studies in preparation for randomised controlled trials: Development of a conceptual framework. PloS One, 11(3), e0150205. https://doi.org/10.1371/journal.pone.0150205
- Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M.-I., Corella, D., Arós, F., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Fiol, M., Lapetra, J., Lamuela-Raventos, R. M., Serra-Majem, L., Pintó, X., Basora, J., Muñoz, M. A., Sorlí, J. V., Martínez, J. A., & Martínez-González, M. A, PREDIMED Study Investigators. (2013). Primary prevention of cardiovascular disease with a Mediterranean diet. The New England Journal of Medicine, 368(14), 1279–1290. https://doi.org/10.1056/NEJMoa1200303
- Fleig, L., Pomp, S., Schwarzer, R., & Lippke, S. (2013). Promoting exercise maintenance: How interventions with booster sessions improve long-term rehabilitation outcomes. Rehabilitation Psychology, 58(4), 323–333. https://doi.org/10.1037/a0033885
- Hagströmer, M., Oja, P., & Sjöström, M. (2006). The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutrition, 9(6), 755–762. https://doi.org/10.1079/phn2005898
- Heffernan, M., Andrews, G., Fiatarone Singh, M. A., Valenzuela, M., Anstey, K. J., Maeder, A. J., McNeil, J., Jorm, L., Lautenschlager, N. T., Sachdev, P. S., Ginige, J. A., Hobbs, M. J., Boulamatsis, C., Chau, T., Cobiac, L., Cox, K. L., Daniel, K., Flood, V. M., Guerrero, Y., … Brodaty, H, Maintain Your Brain Collaborative Team. (2019). Maintain Your Brain: Protocol of a 3-year randomized controlled trial of a personalized multi-modal digital health intervention to prevent cognitive decline among community dwelling 55 to 77 year olds. Journal of Alzheimer’s Disease, 70(s1), S221–S237. https://doi.org/10.3233/JAD-180572
- Hoffman, R., & Gerber, M. (2013). Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: A critical appraisal. Nutrition Reviews, 71(9), 573–584. https://doi.org/10.1111/nure.12040
- IBM Corp. Released. (2019). IBM SPSS Statistics for Windows, Version 26.0. IBM Corp.
- Jeejeebhoy, K., Dhaliwal, R., Heyland, D. K., Leung, R., Day, A. G., Brauer, P., Royall, D., Tremblay, A., Mutch, D. M., Pliamm, L., Rhéaume, C., & Klein, D. (2017). Family physician-led, team-based, lifestyle intervention in patients with metabolic syndrome: Results of a multicentre feasibility project. CMAJ Open, 5(1), E229–E236. https://doi.org/10.9778/cmajo.20160101
- Kuznetsova, A. B., P, B., & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13) https://doi.org/10.18637/jss.v082.i13
- Lachman, M. E., Lipsitz, L., Lubben, J., Castaneda-Sceppa, C., & Jette, A. M. (2018). When adults don’t exercise: Behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innovation in Aging, 2(1), 1–12. https://doi.org/10.1093/geroni/igy007
- Lampit, A., Hallock, H., & Valenzuela, M. (2014). Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11(11), e1001756. https://doi.org/10.1371/journal.pmed.1001756
- Lemstra, M., Bird, Y., Nwankwo, C., Rogers, M., & Moraros, J. (2016). Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Preference and Adherence, 10, 1547–1559. https://doi.org/10.2147/PPA.S103649
- Lenth, R., Love, J., Herve, M. (2017). https://mran.microsoft.com/snapshot/2017-12-11/web/packages/emmeans/emmeans.pdf). Estimated Marginal Means, "emmeans" R package.
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., Ballard, C., Banerjee, S., Burns, A., Cohen-Mansfield, J., Cooper, C., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Larson, E. B., Ritchie, K., Rockwood, K., Sampson, E. L., … Mukadam, N. (2017). Dementia prevention, intervention, and care. Lancet (London, England), 390(10113), 2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6
- Martínez-González, M. Á., Hershey, M. S., Zazpe, I., & Trichopoulou, A. (2017). Transferability of the Mediterranean diet to non-Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients, 9(11), 1226. https://doi.org/10.3390/nu9111226
- McMaster, M., Kim, S., Clare, L., Torres, S. J., Cherbuin, N., DʼEste, C., & Anstey, K. J. (2020). Lifestyle risk factors and cognitive outcomes from the multidomain dementia risk reduction randomized Controlled Trial, Body Brain Life for Cognitive Decline (BBL-CD). Journal of the American Geriatrics Society, 68(11), 2629–2637. https://doi.org/10.1111/jgs.16762
- McMaster, M., Kim, S., Clare, L., Torres, S. J., D’Este, C., & Anstey, K. J. (2018). Body, Brain, Life for Cognitive Decline (BBL-CD): Protocol for a multidomain dementia risk reduction randomized controlled trial for subjective cognitive decline and mild cognitive impairment. Clinical Interventions in Aging, 13, 2397–2406. https://doi.org/10.2147/CIA.S182046
- Middleton, G., Keegan, R., Smith, M. F., Alkhatib, A., & Klonizakis, M. (2015). Implementing a Mediterranean diet intervention into a RCT: Lessons learned from a non-Mediterranean based country. The Journal of Nutrition, Health & Aging, 19(10), 1019–1022. https://doi.org/10.1007/s12603-015-0663-0
- Posit Science. (2022, October 28). BrainHQ. http://www.brainhq.com.
- R Core Team. (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
- Rebok, G. W., Ball, K., Guey, L. T., Jones, R. N., Kim, H.-Y., King, J. W., Marsiske, M., Morris, J. N., Tennstedt, S. L., Unverzagt, F. W., & Willis, S. L, ACTIVE Study Group. (2014). Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62(1), 16–24. https://doi.org/10.1111/jgs.12607
- Rosenberg, A., Mangialasche, F., Ngandu, T., Solomon, A., & Kivipelto, M. (2020). Multidomain interventions to prevent cognitive impairment, alzheimer’s disease, and dementia: From finger to world-wide fingers. The Journal of Prevention of Alzheimer’s Disease, 7(1), 29–36. https://doi.org/10.14283/jpad.2019.41
- Schröder, H., Fitó, M., Estruch, R., Martínez-González, M. A., Corella, D., Salas-Salvadó, J., Lamuela-Raventós, R., Ros, E., Salaverría, I., Fiol, M., Lapetra, J., Vinyoles, E., Gómez-Gracia, E., Lahoz, C., Serra-Majem, L., Pintó, X., Ruiz-Gutierrez, V., & Covas, M.-I. (2011). A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. The Journal of Nutrition, 141(6), 1140–1145. https://doi.org/10.3945/jn.110.135566
- Schulz, K. F., Altman, D. G., & Moher, D, CONSORT Group. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Medicine, 8, 18–19. https://doi.org/10.1186/1741-7015-8-18
- Schwartz, H. E., Bay, C. P., McFeeley, B. M., Krivanek, T. J., Daffner, K. R., & Gale, S. A. (2019). The Brain Health Champion study: Health coaching changes behaviors in patients with cognitive impairment. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 5(1), 771–779. https://doi.org/10.1016/j.trci.2019.09.008
- Schwartz, J., Rhodes, R., Bredin, S., Oh, P., & Warburton, D. (2019). Effectiveness of approaches to increase physical activity behavior to prevent chronic disease in adults: A brief commentary. Journal of Clinical Medicine, 8(3), 295. https://doi.org/10.3390/jcm8030295
- Skinner, J., Carvalho, J. O., Potter, G. G., Thames, A., Zelinski, E., Crane, P. K., Gibbons, L. E., & Initiative, A. S. D. N, for the Alzheimer’s Disease Neuroimaging Initiative. (2012). The Alzheimer’s disease assessment scale-cognitive-plus (ADAS-Cog-Plus): An expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging and Behavior, 6(4), 489–501. https://doi.org/10.1007/s11682-012-9166-3
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org
- Winter, B. (2013). Linear models and linear mixed effects models in R with linguistic applications. arXiv13085499 [https://arxiv.org/ftp/arxiv/papers/1308/1308.5499.pdf].
- World Health Organisation. (2019). Risk reduction of cognitive decline and dementia. WHO Guidelines.
