Abstract
Objectives
Speech, language and communication difficulties are prevalent in all dementia subtypes and are likely to considerably impact the quality-of-life of people with dementia and their families. Communication interventions provided by trained professionals are recommended for this population, but little is known about their quality-of-life outcomes. This review aims to explore the quality-of-life outcomes of communication-related interventions for people with dementia and their families.
Methods
Seven databases were systematically searched. Reference lists from included studies and relevant systematic reviews were also hand-searched. Primary research with quantitative quality-of-life outcomes were included. Narrative analysis was utilised to identify key intervention features and to describe quality-of-life outcomes.
Results
1,174 studies were identified. Twelve studies were eligible for inclusion. Studies were heterogeneous in location, participant group, methodologies, interventions and outcome measures. Four studies reported increased quality-of-life for people with dementia following intervention. No studies reported increased quality-of-life for family members.
Conclusion
Further research is needed in this area. The studies which reported improved quality-of-life involved multi-disciplinary approaches to intervention, involvement of family caregivers, and functional communication intervention. However, data is limited so results should be interpreted with caution. The standardised use of a communication-focused quality-of-life outcome measure would improve sensitivity and comparability of future studies.
Introduction
Fifty-five million people worldwide currently live with dementia, with prevalence expected to rise to 78 million in 2030 (World Health Organisation (WHO), 2021). Dementia is defined by the International Classification of Diseases − 11 (ICD-11) (WHO, Citation2019) as ‘a syndrome—usually of chronic or progressive nature … [that] affects memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement’, all of which can impact a person’s communication. There are many dementia subtypes: Alzheimer’s Disease is the most common (62%), followed by vascular dementia (17%) and mixed dementia (10%), along with rarer dementia subtypes such as primary progressive aphasia (PPA) (Prince et al., Citation2014). While symptoms and progression vary, all dementia subtypes can involve communication difficulties associated with impairments in expressing and comprehending language (aphasia); motor speech (dysarthria); reading and writing; and cognitive communication difficulties, such as difficulty retaining information and staying on topic (Banovic et al., Citation2018; WHO, Citation2019). Communication difficulties often increase as the disease progresses (Banovic et al., Citation2018; Ross et al., Citation1990) and individuals can experience a loss of the ability to communicate thoughts and needs (Woodward, Citation2013). Communication difficulties have a range of implications for people with dementia, such as problems with social interactions and maintaining relationships; reductions in hobbies and leisure activities; withdrawal from occupations; and increased behaviours that challenge, such as aggression (Bourgeois et al., Citation2003; Burgio & Fisher, Citation2000; Schwam, & Xu, Citation2010; Woodward, Citation2013). These issues can considerably impact the quality-of-life of people with dementia and their caregivers (Savundranayagam et al., Citation2005).
Professional bodies for speech and language therapists (SLTs) worldwide recommend communication interventions for people with dementia and the people that support them (American Speech-Language-Hearing Association (ASHA), Citation2017; Royal College of Speech and Language Therapists (RCSLT), 2014). Interventions include professional education; impairment-based interventions such as word retrieval; compensatory-based approaches such as communication strategies; and group education and support for managing communication difficulties (ASHA, Citation2017; RCSLT, Citation2014; Volkmer et al., Citation2020). Whilst there are some studies assessing the outcomes of these interventions, including their effects on quality-of-life, the evidence exploring the impact of communication intervention on quality-of-life has not been synthesised in a systematic review.
The World Health Organisation (WHO, Citation2012, p.11) defines quality-of-life in health as ‘an ‘individual’s’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns’. Quality-of-life is complex and depends on a wide number of factors, with important features of quality-of-life described by people with dementia including feeling accepted, being understood, and enhancing meaning in life (Dröes et al., Citation2006). More recently, the IDEAL study identified factors influencing caregivers’ (Clare, Wu, Jones, et al., Citation2019) and people with dementia’s (Clare, Wu, Quinn, et al. Citation2019) ability to live well. For caregivers the primary factors were psychological health, physical health, social resources and relationship with the person with dementia. The only independent predictor of living well for the person with dementia was psychological health. Communication between the person with dementia and caregiver is likely to influence psychological health, social resources and relationships. Links have also been found between dementia progression, communication changes, increased behaviours that challenge, and caregiver burden, which impact quality-of-life (Savundranayagam et al., Citation2005). Communication is related to improved relationships, social engagement and functional ability, which are also associated with better quality-of-life for people with dementia (Martyr et al., Citation2018). Indeed, communication has been described as a key domain and subdomain within the quality-of-life of this population (Banerjee et al., Citation2010; Brod et al., Citation1999), and communication difficulties also have considerable implications for those who support people with dementia (Olthof-Nefkens et al., Citation2023; Stiadle et al., Citation2014). Olthof-Nefkens et al. (Citation2023) identified an association between self-perceived communication abilities and the quality-of-life of people affected by dementia.
In recent years, there has been a societal shift away from the negative consequences of dementia, towards an improved recognition of quality-of-life, with healthcare policies focusing on ‘living well’ with dementia (Clare, Citation2017; Clarke et al., Citation2020; Department of Health, Citation2020; Quinn et al., Citation2022). The importance of timely psychosocial interventions to reduce disability in dementia is widely acknowledged (Prince et al., Citation2011; WHO, 2015). Some non-pharmacological interventions, such as cognitive stimulation therapy, have been found to improve the quality-of-life of people with dementia and their families in some studies (e.g. Woods et al., Citation2006), but to have no effect in others (e.g. Clare, Kudlicka, et al. Citation2019). With increased recognition of quality-of-life in dementia, it is timely to review the existing evidence exploring the effect of communication interventions on this important outcome. This has implications for clinical decision-making, policy and practice.
Study aims
This systematic literature review aims to explore the effect of communication interventions on the quality-of-life of people with dementia and their families.
Methodology
Study design
A systematic review was conducted to explore the effect of communication interventions on the quality-of-life of people with dementia and their families. The protocol was registered with PROSPERO on 23/06/2021 (registration number 261926).
Literature search
The searches were conducted during May 2020, and repeated August 2022, in the databases: PsycINFO, CINAHL, EMBASE, EMCARE, MEDLINE, BNI and AMED.
The search terms were identified and adapted to corresponding terms depending on the database. Each individual search term was supplemented with relevant free text terms. Where appropriate, the free text terms were truncated so as not to exclude alternative word endings.
The search results were limited to articles written in English, published in or after 2005, and included only adults or older adults as the target population. The full search string is included in Appendix 1. The database searches were supplemented with a manual review of reference lists of relevant articles and systematic reviews.
Eligibility criteria
This review included primary research with quantitative quality-of-life outcome measures, to establish the quality-of-life effects of interventions which target communication. Studies included interventions targeting verbal or non-verbal communication/interaction of people with dementia and/or their family caregivers. Studies were not excluded based on the professional backgrounds of those delivering interventions. Study participants were either people with dementia (of any type and severity, living at home) or their family members. Studies were excluded if participants’ primary diagnosis was not dementia, or if they had other co-morbidities potentially affecting language. Studies published after 2005 were included, to reflect current practice. Please see Appendix 2 for full eligibility criteria and rationale.
Screening
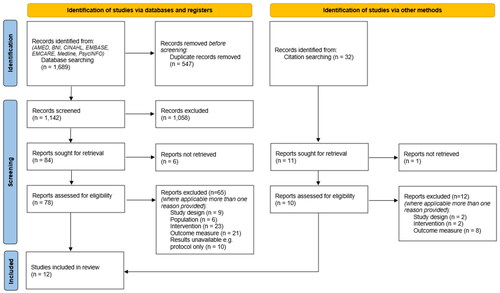
1,689 studies were identified through database searching and 32 through other search methods, for example reference lists of relevant systematic reviews (). 547 duplicate studies were removed. Three reviewers (AH, ZC and JL) screened all titles and abstracts as a team, discussed any disagreements, and came to a consensus. 1,079 studies were excluded based on title and abstract. Six further studies were excluded because full texts were unavailable. Three reviewers (AH, ZC and DM) independently screened the full texts of the remaining 89 studies. If there was uncertainty regarding eligibility, the paper was read independently by another team member and a consensus was reached. Seventy-seven studies were excluded after screening full texts. Twelve studies remained: three randomised controlled trials (RCTs) and nine non-RCTs including case studies, comparison-group studies and pre- post- intervention studies.
Risk of bias
The 12 studies were evaluated using risk of bias tools: the ROB-1 tool for RCTs (Appendix 3), and the ROBINS-1 tool for non-RCTs (Appendix 4), to inform the interpretation of the findings. Two reviewers (AH and DM) assessed each study’s risk of bias independently, then compared their results. Where disagreement arose, a third reviewer’s (ZC) opinion was sought. One author was contacted and additional information on missing and unclear data was obtained.
Data extraction
Two reviewers (AH and DM) extracted data from the included studies. The Template for Intervention Description and Replication (TIDieR) framework (Hoffmann et al., Citation2014) was used to structure extraction of data related to intervention characteristics.
Analysis
Meta-analysis was not appropriate due to heterogeneity in study designs, interventions, control groups and outcome measures. Narrative analysis was therefore conducted using the TIDieR framework (Hoffmann et al., Citation2014) as a structure for data synthesis.
Results
Characteristics of included studies
Twelve studies were included (). Five were conducted in English-speaking countries, with two including at least one UK site. Interventions targeted various dementia diagnoses. Two studies specified mild or mild-moderate stage of dementia. Four included the person with dementia only, and two included informal caregivers only. Six included both the person with dementia and their informal caregiver (dyads). In the studies involving dyads, quality-of-life outcome measurement did not always relate to both the person with dementia and their caregiver. Participant numbers ranged between one individual participant and 255 dyads.
Table 1. Study characteristics.
Table 2. Intervention characteristics.
Risk of bias assessment
Methodological quality was variable across all studies (Appendices 3-4). Of the RCTs, only one study had low risk in three or more domains (Barnes & Markham, Citation2018). Common reasons for bias included a lack of true randomisation, lack of blinding, and reporting bias. For the non-RCTs, there were low risk of bias domains due to selection of participants and classification of interventions. However, bias was introduced due to confounding, outcome measurement and selection of the reported result. All studies demonstrated high or questionable risk of bias across several domains. Although at times this was due to the nature of interventions, such as lack of ability to blind participants to intervention group, findings must be interpreted with caution as a result and the results of the included studies are interpreted within this context in the discussion section.
Intervention characteristics according to the TIDIER checklist ()
Why (goal of intervention)
Three study interventions targeted linguistic or cognitive functioning (Andrade-Calderon, Salvador-Cruz and Soso Ortiz, Citation2015, La Rue et al., Citation2015, and Santos et al., Citation2015). One targeted functional communication of the person with dementia (Cadorio et al., Citation2019). Three targeted skills/strategies for the person with dementia and the caregiver (Jokel et al., Citation2017, Judge et al., Citation2013, and Leroi et al., 2020). The remaining five studies targeted caregiver knowledge.
What (intervention type)
Six studies investigated single component (communication-focused) interventions (Andrade-Calderón et al., Citation2015; Barnes & Markham, Citation2018; Cadorio et al., Citation2019; Haberstroh et al., Citation2011; Jokel et al., Citation2017; Messemaker et al., Citation2017). The other six investigated multi-component interventions of which communication was a part. Studies varied in intervention recipient (caregiver, person with dementia, or both); and intervention type (e.g. language training (impairment-based interventions), communication strategies, counselling, social inclusion (functional interventions)).
Who (intervention provider)
Interventions were delivered by a variety of individuals including psychiatrists, psychologists, SLTs, SLT students, other multi-disciplinary clinicians, and trained volunteers.
How (mode of delivery) and where (location of intervention)
All interventions involved face-to-face contact; this was implied and not explicit in Haberstroh et al.’s (Citation2011) paper. Four included group interventions, one included group and individual sessions, and seven comprised individual sessions. Locations included: domiciliary settings; community spaces; hospital outpatient settings; and service settings that promote independent living. Two did not specify location.
When and how much (duration, number of sessions)
Interventions varied significantly in their duration and intensity, from a one-off hour-long lecture (Han et al., Citation2020) to 50 sessions over a 12-month period (Andrade-Calderón et al., Citation2015).
Tailoring (e.g. individualised to client)
Five interventions were tailored to individual needs. Han et al.’s (Citation2020) intervention involved a lecture which could not be tailored. Six interventions had set themes or topics, but involved some flexibility, for example encouraging identification of individual strategies or goals, or teaching individualised skills.
Modifications/how well (attrition, compliance)
Only Jokel et al. (Citation2017) reported an intervention modification following study commencement (the addition of an orthographic prompt). La Rue et al. (Citation2015) reported limitations in volunteer availability resulting in fewer outings for some participants. Other studies did not report protocol deviations.
Outcomes
Outcome measure used and timing of outcome assessment
Seven quality-of-life outcome measures were utilised in the studies with either the person with dementia or the caregiver (). All the studies completed outcome assessments prior to intervention and soon after intervention completion. Five studies incorporated second follow-ups, the timing of which varied considerably (Appendix 5).
Patient quality-of-life (self-reported or proxy)
Ten studies investigated patient quality-of-life (patient-reported or proxy). Three reported no change in patient quality-of-life (Andrade-Calderón et al., Citation2015; Judge et al, Citation2013; Messemaker et al., Citation2017). One reported a statistically significant decrease in quality-of-life (La Rue et al., Citation2015: (p = 0.048, 95% CI = −0.40 to −5.15). Leroi et al (2020) documented increased patient-reported quality-of-life, but decreased proxy scores, however, these were based on raw scores so statistical significance could not be ascertained. Four studies reported statistically significant increased quality-of-life (Haberstroh et al., Citation2011: p < 0.01; Jokel et al., Citation2017: p < 0.05; Teri et al., 2018: p <.001, 95% CI = 0.50 to 1.56); with Santos et al. (Citation2015) reporting significant increase in patient-reported scores in the mild Alzheimer’s Disease group (p = 0.003) but no change for the moderate Alzheimer’s Disease group or any group’s proxy scores.
Of the four studies reporting statistically significant improvement in patient-reported quality-of-life, none were RCTs, but three involved non-randomised comparison group studies. The fourth (Teri et al., 2018) demonstrated statistically significant positive changes in pre-post treatment comparisons in a staggered multiple baseline design. These four studies varied considerably in: methodology; participant numbers; intervention types and recipients. All four studies included communication strategy training for caregivers and/or people with dementia and involved face-to-face group or individual sessions.
Caregiver quality-of-life
Three studies reported on caregiver quality-of-life. Han et al. (Citation2020) identified a decrease in caregiver quality-of-life following intervention (p = 0.004). Barnes and Markham (Citation2018) and Andrade-Calderón et al. (Citation2015) did not find an overall increase in caregiver quality-of-life scores, however the former reported statistically significant improvement in one caregiver quality-of-life sub-score, value (p = 0.046, 95% CI = −2.3 to − 0.02).
Discussion
This systematic review has examined the evidence relating to quality-of-life outcomes of interventions which target communication for people with dementia and their families. Twelve studies met the eligibility criteria and were heterogeneous in their methodological designs and outcome measures. Conclusions should be made with caution due to the limited number of RCTs, as well as study heterogeneity and risk of bias identified. However, this review highlights several considerations.
Interventions
Several studies included communication as a subsection within more general multi-component interventions. In the present systematic review, some single-component (Haberstroh et al., Citation2011; Jokel et al., Citation2017) and some multi-component interventions (Santos et al., Citation2015; Teri et al., Citation2018) reported positive effects on quality-of-life.
The range of professionals providing communication-related interventions in this review demonstrates the roles of professionals other than SLTs in delivering communication-related interventions. This suggests a value in multidisciplinary approaches. Integrated multidisciplinary approaches to dementia care are beneficial, as no single professional body has the expertise to address the complex range of physical, cognitive, and psychological changes that occur with dementia (Grand et al., Citation2011). However, only four of the 12 studies included in this review had SLT involvement in the communication intervention. As SLTs have particular expertise in communication disorders, their limited representation within this review suggests a need for the SLT profession to develop its evidence-base relating to quality-of-life and communication interventions in dementia. This could include research into current SLT clinical practice, with possible future recommendations for training or more specific clinical guidance.
Many of the interventions involved a family member of the person with dementia, highlighting the important roles of these individuals in the delivery of communication interventions. Brodaty et al.’s (Citation2003) systematic review of psychosocial interventions for caregivers of people with dementia found that caregiver involvement often led to positive outcomes and study success. Of the four studies that demonstrated a statistically significant improvement in quality-of-life, all involved caregivers. This may suggest that dyadic or caregiver interventions for communication can have a positive impact on the quality-of-life of people with dementia; further research is needed in this area.
All the studies that showed statistically significant improvements in quality-of-life focused on functional communication strategies and education, as opposed to impairment-based interventions targeting linguistic abilities. Research suggests that cognitive stimulation therapy, an impairment-based intervention focused on maintaining cognitive function, can lead to improved quality-of-life for people with dementia (Spector et al., Citation2003); it is unclear why the impairment-based intervention approaches in this review did not influence quality-of-life. This could be due to small sample sizes, or that people with dementia experiencing more significant communication difficulties may be at a later stage of disease progression, resulting in difficulty engaging in impairment-based interventions.
Many of the interventions were tailored to the individual needs of participants, and an element of intervention tailoring was found in all studies that showed improvements in quality-of-life. Individual tailoring is likely to be necessary due to the heterogeneity of this population. All studies that demonstrated improvements in quality-of-life involved a block of at least weekly sessions over a 5–12-week period. Research suggests that intensive SLT positively influences outcomes in the stroke population (Breitenstein et al., Citation2017). However, given service limitations for this client group, particularly with prevalence increasing, the delivery of higher-intensity programmes may not be feasible in current service delivery models. All interventions consisted of face-to-face sessions. Further research into the efficacy of remote input for this population would be valuable, given the development of technology in recent years and the increase in remote interventions following the COVID-19 pandemic.
Outcome measures
Seven quality-of-life outcome measures were used across the 12 studies. This decreased study comparability, which contributed to the authors’ inability to meta-analyse the results. Additionally, these outcome measures make minimal reference to communication, which may reduce their sensitivity for this communication-focused review. Communication-related quality-of-life measurement tools have been standardised for the post-stroke aphasic population (e.g. ASHA QCL (Paul et al., Citation2004), SAQOL (Hilari et al., Citation2003)) but have not been standardised for use with the dementia population despite growing evidence of the association between communication difficulties and quality-of-life for this population (Banerjee et al., Citation2010; Brod et al., Citation1999; Martyr et al., Citation2018; Olthof-Nefkens et al., Citation2023; Savundranayagam et al., Citation2005; Stiadle et al., Citation2014). The identification and standardised use of a dementia-specific quality-of-life tool that includes communication-related items would be beneficial. This would facilitate effective and quantifiable measurement of the quality-of-life impact of communication interventions, and increase comparability of studies, which would support future reviews. It is increasingly recognised that quality-of-life is a valuable health outcome measure for this population, due to a lack of a cure for dementia to date, so an effective measure for this population would be valuable (Department of Health, Citation2020; Perneczky, Citation2019).
A further limitation of the included studies is that only two of them completed a follow-up after a period without study intervention. This limits the conclusions that can be drawn relating to the maintenance of intervention gains.
Quality-of-life
There is some evidence relating to the expected trajectory of quality-of-life for people with dementia and their families. Lyketsos et al. (Citation2003) found a small reduction in quality-of-life ratings in long-term care residents with dementia over a two-year period. However, quality-of-life ratings stayed the same or improved for nearly half of these residents. Clare et al. (Citation2022a) found that quality-of-life of people with mild-moderate dementia on average remained stable over a two-year period but with individual differences in particular sub-groups. They found that the quality-of-life of caregivers of people with mild-moderate dementia decreased slightly over a year period (Clare et al. Citation2022b). Quality-of-life outcomes of the interventions under discussion should be interpreted within the context of these varying trajectories and, for example, for some members of the caregiving population in particular, either stabilising or slowing the decline in quality-of-life would be a positive intervention effect. To best analyse the intervention effects within the context of varied quality-of-life trajectories for this population, studies should include large participant numbers and control groups. Nearly half of the studies in this review had under 10 participants and only six studies had control groups; three were RCTs, but none of these demonstrated statistically significant improvements. Furthermore, the risk of bias assessments highlighted several areas for concern, primarily in the lack of true randomisation and blinding within the RCTs. This demonstrates a need for further high-quality research in this area, considering designs that are appropriate to the complex nature of the interventions and that are sensitive to outcomes meaningful to people with dementia and their families. Realist approaches considering the contexts, mechanisms and outcomes of communication interventions may be useful to allow future studies to consider not only ‘what works’, but ‘what works for who, how, in what circumstances and to what extent’ (Pawson et al., Citation2005, p.32).
Strengths and limitations of this review
Four reviewers were involved in the screening process. Reviewers resolved disagreements through discussion and reference to inclusion and exclusion criteria, as advised by Siddaway et al. (Citation2019) best practice guide for systematic reviews. A patient and public involvement group discussed the plan for this systematic review and provided feedback considering lived experiences of dementia-related communication difficulties. This project was also discussed with a group of third-sector dementia professionals, who highlighted challenges in advising clients about the effectiveness of dementia-focused communication interventions.
This study includes English-language papers only, limiting the transferability of findings and potentially excluding important findings from non-English language papers. Additionally, this paper only involves studies with quantitative outcome measurements; qualitative exploration of this topic could provide a broader perspective of communication-related quality-of-life for this population.
Several methodological factors and limitations of the included papers, as well as their heterogeneity, has meant that robust conclusions cannot be drawn about the association between communication interventions and quality-of-life outcomes for this population. These include the variation in (and limitations of) the quality-of-life outcome measures used, and the complex and multi-factorial nature of quality-of-life. This means that non-controlled or non-measured aspects of participants’ lives can impact quality-of-life scores, and improvement in one element of quality-of-life might not be strong enough to affect overall quality-of-life scores. This review highlights considerations for future studies, such as the development and implementation of communication-focused quality-of-life outcome measures across studies exploring interventions targeting communication. Furthermore, it is important that future research measures communication changes as well as quality-of-life in order to establish whether intervention effects relating to quality-of-life are associated with communication changes. It is hoped that this will support the development of further robust and comparable research studies in on this topic, which would result in future systematic reviews drawing firmer conclusions about the link between communication interventions and quality-of-life.
Conclusion
This review has highlighted considerations relating to communication interventions for people with dementia and their families. However, these should be interpreted with caution due to the limited number of studies within this review, as well as the heterogeneity of the studies which limits their comparability. This review suggests the value of multi-disciplinary approaches to communication interventions which involve the families of people with dementia and focus on functional communication strategies. There is a need for further research into the quality-of-life impact of communication interventions for people with dementia, and especially into remote interventions, as these delivery models are becoming more prevalent as technology advances. This review has also highlighted the need for a more standardised approach to outcome measurement for research studies considering the quality-of-life of people with dementia, and the possibility of developing a communication-focused quality-of-life measurement for this population. Future research should also comprehensively study both the intervention and its influencing factors, considering the complex nature of these interventions. Approaches such as realist or process evaluation may be appropriate.
Supplemental Material
Download Zip (257.6 KB)Acknowledgements
The authors would like to thank Oxford Health NHS Foundation Trust Research and Development Team for their support and training opportunities. We would especially like to thank Edoardo Ostinelli for his advice relating to data analysis. The authors would also like to thank the Oxford Health NHS Foundation Trust library team for their support with identifying literature.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- American Speech-Language-Hearing Association. (2017). Dementia (Practice Portal). Retrieved October 13, 2022, www.asha.org/Practice-Portal/Clinical-Topics/Dementia/.ASHA
- Andrade-Calderón, P., Salvador-Cruz, J., & Sosa-Ortiz, A. L. (2015). Positive impact of speech therapy in progressive non-fluent aphasia. Acta Colombiana de Psicología, 18(2), 101–114. https://doi.org/10.14718/ACP.2015.18.2.9
- Banerjee, S., Willis, R., Graham, N., & Gurland, B. J. (2010). The Stroud/ADI dementia quality framework: A cross‐national population‐level framework for assessing the quality of life impacts of services and policies for people with dementia and their family carers. International Journal of Geriatric Psychiatry, 25(3), 249–257. https://doi.org/10.1002/gps.2330
- Banovic, S., Zunic, L. J., & Sinanovic, O. (2018). Communication difficulties as a result of dementia. Materia Socio-Medica, 30(3), 221–224. https://doi.org/10.5455/msm.2018.30.221-224
- Barnes, C. J., & Markham, C. (2018). A pilot study to evaluate the effectiveness of an individualized and cognitive behavioural communication intervention for informal carers of people with dementia: The Talking Sense programme. International Journal of Language & Communication Disorders, 53(3), 615–627. https://doi.org/10.1111/1460-6984.12375
- Bourgeois, M. S., Camp, C., Rose, M., White, B., Malone, M., Carr, J., & Rovine, M. (2003). A comparison of training strategies to enhance use of external aids by persons with dementia. Journal of Communication Disorders, 36(5), 361–378. https://doi.org/10.1016/S0021-9924(03)00051-0
- Breitenstein, C., Grewe, T., Flöel, A., Ziegler, W., Springer, L., Martus, P., Huber, W., Willmes, K., Ringelstein, E. B., Haeusler, K. G., Abel, S., Glindemann, R., Domahs, F., Regenbrecht, F., Schlenck, K.-J., Thomas, M., Obrig, H., de Langen, E., Rocker, R., … Baumgaertner, A., FCET2EC study group (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet (London, England), 389(10078), 1528–1538. https://doi.org/10.1016/S0140-6736(17)30067-3
- Brod, M., Stewart, A. L., Sands, L., & Walton, P. (1999). Conceptualization and measurement of quality of life in dementia: The dementia quality of life instrument (DQoL). The Gerontologist, 39(1), 25–35. https://doi.org/10.1093/geront/39.1.25
- Brodaty, H., Green, A., Sc Hons, B., & Koschera, A. (2003). Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society, 51(5), 657–664. https://doi.org/10.1034/j.1600-0579.2003.00210.x
- Burgio, L. D., & Fisher, S. E. (2000). Application of psychosocial interventions for treating behavioral and psychological symptoms of dementia. International Psychogeriatrics, 12(S1), 351–358. https://doi.org/10.1017/S1041610200007274
- Cadório, I., Figueiredo, D., Martins, P., Cardoso, R., Santos, J., & Lousada, M. (2019). Combined restorative and compensatory treatment for primary progressive aphasia: A case report. Aphasiology, 35(2), 222–239. https://doi.org/10.1080/02687038.2019.1687842
- Clare, L. (2017). Living well with dementia has become a key focus of policy. The Psychologist, 30, 66–69.
- Clare, L., Wu, Y.-T., Jones, I. R., Victor, C. R., Nelis, S. M., Martyr, A., Quinn, C., Litherland, R., Pickett, J. A., Hindle, J. V., Jones, R. W., Knapp, M., Kopelman, M. D., Morris, R. G., Rusted, J. M., Thom, J. M., Lamont, R. A., Henderson, C., Rippon, I., Hillman, A., & Matthews, F. E, IDEAL Study Team (2019). A comprehensive model of factors associated with subjective perceptions of “living well” with dementia: findings from the IDEAL study. Alzheimer Disease and Associated Disorders, 33(1), 36–41. https://doi.org/10.1097/WAD.0000000000000286
- Clare, L., Wu, Y.-T., Quinn, C., Jones, I. R., Victor, C. R., Nelis, S. M., Martyr, A., Litherland, R., Pickett, J. A., Hindle, J. V., Jones, R. W., Knapp, M., Kopelman, M. D., Morris, R. G., Rusted, J. M., Thom, J. M., Lamont, R. A., Henderson, C., Rippon, I., Hillman, A., & Matthews, F. E, IDEAL Study Team (2019). A comprehensive model of factors associated with capability to “live well” for family caregivers of people living with mild-to-moderate dementia: Findings from the IDEAL study. Alzheimer Disease and Associated Disorders, 33(1), 29–35. https://doi.org/10.1097/WAD.0000000000000285
- Clare, L., Kudlicka, A., Oyebode, J. R., Jones, R. W., Bayer, A., Leroi, I., Kopelman, M., James, I. A., Culverwell, A., Pool, J., Brand, A., Henderson, C., Hoare, Z., Knapp, M., Morgan-Trimmer, S., Burns, A., Corbett, A., Whitaker, R., & Woods, B. (2019). Goal-oriented cognitive rehabilitation for early-stage Alzheimer’s and related dementias: The GREAT RCT. Health Technology Assessment (Winchester, England), 23(10), 1–242. https://doi.org/10.3310/hta23100
- Clare, L., Gamble, L. D., Martyr, A., Sabatini, S., Nelis, S. M., Quinn, C., Pentecost, C., Victor, C., Jones, R. W., Jones, I. R., Knapp, M., Litherland, R., Morris, R. G., Rusted, J. M., Thom, J. M., Collins, R., Henderson, C., & Matthews, F. E., on behalf of the IDEAL study team (2022a). Longitudinal trajectories of quality of life among people with mild-to-moderate dementia: A latent growth model approach with IDEAL cohort study data. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 77(6), 1037–1050. https://doi.org/10.1093/geronb/gbac022
- Clare, L., Gamble, L. D., Martyr, A., Sabatini, S., Nelis, S. M., Quinn, C., Pentecost, C., Victor, C., Jones, R. W., Jones, I. R., Knapp, M., Litherland, R., Morris, R. G., Rusted, J. M., Thom, J. M., Collins, R., Henderson, C., & Matthews, F. E., on behalf of the IDEAL study team. (2022b). Living well’ trajectories among family caregivers of people with mild-to-moderate dementia in the IDEAL cohort. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 77(10), 1852–1863. https://doi.org/10.1093/geronb/gbac090
- Clarke, C., Woods, B., Moniz-Cook, E., Mountain, G., Øksnebjerg, L., Chattat, R., Diaz, A., Gove, D., Vernooij-Dassen, M., & Wolverson, E. (2020). Measuring the well-being of people with dementia: A conceptual scoping review. Health and Quality of Life Outcomes, 18(249), 1–14. https://doi.org/10.1186/s12955-020-01440-x
- Department of Health. (2020). Prime Minister’s Challenge on Dementia. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/414344/pm-dementia2020.pdf
- Droes, R.-M., Boelens-Van Der Knoop, E. C. C., Bos, J., Meihuizen, L., Ettema, T. P., Gerritsen, D. L., Hoogeveen, F., De Lange, J., & Scholzel-Dorenbos, C. J. M. (2006). Quality of life in dementia in perspective: An explorative study of variations in opinions among people with dementia and their professional caregivers, and in literature. Dementia, 5(4), 533–558. https://doi.org/10.1177/1471301206069929
- Grand, J. H., Caspar, S., & Macdonald, S. W. (2011). Clinical features and multidisciplinary approaches to dementia care. Journal of Multidisciplinary Healthcare, 4, 125–147. https://doi.org/10.2147/JMDH.S17773
- Haberstroh, J., Neumeyer, K., Krause, K., Franzmann, J., & Pantel, J. (2011). TANDEM: Communication training for informal caregivers of people with dementia. Aging & Mental Health, 15(3), 405–413. https://doi.org/10.1080/13607863.2010.536135
- Haberstroh, J., & Johannes, P. (2011). Kommunikation bei Demenz-TANDEM Trainingsmanual [Communication in dementia – TANDEM training manual]. Berlin, Heidelberg: Springer-Verlag.
- Han, A., Kim, T. H., & Hong, H. (2020). A factorial randomized controlled trial to examine separate and combined effects of a simulation-based empathy enhancement program and a lecture-based education program on family caregivers of people with dementia. Aging & Mental Health, 25(10), 1930–1940. https://doi.org/10.1080/13607863.2020.1768214
- Hilari, K., Byng, S., Lamping, D. L., & Smith, S. C. (2003). Stroke and aphasia quality of life scale-39 (SAQOL-39) evaluation of acceptability, reliability, and validity. Stroke, 34(8), 1944–1950. https://doi.org/10.1161/01.STR.0000081987.46660.ED
- Hoefman, R. J., van Exel, N. J. A., Looren de Jong, S., Redekop, W. K., & Brouwer, W. B. F. (2011). A new test of the construct validity of the Carer Qol instrument: Measuring the impact of informal care giving. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 20(6), 875–887. https://doi.org/10.1007/s11136-010-9829-8
- Hoffmann, T. C., Glasziou, P. P., Boutron, I., Milne, R., Perera, R., Moher, D., Altman, D. G., Barbour, V., Macdonald, H., Johnston, M., Lamb, S. E., Dixon-Woods, M., McCulloch, P., Wyatt, J. C., Chan, A.-W., & Michie, S. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clinical Research ed.), 348, g1687. https://doi.org/10.1136/bmj.g1687
- Jokel, R., Meltzer, J., D R, J., D M, L., J C, J., A N, E., & D T, C. (2017). Group intervention for individuals with primary progressive aphasia and their spouses: Who comes first? Journal of Communication Disorders, 66, 51–64. https://doi.org/10.1016/j.jcomdis.2017.04.002
- Joseph, S., Becker, S., Elwick, H., & Silburn, R. (2012). Adult carers quality of life questionnaire (AC-QoL): Development of an evidence-based tool. Mental Health Review Journal, 17(2), 57–69. https://doi.org/10.1108/13619321211270380
- Judge, K. S., Yarry, S. J., Looman, W. J., & Bass, D. M. (2013). Improved strain and psychosocial outcomes for caregivers of individuals with dementia: Findings from project answers. The Gerontologist, 53(2), 280–292. https://doi.org/10.1093/geront/gns076
- La Rue, A., Felten, K., & Turkstra, L. (2015). Intervention of multi-modal activities for older adults with dementia translation to rural communities. American Journal of Alzheimer’s Disease and Other Dementias, 30(5), 468–477. https://doi.org/10.1177/1533317514568888
- Leroi, I., Simkin, Z., Hooper, E., Wolski, L., Abrams, H., Armitage, C. J., Camacho, E., Charalambous, A. P., Collin, F., Constantinidou, F., Dawes, P., Elliott, R., Falkingham, S., Frison, E., Hann, M., Helmer, C., Himmelsbach, I., Hussain, H., Marié, S., … Yeung, W. K. (2020). Impact of an intervention to support hearing and vision in dementia: The SENSE-Cog Field Trial. International Journal of Geriatric Psychiatry, 35(4), 348–357. https://doi.org/10.1002/gps.5231
- Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L. (1999). Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging, 5(1), 21–32.
- Lyketsos, C. G., Gonzales-Salvador, T., Chin, J. J., Baker, A., Black, B., & Rabins, P. (2003). A follow-up study of change in quality of life among persons with dementia residing in a long-term care facility. International Journal of Geriatric Psychiatry, 18(4), 275–281. https://doi.org/10.1002/gps.796
- Martyr, A., Nelis, S. M., Quinn, C., Wu, Y.-T., Lamont, R. A., Henderson, C., Clarke, R., Hindle, J. V., Thom, J. M., Jones, I. R., Morris, R. G., Rusted, J. M., Victor, C. R., & Clare, L. (2018). Living well with dementia: A systematic review and correlational meta-analysis of factors associated with quality of life, well-being, and life satisfaction in people with dementia. Psychological Medicine, 48(13), 2130–2139. https://doi.org/10.1017/S0033291718000405
- Messemaker, A., Schall, A., Haberstroh, J., & Pantel, J. (2017). MultiTANDEM: Training the trainer to improve homecare for people with dementia. The Journal of Gerontopsychology and Geriatric Psychiatry, 30(4), 165–175. https://doi.org/10.1024/1662-9647/a000178
- Mulhern, B., Rowen, D., Brazier, J., Smith, S., Romeo, R., Tait, R., Watchurst, C., Chua, K.-C., Loftus, V., Young, T., Lamping, D., Knapp, M., Howard, R., & Banerjee, S. (2013). Development of DEMQOL-U and DEMQOL-PROXY-U: Generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technology Assessment (Winchester, England), 17(5), v. https://doi.org/10.3310/hta17050
- Negri, L., Piazza, G., Sartori, R. D. G., Cocchi, M. G., & Delle Fave, A. (2019). The adult carer quality of life questionnaire (AC-QoL): Comparison with measures of burden and well-being, and Italian validation. Disability and Rehabilitation, 41(10), 1207–1216. https://doi.org/10.1080/09638288.2017.1423519
- Olthof-Nefkens, M. W., Derksen, E. W., Lambregts, B., de Swart, B. J., Nijhuis-van der Sanden, M. W., & Kalf, J. G. (2023). Clinimetric evaluation of the experienced communication in dementia questionnaire. The Gerontologist, 63(1), 40–51. https://doi.org/10.1093/geront/gnab187
- Paul, D. R., Frattali, C. M., Holland, A. L., Thompson, C. K., Caperton, C. J., & Slater, S. C. (2004). Quality of communication life scale. Rockville, MD: American Speech-Language-Hearing Association.
- Pawson, R., Greenhalgh, T., Harvey, G., & Walshe, K. (2005). Realist review – A new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy, 10(1_suppl), 21–34. https://doi.org/10.1258/1355819054308530
- Perneczky, R. (2019). Dementia treatment versus prevention. Dialogues in Clinical Neuroscience, 21(1), 43–51. https://doi.org/10.31887/dcns.2019.21.1/rperneczky
- Prince, M., Bryce, R., & Ferri, C. (2011). Alzheimer’s Disease International World Alzheimer Report 2011. The benefits of early diagnosis and intervention. Alzheimer’s Disease International.
- Prince, M., Albanese, E., Guerchet, M., & Prina, M. (2014). World Alzheimer Report 2014: Dementia and risk reduction: An analysis of protective and modifiable risk factors.
- Quinn, C., Pickett, J. A., Litherland, R., Morris, R. G., Martyr, A., & Clare, L. On behalf of the IDEAL Programme Team. (2022). Living well with dementia: What is possible and how to promote it. International Journal of Geriatric Psychiatry, 37(1), 1–7. https://doi.org/10.1002/gps.5627
- Ross, G. W., Cummings, J. L., & Benson, D. F. (1990). Speech and language alterations in dementia syndromes: Characteristics and treatment. Aphasiology, 4(4), 339–352. https://doi.org/10.1080/02687039008249087
- Royal College of Speech and Language Therapists. (2014). Speech and language therapy provision for people with dementia RCSLT Position Paper. https://www.rcslt.org/wp-content/uploads/media/Project/RCSLT/dementia-position-paper-2014.pdf
- Rüttinger, B., & Sauer, J. (2000). Konflikt und Konfliktlösen. Springer Gabler, Wiesbaden: Rosenberger Fachverlag.
- Santos, G. D., Nunes, P. V., Stella, F., Brum, P. S., Yassuda, M. S., Ueno, L. M., Gattaz, W. F., & Forlenza, O. V. (2015). Multidisciplinary rehabilitation program: Effects of a multimodal intervention for patients with Alzheimer’s disease and cognitive impairment without dementia. Archives of Clinical Psychiatry (São Paulo), 42(6), 153–156. https://doi.org/10.1590/0101-60830000000066
- Savundranayagam, M. Y., Hummert, M. L., & Montgomery, R. J. V. (2005). Investigating the effects of communication problems on caregiver burden. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(1), S48–S55. https://doi.org/10.1093/geronb/60.1.S48
- Schwam, E., & Xu, Y. (2010). Cognition and function in Alzheimer’s disease: Identifying the transitions from moderate to severe disease. Dementia and Geriatric Cognitive Disorders, 29(4), 309–316. https://doi.org/10.1159/000269837
- Siddaway, A. P., Wood, A. M., & Hedges, L. V. (2019). How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annual Review of Psychology, 70(1), 747–770. https://doi.org/10.1146/annurev-psych-010418-102803
- Smith, S. C., Lamping, D. L., Banerjee, S., Harwood, R. H., Foley, B., Smith, P., Cook, J. C., Murray, J., Prince, M., Levin, E., Mann, A., & Knapp, M. (2007). Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychological Medicine, 37(5), 737–746. https://doi.org/10.1017/S0033291706009469
- Spector, A., Thorgrimsen, L., Woods, B. O. B., Royan, L., Davies, S., Butterworth, M., & Orrell, M. (2003). Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: Randomised controlled trial. The British Journal of Psychiatry: The Journal of Mental Science, 183(3), 248–254. https://doi.org/10.1192/bjp.183.3.248
- Stiadle, J. M., Scholar, M., & Zarit, S. H. (2014). Communication problems between caregivers and individuals with dementia: Implications for caregiver well-being [Doctoral dissertation]. Pennsylvania State University.
- Teri, L., Logsdon, R. G., McCurry, S. M., Pike, K. C., & McGough, E. L. (2018). Translating an evidence-based multicomponent intervention for older adults with dementia and caregivers. The Gerontologist, 60(3), 548–557. https://doi.org/10.1093/geront/gny122
- The WHOQOL Group. (1998). WHO Quality of life scale (WHOQOL-BREF). Psychological Medicine, 28(3), 551–558. https://doi.org/10.1017/S0033291798006667
- Thorgrimsen, L., Selwood, A., Spector, A., Royan, L., de Madariaga Lopez, M., Woods, R. T., & Orrell, M. (2003). Whose quality of life is it anyway? The validity and reliability of the quality of life-Alzheimer’s disease (QoL-AD) scale. Alzheimer Disease and Associated Disorders, 17(4), 201–208. https://doi.org/10.1097/00002093-200310000-00002
- Volkmer, A., Spector, A., Meitanis, V., Warren, J. D., & Beeke, S. (2020). Effects of functional communication interventions for people with primary progressive aphasia and their caregivers: A systematic review. Aging & Mental Health, 24(9), 1381–1393. https://doi.org/10.1080/13607863.2019.1617246
- Woods, B., Thorgrimsen, L., Spector, A., Royan, L., & Orrell, M. (2006). Improved quality of life and cognitive stimulation therapy in dementia. Aging & Mental Health, 10(3), 219–226. https://doi.org/10.1080/13607860500431652
- Woodward, M. (2013). Aspects of communication in Alzheimer’s disease: Clinical features and treatment options. International Psychogeriatrics, 25(6), 877–885. https://doi.org/10.1017/S1041610213000318
- World Health Organisation. (2012). WHOQOL User Manual. Retrieved October 13, 2022, from https://www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03
- World Health Organization. (2019). ICD-11: International classification of diseases (11th revision). Retrieved February 12, 2021, from, https://icd.who.int/
- World Health Organisation. (2021). Global status report on the public health response to Dementia. Retrieved October 13, 2022, from https://www.who.int/publications/i/item/9789240033245