Abstract
Objectives
This study tested the association between neuroticism and six cognitive measures, and examined the potential mediating roles of social connection (social isolation and loneliness) among middle-aged and older adults.
Methods
This cross-sectional study was a secondary analysis of the Canadian Longitudinal Study on Aging (CLSA) Comprehensive Cohort, a sample of Canadians aged 45–85 years at baseline. Respondents with data collected at the first follow-up, between 2015 and 2018, were included (n = 27,765). Structural equation modelling was used to assess the association between neuroticism and six cognitive measures (Rey Auditory Verbal Learning Test immediate recall and delayed recall, Animal Fluency Test, Mental Alternation Test, Controlled Oral Word Association Test and Stroop Test interference ratio), with direct and indirect effects (through social isolation and loneliness). All analyses were stratified by sex, including females (n = 14,133) and males (n = 13,632).
Results
In unadjusted models, there was evidence of associations between neuroticism and all cognitive measures, except the Stroop Test interference ratio, suggesting higher neuroticism was associated with lower scores on memory and executive function tests. In the models of these other five outcomes, there was consistent evidence of indirect effects (through social isolation and loneliness) and, in some cases, direct effects. The results are discussed in context with limitations, including the use of cross-sectional design and alternative hypotheses to explain the association between personality and cognition.
Conclusion
Among middle-aged and older adults, for both males and females, the findings suggest that the association between neuroticism and cognitive outcomes may be mediated by aspects of social connection.
Introduction
Social connection is associated with better health, including cognitive outcomes. Recent systematic reviews have linked both subjective and objective measures of social connection, e.g. loneliness and social isolation, with incident dementia (Kuiper et al., Citation2015; Lara et al., Citation2019; Penninkilampi et al., Citation2018) and worse cognitive function (Evans et al., Citation2019; Kelly et al., Citation2017; Kuiper et al., Citation2016). However, while the overlap between social isolation and loneliness is only partial (Menec et al., Citation2020), the mechanisms for the associations between the different aspects of social connection and the risk of dementia (Sommerlad et al., Citation2023) have been hypothesized to include health-related behaviours (e.g. physical inactivity) (Malcolm et al., Citation2019), psychological mediators (e.g. stress) (Holt-Lunstad & Smith, Citation2016) and cognitive reserve (Stern et al., Citation2020). Emerging evidence from studies of neuro-immune markers (e.g. suggesting social isolation and loneliness are distinctly associated with levels and regulation of inflammation, respectively) (Walker et al., Citation2019) may help to substantiate and distinguish biological pathways. Still, there is growing consensus that the health impacts of objective and subjective aspects of social connection should be studied together—but as distinct phenomena (Cornwell & Waite, Citation2009; Holt-Lunstad et al., Citation2015; Malcolm et al., Citation2019; Menec et al., Citation2020).
Personality traits are also thought to influence cognitive outcomes. For example, a linked cohort study found that high school personality could predict dementia risk almost five decades later (Chapman et al., Citation2020). Analysis of the Health and Retirement Study, a large population-based longitudinal study of Americans aged 50 years or older, found that personality traits were associated with the risk of dementia even after controlling for demographic characteristics and other risk factors for dementia (Terracciano et al., Citation2017). The findings related to neuroticism (a personality trait characterized by emotional instability), have been substantiated in other studies with outcomes including lower cognitive function (Sutin et al., Citation2019) as well as increased risk of mild cognitive impairment (Yoneda et al., Citation2023) and dementia (Johansson et al., Citation2014; Low et al., Citation2013; Terracciano et al., Citation2014). A 2021 meta-analysis pooled the results of 12 prospective studies to show that, among the five personality traits (neuroticism, extraversion, openness, agreeableness and conscientiousness), lower conscientiousness and higher neuroticism were associated with increased dementia risk; for the latter, every standard deviation increase in neuroticism, the risk of dementia increased by 24% (hazard ratio = 1.24, 95% CI [1.17, 1.31], p < .001), a potential effect size likened to that of other risk factors including diabetes, hypertension, smoking and physical inactivity (Aschwanden et al., Citation2021). Potential causal and non-causal explanations have been proposed for this observed relationship. For example, considering the latter, neuroticism may lead to more care-seeking (Friedman et al., Citation2013; Hajek et al., Citation2017) and thus greater detection of cognitive disorders, thereby explaining the observed associations. However, findings from prospective studies with structured clinical evaluations (Yoneda et al., Citation2023) suggest that this bias does not explain the link between neuroticism and cognition. Common cause (e.g. personality and dementia have a shared genetic etiology), predisposition (e.g. mediation through health-related behaviors) and pathoplastic (e.g. resistance to the effects of neuropathology) hypotheses have been highlighted for future research (Segerstrom, Citation2020; Terracciano & Sutin, Citation2019).
Personality traits and social connection are also linked to each other. Personality traits are characterized by social attributes (e.g. extraverted individuals are more sociable). Mechanisms initially proposed to explain the relationship between personality and social support may extend to other aspects of social connection; that is, one’s personality might influence how they build their social environment, how others react to them and how they evaluate their social relationships (Pierce et al., Citation1997). Although neuroticism is not most closely characterized by social attributes, it is associated with aspects of social connection. A 2019 meta-analysis reported that lower neuroticism was associated with greater perceived availability of social support (Barańczuk, Citation2019). Moreover, a 2020 meta-analysis estimated the correlations between the ‘big five’ personality traits and loneliness, identifying neuroticism as one of the strongest associations (second only to extraversion) (Buecker et al., Citation2020). Both studies implicated roles for stress, including vulnerability and reactivity linked to neuroticism, as well as social responses from others.
Taken together, there is evidence that the potential effects of personality and social connection on cognitive outcomes may be linked. However, very few studies have simultaneously examined the relationships between personality, objective, and subjective aspects of social connection and health (Mund & Neyer, Citation2016). The objective of this study was to test the association between neuroticism and cognition and the potential mediating roles of social isolation and loneliness among middle-aged and older adults. Neuroticism is relatively stable in this age range (Steunenberg et al., Citation2005), and this demographic group has particular risks related to both social connection (e.g. retirement and loss of social networks) as well as cognitive outcomes (e.g. increasing risk of dementia). The analysis focused on neuroticism as it is the strongest (personality) predictor of both loneliness and cognitive outcomes. Little is known about how the association between neuroticism and cognition may differ between males and females. However, given the evidence of potential sex or gender differences in the association between neuroticism and loneliness (Ormstad et al., Citation2020), potential differences were also explored in this study. Understanding the associations between personality, social connection, and cognition will help identify strategies for maintaining cognitive function. Evidence to further a conceptual model linking objective and subjective measures of social connection to health, while also acknowledging potential differential effects across populations (Courtin & Knapp, Citation2017) (e.g. personality traits and/or by sex), will help to develop, tailor and test interventions targeting social connection as a means of improving health outcomes (Cohen-Mansfield & Perach, Citation2015; Gardiner et al., Citation2018).
Methods
This was a secondary analysis of the Canadian Longitudinal Study on Aging (CLSA) Comprehensive Cohort, a sample of Canadians aged 45–85 years at the time of recruitment, between 2011 and 2015 (Raina et al., Citation2009, Citation2019). To be eligible for the Comprehensive Cohort, participants were required to live within 25–50 km of one of 10 CLSA data collection sites across Canada. The CLSA was restricted to those who were able to complete the interviews in English or French. Individuals living in long-term care institutions and those who had self-reported overt cognitive impairment at the time of recruitment were excluded at baseline. For this cross-sectional study, only those respondents who provided data at the first follow-up, between 2015 and 2018 (n = 27,765) were included. Although the study intended to use two waves of data (baseline and first follow-up) to model changes in cognitive outcomes, very little change was observed in the data, thus, a cross-sectional design was adopted. The first follow-up data were selected as they included the three-item loneliness measure (described below).
Measures
Neuroticism was assessed at the first follow-up using an adaptive administration of the Ten Item Personality Inventory (TIPI) (Gosling et al., Citation2003). The CLSA created derived variables by taking the mean score of the two questions on emotional stability, which scored 1–7 (with higher scores indicating greater endorsement of the trait).
Loneliness, a measure of subjectively feeling alone (Holt-Lunstad, Citation2021), was assessed at the first follow-up using a three-item loneliness scale (Hughes et al., Citation2004), with questions on the frequency of feeling a lack of companionship, left out and isolated from others and where each of these items was recoded as 0 (hardly ever), 1 (some of the time) or 2 (often) and then added to generate a loneliness score from 0 to 6 (with higher scores indicating greater loneliness).
Social isolation, a measure of objectively being alone (Holt-Lunstad, Citation2021), was assessed using data collected at baseline and first follow-up with a social isolation index. The index was derived from indicators for each of (1) living alone and not married or in a common law relationship; (2) got together with friends or neighbours within the past 6 months or less frequently, or reported having no friends or neighbours; (3) got together with relatives/siblings within the past 6 months or less frequently, or reported having no relatives or siblings; (4) got together with children within the past 6 months or less frequently, or had no children; (5) being retired and having participated in none or only one of eight social activities at least once a month or more often. The indicators were added to generate the social isolation score from 0 to 5 (with higher scores indicating greater social isolation) (Menec et al., Citation2020).
Cognition was assessed at the first follow-up using six assessments of memory and executive function (Tuokko et al., Citation2020). As part of the CLSA protocol, trained interviewers administered all cognitive measures in a standardized order and scoring was standardized with the aid of computerized algorithms.
Memory In the modified Rey Auditory Verbal Learning Test (Rey, Citation1964), respondents are asked to recall a list of 15 common words immediately (Rey 1) and after a 5-min delay (Rey 2). Higher scores indicate better test performance.
Executive function In the Animal Fluency Test (AFT) (Read, Citation1987), respondents were asked to name as many animals as possible in 60 s. CLSA had two scoring methods and, consistent with how this test is scored clinically, the second score was used in this analysis. Higher scores indicate better test performance.
In the Mental Alternation Test (MAT) (Teng, Citation1995), respondents were asked to complete three sub-tasks involving letters and numbers (count from 1 to 20, recite the letters of the alphabet and alternate between numbers and in order), and the score is the number of correct consecutive numeric and alphabetical alternations in 30 s. Higher scores indicate better test performance.
In the Controlled Oral Word Association Test (COWAT) (Lezak et al., Citation2004), respondents were asked to complete three sub-tasks involving naming words starting with a particular letter within 60 s. The scores from the three tests were summed for the analysis. Higher scores indicate better test performance.
In the Victoria Version of the Stroop Test (Bayard et al., Citation2011; Troyer et al., Citation2006) (interference ratio), respondents were asked to complete three sub-tasks involving colours and words printed on three cards: dot (naming the colour of the ink in dots); word (naming the colour of the ink in common, non-colour words); and colour (naming the colour of the ink where colour words (e.g. ‘blue’) are printed in non-corresponding colours of ink). The test was scored with time to completion and the number of errors on each of the three parts of the test. The interference ratio was calculated by dividing the time required to complete the last card (i.e. colour) by the time required to complete the first card (i.e. dot). Unlike the other cognitive tests (Rey, AFT, MAT, and COWAT), with the Stroop Test (interference ratio), lower scores indicate better test performance.
Adjustment variables were selected as potential confounders based on existing literature, namely: age (in years, self-reported at first follow-up); educational attainment (highest level of education, self-reported at baseline) and hearing (assessed using air-conduction hearing thresholds, measured for 8 frequencies in both ears by trained technicians with a semi-automated screening audiometer in a quiet office room, and calculated as the bilateral mid-frequency (1000, 2000, 3000, and 4000 Hz) pure tone threshold average) (Phillips et al., Citation2022). Each of these variables (i.e. age, educational attainment, and hearing) have been associated with the risk of dementia (Livingston et al., Citation2020) as well as social connection (Menec et al., Citation2019; Shukla et al., Citation2020). Other risk factors for dementia—including measures of health-related behaviours (e.g. smoking) and health status (e.g. hypertension) as well as physiological measures (e.g. inflammation)—were not included as potential confounders since they may lie on the causal pathways between neuroticism, social connection, and brain health (Cené et al., Citation2022; Segerstrom, Citation2020).
All analyses were stratified by self-reported sex (male or female, reported at baseline).
Statistical analysis
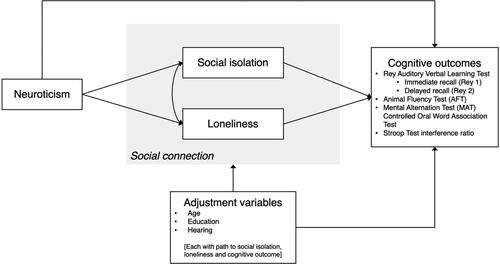
First, a path diagram was prepared to articulate the hypothesized associations and mediation process, including direct and indirect effects (see ); although all analyses were stratified by sex, the path diagrams were not sex-specific. Second, the associations between neuroticism and each of the six cognitive measures were estimated using linear regression. Third, structural equation modelling (SEM) was used to test the association between neuroticism and each of the six measures of cognition and evaluate the mediation models. None of the measures were treated as latent variables in the model. Full-information maximum likelihood (FIML) estimation was used to handle missing values. Fourth, sex differences were explored by computing tests for group invariance of SEM parameter estimates for the pathways between each of neuroticism, loneliness, and social isolation and the cognitive outcome as well as for the pathway between neuroticism and each of loneliness and social isolation, from sex-stratified (male and female) models. All analyses were conducted using STATA 16 and with statistical significance set at p = 0.05.
Figure 1. Path diagram of hypothesized associations and mediation process, including direct and indirect effects.
Sensitivity analyses: given potential differences in the appropriateness of measures for use in language-based subsamples and recommendations to examine such subsamples separately when using the cognitive measures from the CLSA (Tuokko et al., Citation2020), analyses were repeated excluding respondents where language of response was French (the smaller of the language-based subsamples) on any of the cognitive measures, leaving n = 11,144 females and n = 10,849 males.
Results
The study sample consisted of 14,133 females and 13,632 males (see for description). Compared to males, females reported higher mean levels of neuroticism (2.43 vs. 2.16; p < 0.001), loneliness (0.90 vs. 0.72; p < 0.001), and social isolation (1.70 vs. 1.67; p = 0.017). Sex differences were also apparent in the cognitive measures, where females had higher mean scores on measures of memory (Rey 1: 7.08 vs. 6.05; p < 0.001 and Rey 2: 5.27 vs. 4.08; p < 0001) whereas mean scores on executive function were sometimes higher (COWAT: 41.48 vs. 39.35; p < 0.001), lower (AFT: 21.26 vs. 21.71; p < 0.001 and MAT: 25.72 vs. 27.15; p < 0.001) or showed no difference (Stroop: 2.14 vs. 2.12; p = 0.082).
Table 1. Summary of study sample characteristics, stratified by sex.
The results of the unadjusted regression and SEMs are reported for memory (see ) and executive function (see ) outcomes.
Table 2. Results of unadjusted and structural equation modelling, for memory outcomes.
Table 3. Results of unadjusted and structural equation modelling, for executive functioning outcomes.
In the unadjusted models, there were statistically significant inverse associations between loneliness and five of six cognitive outcomes (Rey 1, Rey 2, AFT, MAT, and COWAT); for the exception, Stroop interference, the association was positive in both females and males but only statistically significant in females. Unadjusted models for the association between social isolation and cognitive outcomes were less consistent. For both males and females, there were statistically significant positive associations between social isolation and four of six cognitive outcomes (Rey 1, Rey 2, AFT, and MAT). Conversely, the positive association with COWAT was only statistically significant among males and there were statistically significant inverse associations between social isolation and Stroop (for both males and females). There were inverse associations between neuroticism and cognitive outcomes, except for Stroop interference, although not all the associations were statistically significant.
In the SEMs, the inverse associations between loneliness and cognitive outcomes were statistically significant except for the Stroop interference (both females and males). The inverse associations between social isolation and cognitive outcomes were all statistically significant except for AFT (females) and Rey 2 (males). The parameter estimates for the pathways between neuroticism and loneliness were positive and statistically significant for both females (0.306, p < 0001) and males (0.273, p < 0.001), and the test for group invariance of parameters suggested the association was stronger for females (p = 0.002). The parameter estimates for the pathways between neuroticism and social isolation were similarly positive and statistically significant for females (0.025, p = 0.001) and males (0.059, p < 0.001), but this time the test for group invariance of parameters suggested the association was stronger for males (p = 0.002).
In each of the SEMs, the parameter estimates for neuroticism (total effects) were statistically significant, except for the Stroop interference (both females and males) and COWAT (for males). Aside from these three models, the findings suggest an inverse association between neuroticism and cognitive outcomes that is at least partially mediated by loneliness and social isolation; in this subset of nine models, the parameter estimates for neuroticism (indirect effects) were statistically significant and, in six of these models, the parameter estimates for neuroticism (direct effects) were statistically significant. Comparing SEM models from males and females, the only statistically significant tests for group invariance of parameters for the pathways with cognitive outcomes were between neuroticism and Rey 1, as well as social isolation and AFT (see ). Sensitivity analyses that excluded those who responded to cognitive assessments in French produced similar results, except that the total effects for neuroticism were not statistically significant for either males or females in the COWAT models (see Supplementary Material).
Table 4. Results of structural equation modelling, tests for (male/female) group invariance of parameters, for memory and executive functioning outcomes.
Discussion
These study findings suggested that the association between neuroticism and cognition may be partially mediated through subjective and objective aspects of social connection, namely, loneliness and social isolation. The indirect effect of neuroticism, that is, the pathway from neuroticism to cognition through loneliness and social isolation, was statistically significant for five of the six cognitive outcomes (all except the Stroop interference ratio). This pattern of indirect effects was apparent among both females and males, and there was little evidence that the association between neuroticism and cognition differed by sex.
These findings are consistent with previous research linking neuroticism to cognitive outcomes. A 2019 study of 2865 older adults who participated in the Health and Retirement Study tested the associations between five-factor model personality traits and found that neuroticism was associated with worse performance on all of the cognitive tasks and there was little evidence of sex differences in these associations; potential explanations for the associations included the impact of self-consciousness and performance anxiety related to higher neuroticism as well as similar mechanisms proposed for the relationship between neuroticism and dementia risk (i.e. the impact of neuroticism on health-related behaviours and vulnerability to stress) (Sutin et al., Citation2019). Others have also examined this apparent association, including finding that intrusive thoughts mediated the relationship between neuroticism and cognitive performance (Munoz et al., Citation2013) and equivocal support for a moderating role for depression (Boyle et al., Citation2010). Conversely, others have tested the interaction between personality and social connection in predicting cognitive outcomes. In their cohort study, Wang et al. found that neuroticism was associated with an increased risk of dementia only among those who were socially isolated and proposed that the association between neuroticism and the risk of dementia may be buffered by social connection (Wang et al., Citation2009). In their study, Segel-Karpas and Lachman proposed that the beneficial effects of social contact depend on personality traits. In particular, their findings suggested that neuroticism was inversely associated with both episodic memory and executive functioning but moderated the association between social contact and episodic memory such that the beneficial impact of social contact on episodic memory was not apparent among those with high neuroticism (Segel-Karpas & Lachman, Citation2018). This study builds on previous research examining cognitive outcomes by considering the evidence linking neuroticism to social and cognitive outcomes, to a priori hypothesize and test a mediation model in the analysis. The findings further inform research linking neuroticism to the risk of dementia by providing evidence for the predisposition hypothesis (Segerstrom, Citation2020; Terracciano & Sutin, Citation2019), where the risk is at least partially conferred by mediation through health-related behaviors and, in particular, social connection.
Evidence of mediation effects through objective and subjective aspects of social connection has implications for policy, clinical practice, and research. The findings highlight these potentially modifiable risk factors and underscore the potential of addressing social connection to promote brain health in aging. Still, it is important not to overestimate the importance of individual factors, including personality, in predicting social connection (de Jong-Gierveld, Citation1987) and instead consider the potential for policy (Umberson & Montez, Citation2010) and public health action (Holt-Lunstad et al., Citation2017). Potential population approaches to address social connection have been proposed, including optimizing the built environment to enable social connection and increasing public awareness about the benefits of social connection, such as through the establishment of guidelines similar to those for diet and exercise (Holt-Lunstad, Citation2023). Still, research to substantiate the biological mechanisms underpinning the associations between social connection and health, as well as the roles of individual- and population-level characteristics and evidence to guide recommendations (e.g. frequency and context of social engagement) would help to drive such initiatives (Holt-Lunstad et al., Citation2017). These findings highlight personality among individual-level factors to consider as a potential predictor of social connection but do not explain the mechanisms for the apparent association between social connection and cognitive outcomes. Neuroimaging studies suggest neuroticism may be associated with amyloid and tau neuropathology (Terracciano et al., Citation2022). There is also evidence suggesting effects of personality on brain structure (DeYoung et al., Citation2010), including that hippocampal volume may mediate the association between neuroticism and dementia (Duchek et al., Citation2020).
This study also highlights areas for future research, including to investigate the potential health effects of other aspects of personality and social connection. In particular, while the analysis focused on one aspect of personality (i.e. neuroticism), higher conscientiousness has also been linked to a reduced risk of dementia. However, the mechanisms to explain this association are unclear. Further, there is evidence of associations between personality traits and social support (Barańczuk, Citation2019) as well as preliminary evidence of a link between social support and cognition (Costa-Cordella et al., Citation2021). Although this study examined loneliness and social isolation as the aspects of social connection that have been linked to the risk of dementia and cognitive function (Evans et al., Citation2019; Kelly et al., Citation2017; Kuiper et al., Citation2015, Citation2016; Lara et al., Citation2019; Penninkilampi et al., Citation2018), it is possible social support may influence cognitive outcomes, such as through the impact on stress. These findings have implications for research on other health outcomes. Personality (Graham et al., Citation2017) as well as objective and subjective social connection (Cené et al., Citation2022) have been similarly identified as risk factors for mortality; for personality, mediation through aspects of social connection may also explain some of the apparent association.
Limitations
The CLSA is a large sample of middle-aged and older Canadian adults with rich data to investigate personality, social connection, and health (Menec et al., Citation2019, Citation2020). The results of this study build on previous research by including distinct measures of both objective and subjective social connection and specifically testing a mediation hypothesis to explain the association between personality and health. Still, the study has several important limitations. First, the data were cross-sectional; although the hypothesized associations and mediation process were premised on the temporal ordering of neuroticism, social connection, and cognition, alternative pathways are plausible. In particular, there may be bidirectional effects between personality and loneliness (Buecker et al., Citation2020), and although the CLSA excluded individuals with cognitive impairment, personality traits may be impacted by prodromal dementia. Second, the study could also not rule out other alternative hypotheses to explain the association between personality and cognition; the mediation model tested here assumes a predisposition model, but common cause and pathoplastic (e.g. the influence of personality on resilience and susceptibility to neuropathology) mechanisms have also been described (Segerstrom, Citation2020). Third, these findings are limited by the nature of the assessment of cognition in the CLSA. The memory assessment did not include learning, and has no visual or psychomotor measures, and the assessment of inhibition (Stroop interference ratio) only measured time-based evidence of interference and not errors in performance. Indeed, the lack of accounting for errors in Stroop performance could have accounted for the findings from the Stroop that were not statistically significant, which is contrasted with the findings with the other cognitive measures. Further, while these cognitive assessments are supported for use in large studies of aging and may predict cognitive decline and dementia (Tuokko et al., Citation2017, Citation2020)—they do not equate to a clinical diagnosis and, in fact, those with overt cognitive impairment at baseline were excluded from the CLSA. Fourth, although it seems more plausible that socially constructed roles, behaviours, expressions, and identities (i.e. gender) affect these relationships rather than biological differences (i.e. sex), 99.9% of the sample reported current gender identity as male or female and without more detailed measures of gender, these differences were explored stratifying by sex. Fifth, the measurement of personality was through a necessarily brief, self-reported assessment that was not initially developed for older adults so undoubtedly included some measurement error. In fact, even using the more detailed NEO Five-Factor Inventory, in a sample of cognitively unimpaired older adults informant-reported neuroticism had stronger associations with cognitive outcomes than the self-reported measure (Duchek et al., Citation2020). Finally, if the association between neuroticism and cognitive outcomes differs across populations, CLSA sampling criteria (e.g. able to respond in English or French, no overt cognitive impairment precluding ability to provide consent) may limit the generalizability of the results. It is notable, however, that the age-stratified random sampling approach used by the CLSA makes it one of the more representative studies available.
Conclusion
This study found that, among middle-aged and older adults, neuroticism was inversely associated with five (of six) measures of cognition and evidence suggesting that social connection may partially mediate the association in both males and females. These findings reiterate others’ calls to consider the importance of including personality in conceptual models of health, including cognitive functioning (Low et al., Citation2013; Segel-Karpas & Lachman, Citation2018). Evidence of effects through social connection has potential implications for both research and clinical practice. The findings highlight a modifiable mediator of the potential effects of personality on health, suggesting the potential for tailored prevention approaches (Aschwanden et al., Citation2021; Low et al., Citation2013). Given the reliance on cross-sectional data in this study, future prospective research is needed to substantiate these findings; such research must incorporate assessments of personality, objective and subjective social connection, and cognitive outcomes, including the risk of dementia. The findings of direct effects also call for research into other potential mediators. Such studies would be strengthened by measures of neuropathology to investigate potential moderation, thereby testing an alternative, pathoplastic model to explain the association between personality and dementia (Aschwanden et al., Citation2021; Segerstrom, Citation2020).
Ethical approval
The study was approved by the Research Ethics Board at University Health Network.
Supplemental Material
Download MS Word (33.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available from the Canadian Longitudinal Study on Aging (www.clsa-elcv.ca) for researchers who meet the criteria for access to de-identified CLSA data.
Additional information
Funding
References
- Aschwanden, D., Strickhouser, J. E., Luchetti, M., Stephan, Y., Sutin, A. R., & Terracciano, A. (2021). Is personality associated with dementia risk? A meta-analytic investigation. Ageing Research Reviews, 67, 101269. https://doi.org/10.1016/j.arr.2021.101269
- Barańczuk, U. (2019). The five factor model of personality and social support: A meta-analysis. Journal of Research in Personality, 81, 38–46. https://doi.org/10.1016/j.jrp.2019.05.002
- Bayard, S., Erkes, J., Moroni, C., & Collège des Psychologues Cliniciens spécialisés en Neuropsychologie du Languedoc Roussillon (2011). Victoria Stroop Test: Normative data in a sample group of older people and the study of their clinical applications in the assessment of inhibition in Alzheimer’s disease. Archives of Clinical Neuropsychology, 26(7), 653–661. https://doi.org/10.1093/arclin/acr053
- Boyle, L. L., Lyness, J. M., Duberstein, P. R., Karuza, J., King, D. A., Messing, S., & Tu, X. (2010). Trait neuroticism, depression, and cognitive function in older primary care patients. The American Journal of Geriatric Psychiatry, 18(4), 305–312. https://doi.org/10.1097/JGP.0b013e3181c2941b
- Buecker, S., Maes, M., Denissen, J. J. A., & Luhmann, M. (2020). Loneliness and the big five personality traits: A meta-analysis. European Journal of Personality, 34(1), 8–28. https://doi.org/10.1002/per.2229
- Cené, C. W., Beckie, T. M., Sims, M., Suglia, S. F., Aggarwal, B., Moise, N., Jiménez, M. C., Gaye, B., & McCullough, L. D. (2022). Effects of objective and perceived social isolation on cardiovascular and brain health: A scientific statement from the American Heart Association. Journal of the American Heart Association, 11(16), e026493. https://doi.org/10.1161/JAHA.122.026493
- Chapman, B. P., Huang, A., Peters, K., Horner, E., Manly, J., Bennett, D. A., & Lapham, S. (2020). Association between high school personality phenotype and dementia 54 years later in results from a national US sample. JAMA Psychiatry, 77(2), 148–154. https://doi.org/10.1001/jamapsychiatry.2019.3120
- Cohen-Mansfield, J., & Perach, R. (2015). Interventions for alleviating loneliness among older persons: A critical review. American Journal of Health Promotion, 29(3), e109–e125. https://doi.org/10.4278/ajhp.130418-LIT-182
- Cornwell, E. Y., & Waite, L. J. (2009). Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior, 50(1), 31–48. https://doi.org/10.1177/002214650905000103
- Costa-Cordella, S., Arevalo-Romero, C., Parada, F. J., & Rossi, A. (2021). Social support and cognition: A systematic review. Frontiers in Psychology, 12, 637060. https://doi.org/10.3389/fpsyg.2021.637060
- Courtin, E., & Knapp, M. (2017). Social isolation, loneliness and health in old age: A scoping review. Health & Social Care in the Community, 25(3), 799–812. https://doi.org/10.1111/hsc.12311
- de Jong-Gierveld, J. (1987). Developing and testing a model of loneliness. Journal of Personality and Social Psychology, 53(1), 119–128. https://doi.org/10.1037//0022-3514.53.1.119
- DeYoung, C. G., Hirsh, J. B., Shane, M. S., Papademetris, X., Rajeevan, N., & Gray, J. R. (2010). Testing predictions from personality neuroscience. Brain structure and the big five. Psychological Science, 21(6), 820–828. https://doi.org/10.1177/0956797610370159
- Duchek, J. M., Aschenbrenner, A. J., Fagan, A. M., Benzinger, T. L. S., Morris, J. C., & Balota, D. A. (2020). The relation between personality and biomarkers in sensitivity and conversion to Alzheimer-type dementia. Journal of the International Neuropsychological Society, 26(6), 596–606. https://doi.org/10.1017/s1355617719001358
- Evans, I. E. M., Martyr, A., Collins, R., Brayne, C., & Clare, L. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–s144. https://doi.org/10.3233/jad-180501
- Friedman, B., Veazie, P. J., Chapman, B. P., Manning, W. G., & Duberstein, P. R. (2013). Is personality associated with health care use by older adults? The Milbank Quarterly, 91(3), 491–527. https://doi.org/10.1111/1468-0009.12024
- Gardiner, C., Geldenhuys, G., & Gott, M. (2018). Interventions to reduce social isolation and loneliness among older people: An integrative review. Health & Social Care in the Community, 26(2), 147–157. https://doi.org/10.1111/hsc.12367
- Gosling, S. D., Rentfrow, P. J., & Swann, W. B. (2003). A very brief measure of the big-five personality domains. Journal of Research in Personality, 37(6), 504–528. https://doi.org/10.1016/S0092-6566(03)00046-1
- Graham, E. K., Rutsohn, J. P., Turiano, N. A., Bendayan, R., Batterham, P. J., Gerstorf, D., Katz, M. J., Reynolds, C. A., Sharp, E. S., Yoneda, T. B., Bastarache, E. D., Elleman, L. G., Zelinski, E. M., Johansson, B., Kuh, D., Barnes, L. L., Bennett, D. A., Deeg, D. J. H., Lipton, R. B., … Mroczek, D. K. (2017). Personality predicts mortality risk: An integrative data analysis of 15 international longitudinal studies. Journal of Research in Personality, 70, 174–186. https://doi.org/10.1016/j.jrp.2017.07.005
- Hajek, A., Bock, J. O., & König, H. H. (2017). The role of personality in health care use: Results of a population-based longitudinal study in Germany. PLOS One, 12(7), e0181716. https://doi.org/10.1371/journal.pone.0181716
- Holt-Lunstad, J. (2021). A pandemic of social isolation? World Psychiatry, 20(1), 55–56. https://doi.org/10.1002/wps.20839
- Holt-Lunstad, J. (2023). National health guidelines for social connection: What is the evidence in support and what might the guidelines say? Policy Insights from the Behavioral and Brain Sciences, 10(1), 41–50. https://doi.org/10.1177/23727322221150204
- Holt-Lunstad, J., Robles, T. F., & Sbarra, D. A. (2017). Advancing social connection as a public health priority in the United States. The American Psychologist, 72(6), 517–530. https://doi.org/10.1037/amp0000103
- Holt-Lunstad, J., & Smith, T. B. (2016). Loneliness and social isolation as risk factors for CVD: Implications for evidence-based patient care and scientific inquiry. Heart, 102(13), 987–989. https://doi.org/10.1136/heartjnl-2015-309242
- Holt-Lunstad, J., Smith, T. B., Baker, M., Harris, T., & Stephenson, D. (2015). Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspectives on Psychological Science, 10(2), 227–237. https://doi.org/10.1177/1745691614568352
- Hughes, M. E., Waite, L. J., Hawkley, L. C., & Cacioppo, J. T. (2004). A short scale for measuring loneliness in large surveys: Results from two population-based studies. Research on Aging, 26(6), 655–672. https://doi.org/10.1177/0164027504268574
- Johansson, L., Guo, X., Duberstein, P. R., Hällström, T., Waern, M., Östling, S., & Skoog, I. (2014). Midlife personality and risk of Alzheimer disease and distress. Neurology, 83(17), 1538–1544. https://doi.org/10.1212/WNL.0000000000000907
- Kelly, M. E., Duff, H., Kelly, S., McHugh Power, J. E., Brennan, S., Lawlor, B. A., & Loughrey, D. G. (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. https://doi.org/10.1186/s13643-017-0632-2
- Kuiper, J. S., Zuidersma, M., Oude Voshaar, R. C., Zuidema, S. U., van den Heuvel, E. R., Stolk, R. P., & Smidt, N. (2015). Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Research Reviews, 22, 39–57. https://doi.org/10.1016/j.arr.2015.04.006
- Kuiper, J. S., Zuidersma, M., Zuidema, S. U., Burgerhof, J. G., Stolk, R. P., Oude Voshaar, R. C., & Smidt, N. (2016). Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology, 45(4), 1169–1206. https://doi.org/10.1093/ije/dyw089
- Lara, E., Martín-María, N., De la Torre-Luque, A., Koyanagi, A., Vancampfort, D., Izquierdo, A., & Miret, M. (2019). Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Research Reviews, 52, 7–16. https://doi.org/10.1016/j.arr.2019.03.002
- Lezak, M. D., Howieson, D. B., Loring, D. W., & Fischer, J. S. (2004). Neuropsychological assessment. Oxford University Press.
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396(10248), 413–446. https://doi.org/10.1016/s0140-6736(20)30367-6
- Low, L.-F., Harrison, F., & Lackersteen, S. M. (2013). Does personality affect risk for dementia? A systematic review and meta-analysis. The American Journal of Geriatric Psychiatry, 21(8), 713–728. https://doi.org/10.1016/j.jagp.2012.08.004
- Malcolm, M., Frost, H., & Cowie, J. (2019). Loneliness and social isolation causal association with health-related lifestyle risk in older adults: A systematic review and meta-analysis protocol. Systematic Reviews, 8(1), 48. https://doi.org/10.1186/s13643-019-0968-x
- Menec, V. H., Newall, N. E., Mackenzie, C. S., Shooshtari, S., & Nowicki, S. (2019). Examining individual and geographic factors associated with social isolation and loneliness using Canadian Longitudinal Study on Aging (CLSA) data. PLOS One, 14(2), e0211143. https://doi.org/10.1371/journal.pone.0211143
- Menec, V. H., Newall, N. E., Mackenzie, C. S., Shooshtari, S., & Nowicki, S. (2020). Examining social isolation and loneliness in combination in relation to social support and psychological distress using Canadian Longitudinal Study of Aging (CLSA) data. PLOS One, 15(3), e0230673. https://doi.org/10.1371/journal.pone.0230673
- Mund, M., & Neyer, F. J. (2016). The winding paths of the lonesome cowboy: Evidence for mutual influences between personality, subjective health, and loneliness. Journal of Personality, 84(5), 646–657. https://doi.org/10.1111/jopy.12188
- Munoz, E., Sliwinski, M. J., Smyth, J. M., Almeida, D. M., & King, H. A. (2013). Intrusive thoughts mediate the association between neuroticism and cognitive function. Personality and Individual Differences, 55(8), 898–903. https://doi.org/10.1016/j.paid.2013.07.019
- Ormstad, H., Eilertsen, G., Heir, T., & Sandvik, L. (2020). Personality traits and the risk of becoming lonely in old age: A 5-year follow-up study. Health and Quality of Life Outcomes, 18(1), 47. https://doi.org/10.1186/s12955-020-01303-5
- Penninkilampi, R., Casey, A., Singh, M., & Brodaty, H. (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 66(4), 1619–1633. https://doi.org/10.3233/JAD-180439
- Phillips, N. A., Isler, L., Kabir, R., Hämäläinen, A., Wittich, W., Pichora-Fuller, M. K., & Mick, P. (2022). Hearing and visual acuity predict cognitive function in adults aged 45–85 years: Findings from the baseline wave of the Canadian Longitudinal Study on Aging (CLSA). Psychology and Aging, 37(8), 891–912. https://doi.org/10.1037/pag0000716
- Pierce, G. R., Lakey, B., Sarason, I. G., Sarason, B. R., & Joseph, H. J. (1997). Personality and social support processes. In G. R. Pierce, B. Lakey, I. G. Sarason, & B. R. Sarason (Eds.), Sourcebook of social support and personality (pp. 3–18). Plenum Press. https://doi.org/10.1007/978-1-4899-1843-7
- Raina, P., Wolfson, C., Kirkland, S., Griffith, L. E., Balion, C., Cossette, B., Dionne, I., Hofer, S., Hogan, D., van den Heuvel, E. R., Liu-Ambrose, T., Menec, V., Mugford, G., Patterson, C., Payette, H., Richards, B., Shannon, H., Sheets, D., Taler, V., … Young, L. (2019). Cohort profile: The Canadian Longitudinal Study on Aging (CLSA). International Journal of Epidemiology, 48(6), 1752–1753j. https://doi.org/10.1093/ije/dyz173
- Raina, P. S., Wolfson, C., Kirkland, S. A., Griffith, L. E., Oremus, M., Patterson, C., Tuokko, H., Penning, M., Balion, C. M., Hogan, D., Wister, A., Payette, H., Shannon, H., & Brazil, K. (2009). The Canadian longitudinal study on aging (CLSA). Canadian Journal on Aging, 28(3), 221–229. https://doi.org/10.1017/s0714980809990055
- Read, D. E. (1987). Neuropsychological assessment of memory in the elderly. Canadian Journal of Experimental Psychology, 41, 158.
- Rey, A. (1964). L’examen clinique en psychologie, Paris: Presses Universitaires de France, 1964. Chemotherapy and Objective Cognitive Functioning, 95.
- Segel-Karpas, D., & Lachman, M. E. (2018). Social contact and cognitive functioning: The role of personality. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 73(6), 974–984. https://doi.org/10.1093/geronb/gbw079
- Segerstrom, S. C. (2020). Personality and incident Alzheimer’s disease: Theory, evidence, and future directions. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 75(3), 513–521. https://doi.org/10.1093/geronb/gby063
- Shukla, A., Harper, M., Pedersen, E., Goman, A., Suen, J. J., Price, C., Applebaum, J., Hoyer, M., Lin, F. R., & Reed, N. S. (2020). Hearing loss, loneliness, and social isolation: A systematic review. Otolaryngology-Head and Neck Surgery, 162(5), 622–633. https://doi.org/10.1177/0194599820910377
- Sommerlad, A., Kivimäki, M., Larson, E. B., Röhr, S., Shirai, K., Singh-Manoux, A., & Livingston, G. (2023). Social participation and risk of developing dementia. Nature Aging, 3(5), 532–545. https://doi.org/10.1038/s43587-023-00387-0
- Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., Ewers, M., Franzmeier, N., Kempermann, G., Kremen, W. S., Okonkwo, O., Scarmeas, N., Soldan, A., Udeh-Momoh, C., Valenzuela, M., Vemuri, P., & Vuoksimaa, E. (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. https://doi.org/10.1016/j.jalz.2018.07.219
- Steunenberg, B., Twisk, J. W. R., Beekman, A. T. F., Deeg, D. J. H., & Kerkhof, A. J. F. M. (2005). Stability and change of neuroticism in aging. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(1), P27–P33. https://doi.org/10.1093/geronb/60.1.P27
- Sutin, A. R., Stephan, Y., Luchetti, M., & Terracciano, A. (2019). Five-factor model personality traits and cognitive function in five domains in older adulthood. BMC Geriatrics, 19(1), 343. https://doi.org/10.1186/s12877-019-1362-1
- Teng, E. (1995). The mental alternations test (MAT). Clinical Neuropsychology, 9(3), 287.
- Terracciano, A., Bilgel, M., Aschwanden, D., Luchetti, M., Stephan, Y., Moghekar, A. R., Wong, D. F., Ferrucci, L., Sutin, A. R., & Resnick, S. M. (2022). Personality associations with amyloid and tau: Results from the Baltimore longitudinal study of aging and meta-analysis. Biological Psychiatry, 91(4), 359–369. https://doi.org/10.1016/j.biopsych.2021.08.021
- Terracciano, A., Stephan, Y., Luchetti, M., Albanese, E., & Sutin, A. R. (2017). Personality traits and risk of cognitive impairment and dementia. Journal of Psychiatric Research, 89, 22–27. https://doi.org/10.1016/j.jpsychires.2017.01.011
- Terracciano, A., & Sutin, A. R. (2019). Personality and Alzheimer’s disease: An integrative review. Personality Disorders, 10(1), 4–12. https://doi.org/10.1037/per0000268
- Terracciano, A., Sutin, A. R., An, Y., O’Brien, R. J., Ferrucci, L., Zonderman, A. B., & Resnick, S. M. (2014). Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & Dementia, 10(2), 179–186. https://doi.org/10.1016/j.jalz.2013.03.002
- Troyer, A. K., Leach, L., & Strauss, E. (2006). Aging and response inhibition: Normative data for the Victoria Stroop Test. Aging, Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 13(1), 20–35. https://doi.org/10.1080/138255890968187
- Tuokko, H., Griffith, L. E., Simard, M., & Taler, V. (2017). Cognitive measures in the Canadian Longitudinal Study on Aging. The Clinical Neuropsychologist, 31(1), 233–250. https://doi.org/10.1080/13854046.2016.1254279
- Tuokko, H., Griffith, L. E., Simard, M., Taler, V., O’Connell, M. E., Voll, S., Kadlec, H., Wolfson, C., Kirkland, S., & Raina, P. (2020). The Canadian longitudinal study on aging as a platform for exploring cognition in an aging population. The Clinical Neuropsychologist, 34(1), 174–203. https://doi.org/10.1080/13854046.2018.1551575
- Umberson, D., & Montez, J. K. (2010). Social relationships and health: A flashpoint for health policy. Journal of Health and Social Behavior, 51(Suppl), S54–S66. https://doi.org/10.1177/0022146510383501
- Walker, E., Ploubidis, G., & Fancourt, D. (2019). Social engagement and loneliness are differentially associated with neuro-immune markers in older age: Time-varying associations from the English Longitudinal Study of Ageing. Brain, Behavior, and Immunity, 82, 224–229. https://doi.org/10.1016/j.bbi.2019.08.189
- Wang, H. X., Karp, A., Herlitz, A., Crowe, M., Kåreholt, I., Winblad, B., & Fratiglioni, L. (2009). Personality and lifestyle in relation to dementia incidence. Neurology, 72(3), 253–259. https://doi.org/10.1212/01.wnl.0000339485.39246.87
- Yoneda, T., Graham, E., Lozinski, T., Bennett, D. A., Mroczek, D., Piccinin, A. M., Hofer, S. M., & Muniz-Terrera, G. (2023). Personality traits, cognitive states, and mortality in older adulthood. Journal of Personality and Social Psychology, 124(2), 381–395. https://doi.org/10.1037/pspp0000418
