Abstract
Objectives
To evaluate the effectiveness of the SPAN-intervention, a psychosocial intervention aiming at improving a sense of usefulness and engaging in meaningful activities, for community-dwelling people living with young-onset dementia (YOD) and their family caregivers.
Methods
A cluster-randomized controlled trial with two parallel groups (SPAN-intervention vs. care as usual) with assessments at baseline and five-month follow-up was performed. Sixty-one persons living with YOD and their family caregivers were included (SPAN-intervention group: n = 35; care as usual group: n = 26). Outcomes included, for the person living with YOD, empowerment (operationalized by self-management abilities using the SMAS-30; primary outcome), quality of life, neuropsychiatric symptoms, disability, apathy; and, for the family caregiver, quality of life, emotional distress, sense of competence. Data were analyzed using linear mixed models.
Results
We found no statistically significant effects of the SPAN-intervention on empowerment, nor on the secondary outcome measures for persons living with YOD or their family caregivers.
Conclusion
Although the SPAN-intervention may provide concrete opportunities to engage in activities and stimulate reciprocity, such as meaningful social activities, this study did not demonstrate intervention effects. Additional qualitative evaluations may provide more insight into the implementation process and experiences of people living with YOD and their family caregivers.
This trial was registered at ClinicalTrials.gov (NCT02937883).
Introduction
In people living with young-onset dementia (YOD), dementia symptoms start before the age of 65 (van de Veen et al., Citation2021). The global prevalence of YOD is 119 per 100.000 population (Hendriks et al., Citation2021). They lose abilities in many domains and especially the loss of important social roles, such as being a parent or financial provider, reduces their sense of identity and self-esteem (Busted et al., Citation2020; van Vliet et al., Citation2010). It has been found that the needs of people with YOD are often unmet for daytime activities, social company, and intimate relationships (Bakker et al., Citation2014a). This was associated with high levels of neuropsychiatric symptoms (Bakker et al., Citation2014b). Furthermore, previous research has shown that feeling useful is very important to preserve a sense of control and self-esteem (Roach & Drummond, Citation2014; Van Vliet et al., Citation2017). Especially in the early stages of dementia, retaining a sense of usefulness was identified as important, while in the later stages pleasant activities, such as hobbies are found to be more important. Having social roles or functional activities has been found to contribute to feelings of usefulness (Van Vliet et al., Citation2017). Therefore, it seems valuable to support them in finding suitable activities to enhance a sense of usefulness and engagement in daily life. Focusing on strengths and creating opportunities to feel useful may provide them with a greater sense of control, improved self-esteem, and may empower them. Furthermore, caring for a person with dementia at home has adverse effects on the psychological and physical health of family caregivers (de Vugt et al., Citation2005; Pinquart & Sörensen, Citation2003), and many experience high levels of burden, depressive symptoms, and a variety of psychosocial problems (Kimura et al., Citation2019; van Vliet et al., Citation2010). Yet, supportive family caregivers who stimulate abilities of the person living with dementia experience less burden than non-adapting caregivers (de Vugt et al., Citation2004), implying that their involvement in interventions for people living with dementia may also be beneficial for themselves and increase feelings of competence.
Nevertheless, people living with YOD and their family caregivers experience barriers or reluctance towards using formal community services (Bakker et al., Citation2013b; Cations et al., Citation2017). However, since they remain living at home for a long time (Bakker et al., Citation2013a), there is a need for accessible and tailored services in the community. The availability of healthcare services specialized for this group remains limited in most countries. In the Netherlands, several services are available, including daily care for those living at home provided by (young-onset) dementia casemanagers, or specialized day care centers (Dutch Young Onset Dementia Knowledge Centre, Citation2015). At present, there are no programs available for these healthcare professionals to support people living with YOD and family caregivers in finding suitable activities to meaningfully spend their days, indicating the need for the development and evaluation of such an intervention.
The concept of empowerment provides a frame for developing an intervention for people living with YOD and their family caregivers. Empowerment can be described as the process through which persons perceive that they control situations (Rogers and Singhal, Citation2003). Empowerment focuses on a person’s strengths and capacities. Family caregivers and healthcare professionals can function as facilitators to enable the empowerment process and encourage the person’s capacity (Rogers & Singhal, Citation2003). Based on this vantage point and a qualitative study into the sense of usefulness in people living with YOD (Van Vliet et al., Citation2017), we developed an intervention aiming to support themselves in being useful to maintain or recapture a sense of control and restore self-esteem. This intervention, called SPAN, comprises the aspects that were considered important by people living with YOD in daily life according to Van Vliet et al. (Citation2017), namely being Socially involved, being engaged in Pleasant activities, being Active, and feeling useful and Needed.
In this study, we investigated the effectiveness of our SPAN-intervention for community-dwelling people living with YOD on (1) empowerment, quality of life and neuropsychiatric symptoms of the person living with YOD, and (2) sense of competence in dealing with the caregiving situation and the level of emotional distress experienced by the primary family caregiver.
Materials and methods
Study design
We used a cluster-randomized controlled design with two parallel groups (intervention versus usual care), with assessments at baseline and five-months follow-up. The Consolidated Standards of Reporting Trials (CONSORT) were followed in this article, see Additional file 1.
Setting and participants
Persons living with YOD and their family caregivers were recruited from various regions of the Netherlands. Persons living with YOD were eligible for participation if dementia symptoms started before the age of 65, they were living at home, they were capable of speaking and understanding the Dutch language, and if a family caregiver gave consent to participate in this study. Exclusion criteria were dementia caused by HIV, traumatic brain injury, Down’s syndrome, Huntington’s disease or alcohol-related dementia. Family caregivers were eligible if they provided care or support for their loved one multiple times a week. The family caregiver could be a partner, an involved child or another family member.
Participants were recruited in collaboration with nineteen healthcare organizations offering dedicated young-onset dementia services throughout the Netherlands. These healthcare organizations were recruited through the University Knowledge network for Old age care Nijmegen (UKON) and the Dutch Young-onset Dementia Knowledge Center. Overall, 33 dementia casemanagers and employees of the day care centers approached their clients for participation, using an information package provided by the researchers. Participants could apply for the study by e-mail, telephone or reply form.
Intervention
The SPAN-intervention addresses current capacities for increasing opportunities to engage in meaningful activities, to focus on their strengths and capabilities, and to feel useful. SPAN was developed in close collaboration with people with YOD and their family caregivers. Based on focus group interviews with people living with young onset dementia and informal caregivers (Van Vliet et al., Citation2017) and a literature review, the intervention was developed. After panel discussion and pilot-testing (n = 11) the SPAN intervention was finalized.
The SPAN-intervention makes use of a SPAN guide and workbook. The SPAN guide includes generic information on how to adapt and structure activities and a list of help and support services available. This guide emphasizes the importance of focusing on strengths and usefulness in daily life to preserve self-esteem and involvement in society. The SPAN-workbook is directed at the person with dementia and offers them and their caregiver guidelines and a roadmap to work out their personal action plan tailored to their specific needs and preferences. The person living with YOD and their family caregiver are supported in the use of the SPAN-workbook by a dementia casemanager or an employee of the day care center.
First, the person living with YOD and their family caregiver have an introductory face-to-face consultation together with the healthcare professional. Thereafter, they enter into conversation about current and desired activities using the SPAN-workbook. Examples of questions are: What activities do I like?, What gives me energy? Three weeks later, there is a telephone consultation with the healthcare professional to answer questions if necessary, after which the dyad continues with concretizing activities, and entering them into a new week schedule. During the second face-to-face consultation, six weeks after the start of the intervention, the healthcare professional examines the needs and wishes of the participating dyad using the answers in the SPAN-workbook, and helps to translate them to concrete activities in the week schedules, with focusing on what is still possible instead of what is no longer possible. Thereafter, the person living with YOD and their family caregiver use the new week scheme in their daily routine for six weeks, with an intermediate telephonic consultation by the healthcare professional. In the third face-to-face consultation, twelve weeks after the start of the intervention, they evaluate the week schedule together with the healthcare professional, and make adjustments, if necessary. Again, the person living with YOD and their family caregiver use the new week schedule for six weeks, and integrate the new or adjusted activities into their daily routine. In a final face-to-face consultation, the SPAN-intervention is evaluated. shows the time line of the SPAN-intervention.
Table 1. Time line of the SPAN-intervention.
Cluster randomization
Participants in the intervention group worked with the SPAN-intervention, in addition to care as usual. Participants in the control group only received care as usual. Healthcare professionals were randomized to either the intervention or control group to avoid contamination. Allocation was conducted by the first author (AB) in the presence of an independent research assistant into the control or intervention group with a 1:1 allocation ratio. Sealed envelopes were used as a method to conceal allocation. Randomization of healthcare professionals was performed within the healthcare organization to obtain an equal distribution between groups in terms of location (e.g. rural and urban) and number of participants. When the organization expected to recruit fewer than five participants, all healthcare professionals from that organization were randomized into one group.
Data collection
Data collection took place at the start of the intervention (baseline) and after five months (follow-up). Persons living with YOD and their family caregiver were interviewed by a researcher (AB, CVC, MW) using validated questionnaires and structured interviews at a place most comfortable for them (mostly at home or at the day care center). Researchers had received training in interviewing techniques and experience interviewing people living with dementia. Data were collected between November 2016 and March 2018.
Baseline characteristics
Baseline characteristics were provided by participants at baseline, using questions of the Older Persons and Informal Caregivers Survey (TOPICS-MDS) (Melis et al., Citation2019). Dementia severity was rated by the healthcare professional with the Global Deterioration Scale (GDS) (C. P. Hughes et al., Citation1982; Reisberg et al., Citation1982).
Primary outcome measure
The primary outcome of this study was self-rated feelings of empowerment in the person living with YOD. Empowerment was operationalized by self-management abilities, assessed using the Self-Management Ability Scale (SMAS-30) (Schuurmans et al., Citation2005; Steverink, Citation2009; Steverink et al., Citation2005). This 30-item questionnaire comprises six five-item subscales: (1) taking initiative; (2) investing; (3) self-efficacy; (4) positive frame of mind; (5) variety; and (6) multifunctionality. The five response categories of the individual items range from ‘never’ to ‘always’. All subscale scores range from 0 to 100. The total SMAS-30 score is the average of the five subscales. Higher scores implicate better self-management capabilities. The reliability of SMAS-30 was considered good for both total scores (Cronbach’s α = 0.90) and subscale scores (α = 0.63 to 0.77) in populations of elderly above the age of 65 (Steverink, Citation2009). The total SMAS-30 score was considered as valid for older people (Cramm et al., Citation2012). The SMAS-30 was valuated as acceptable for people living with dementia and holds strong potential as primary outcome measure (Csipke et al., Citation2021).
Secondary outcomes for person living with YOD
Quality of life was assessed with the 13-item QOL-AD questionnaire administered to the person living with YOD (self-rated version) and the family caregiver (proxy version) separately (Logsdon et al., Citation1999, Citation2002). These scores are combined into a single score (range 13 to 52), weighting the person with dementia’s own quality of life score twice as heavily as the caregiver proxy scores. Higher scores indicate a higher quality of life. The scale has good psychometric properties, appropriate for varying disease stages (Thorgrimsen et al., Citation2003).
Health-related quality of life was assessed using the 5-item EQ-5D-5L (Herdman et al., Citation2011). Each question represents a quality of life dimension: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. An index value is calculated (range 0 to 1) following EQ-5D-5L user guide (2019). In addition, the questionnaire contains a visual analogue scale, which reflects the current self-rated health with end points labeled ‘the best health you can imagine’ and ‘the worst health you can imagine’ (range 0 to 100).
Neuropsychiatric symptoms were assessed by the family caregiver using the 12-item Neuropsychiatric Inventory Questionnaire (NPI-Q) measuring delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, aberrant motor behavior, night-time behavior disturbances, and appetite/eating abnormalities over the past month (De Jonghe et al., Citation2003; Kat et al., Citation2002; Kaufer et al., Citation2000). For each symptom, a screening question is used to determine whether the symptom was present in the last four weeks, and the severity was rated (mild, moderate or severe). Higher scores indicate higher prevalence-rates of neuropsychiatric symptoms (range 0 to 36).
Everyday disability was assessed by the family caregiver using the 20-item Interview for Deterioration in Daily living Activities in Dementia (IDDD) (Teunisse & Derix, Citation1991, Citation1997). The IDDD comprises two subscales concerning the initiative to perform activities and the actual performance, which are combined in a total score. Lower scores indicating less need for help (range 20 to 80).
Apathy was assessed by the family caregiver using the 10-item Apathy Evaluation Scale (AES-10) (Lueken et al., Citation2007). Higher scores indicating more apathetic behavior (range 10 to 40).
Secondary outcomes for family caregiver
Perceived quality of life was measured using a question to rate their quality of life on a scale from 1 to 10. Health-related quality of life of family caregivers was measured using a visual analogue scale ranging from 0 to 100 with endpoints labeled ‘the best health you can imagine’ and ‘the worst health you can imagine’. Caregiver burden was assessed by a visual analogue scale ranging from 0 to 10 with endpoints labeled as ‘not heavy at all’ and ‘way too heavy’ to rate a caregiver’s level of burden in providing care and support.
Caregiver emotional distress was assessed using the NPI-Q distress score (De Jonghe et al., Citation2003; Kaufer et al., Citation2000). The family caregiver rated their experienced level of distress for each existing behavior from no distress to extreme distress. Higher scores indicating more caregiver distress (range 0 to 36).
Caregivers’ sense of competence was assessed using the 7-item Short Sense of Competence Questionnaire (SSCQ) (Vernooij-Dassen et al., Citation1999). This scale assesses the family caregivers’ feeling of being capable of caring for a person with dementia. Higher scores indicating a higher sense of competence (range 0 to 7).
Sample size calculation
We assumed that a healthcare professional would recruit three persons living with dementia on average, and that the SPAN-intervention might lead to an increase of 0.5 standard deviations on the primary outcome measure (SMAS-30). Based on these assumptions, and a significance level alpha of 0.05 and a power of 0.80, a conservative estimated correlation between baseline and follow-up of 0.5 and an intraclass correlation coefficient of 0.05, we calculated that a sample size of 60 people living with dementia was necessary to detect intervention effects (Teerenstra et al., Citation2012). With twenty clusters, we would reach this sample size.
Data analysis
The data collected was analyzed using IBM SPSS Statistics software (version 25.0). Means and standard deviations (SD) were used to describe the baseline characteristics of the participants. Differences in baseline characteristics between intervention and control groups were tested using a Chi2-test or Fisher Exact test for categorical data, a Mann-Whitney U test for ordinal data, and an independent t-test for continuous data.
A linear mixed models analysis was used, with clustering of participants to healthcare professionals taken into account. When the variance within a cluster was estimated as 0.0, no cluster analysis was possible. The interaction term of group x time was included in the analysis to examine differences over time between the intervention and control groups. An intention-to-treat analysis was applied for all outcome measures. Analyses were repeated with neuropsychiatric symptoms as a confounder, because we hypothesized that neuropsychiatric symptoms, such as depression, agitation and apathy, may influence both intervention use and the primary outcome self-management abilities, as was found in a population of older adults (Jane Murray Cramm et al., Citation2012). Furthermore, if potential covariates emerged from the comparisons of the groups at baseline, analyses were repeated with these variables as covariates. A p-value lower than 0.05 was found to be significant.
Ethical considerations
The study protocol was reviewed by the local Medical Ethics Review Committee of the CMO Regio Arnhem Nijmegen (2016-2574), which stated that the study was not subject to the Medical Research Involving Human Subjects Act. The study was conducted in accordance with Dutch Law and the Declaration of Helsinki. The SPAN effect study is registered at the Clinical Trial register NCT02937883 (www.clinicaltrials.gov). All participants gave their written informed consent individually.
Results
Participants
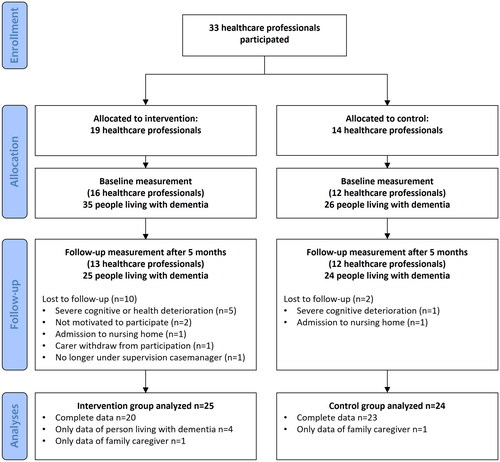
Overall, 61 persons living with YOD and their family caregivers participated in this study, with 35 participants in the intervention group and 26 participants in the control group. Thirty-six participants were recruited by their dementia casemanager, and 25 participants were recruited by their day care center. In total, twelve participants were lost to attrition due to severe deterioration in cognition or health (n = 6), admission to a nursing home (n = 2), lack of motivation (n = 2), family caregiver withdrew from participation (n = 1) or no longer under supervision casemanager (n = 1). A flowchart of participants can be found in .
The mean age of participants was 64.1 years (). More men than women participated (70.5% versus 29.5%, respectively). Almost all participants were living together with their family caregiver (92%). More than one-third of the participating people with dementia lived with dementia caused by Alzheimer’s disease (39%), followed by frontotemporal dementia (21%) and vascular dementia (18%). Most participants lived with very mild to moderate dementia (85%). shows all outcome measures for baseline and five-month follow-up for the intervention and control groups. There were no differences regarding outcome variables at baseline between participants in the intervention and control groups. Participants who were lost to follow-up significantly differed from other participants at baseline in terms of quality of life of the family caregiver (estimate = 0.7; 95% CI, −1.5 to −0.3; p = 0.040) and caregiver burden (estimate = 0.7; 95% CI, 0.3 to 1.5; p = 0.011).
Table 2. Baseline characteristics of participants in the intervention and control groups.
Table 3. Outcomes for control and intervention group participants.
Effects of the SPAN-intervention
People living with young-onset dementia
No statistically significant difference over time was found on the primary outcome of self-management abilities (SMAS-30) between the intervention and control groups (p = 0.485) as shown in . Also on the subscales of the SMAS-30, no intervention effect was found (p = 0.208-0.807). Furthermore, changes over time of the secondary outcome measures quality of life, health-related quality of life, daily functioning, apathy and neuropsychiatric symptoms did not differ between the conditions (p = 0.208-0.807).
Table 4. Intervention effects of the SPAN-intervention on outcome measures.
Table A1. CONSORT 2010 Checklist of information to include when reporting a randomized trial.
The analyses were repeated with neuropsychiatric symptoms as a confounder and revealed similar results for the primary outcome measure self-management. For the secondary outcome measures, with neuropsychiatric symptoms as confounder, the reduction in daily functioning over time was significantly higher in the intervention group compared to the control group (p = 0.029). The reduction among participants in the intervention group was 4.4 points higher than among those in the control group on the 20- to 80-point scale.
Family caregivers
No significant differences over time were found for family caregivers in the intervention and control groups for the outcome measures quality of life (p = 0.132; ), health-related quality of life (p = 0.787), caregiver burden (p = 0.327), sense of competence (p = 0.495), and emotional distress (p = 0.523).
The analyses were repeated with neuropsychiatric symptoms as a confounder, which did not change the results.
Discussion
To our knowledge, the SPAN-intervention is the first psychosocial program designed for people with YOD that focuses on their strengths and creates opportunities to feel useful. This study did not show effects of the SPAN-intervention on empowerment, quality of life and behavior of the person living with YOD, nor on quality of life, emotional distress, and feelings of competence of family caregivers. Although the intervention might indeed not be effective, alternative explanations for not finding an effect of the SPAN-intervention in this study may lie in the measurements used and/or the implementation of the intervention.
First, we operationalized empowerment as self-management abilities, because an instrument to measure empowerment in people with dementia was lacking. However, although self-management abilities may be necessary for engaging meaningfully, the SPAN-intervention did not specifically address these abilities. The SPAN-intervention mainly focused on engaging in meaningful activities in the domains social involvement, pleasant activities, being active, and feeling useful and needed. Self-management abilities may perhaps be considered a prerequisite to engage in these activities. Members of our group recently developed a conceptual framework of empowerment for older people living with dementia (van Corven et al., Citation2021). The SPAN-intervention is in line with the four empowerment domains of this recently developed framework, as it aims to enhance a sense of usefulness (domain 1), and to maintain or regain a sense of control (domain 2) and sense of worth (domain 3). Moreover, a sense of personal identity (domain 4) is addressed, since the program is tailored to each individual. Future studies are recommended to measure empowerment directly; for example, by using the newly developed Engagement and Independence in Dementia Questionnaire (EID-Q) (Stoner et al., Citation2017; Citation2018), whose questions seem to be in line with the domains of empowerment for older people living with dementia (van Corven et al., Citation2021).
Second, regarding perceived quality of life of people living with YOD, dementia-specific quality of life measures, as the QOL-AD, may not be able to detect small changes in quality of life (Ettema et al., Citation2005; L. Hughes et al., Citation2021). Moreover, a recent study found rather high quality of life scores that remained stable over time in people with mild-to-moderate dementia (Clare et al., Citation2022). These findings may indicate that the expected effects on perceived quality of life were smaller than estimated, and thus the power may have been insufficient to identify changes.
In addition, the intervention may not have been implemented according to protocol; despite the things we have done to improve fidelity, such as the use of an intervention manual and a tailored training for healthcare professionals in the intervention group. For instance, certain elements of the intervention may not have been fully implemented; at the follow-up assessment it appeared that not all SPAN participants had yet incorporated meaningful activities into their weekly schedule. It is also possible that the dose of the intervention was not sufficient to induce a change in actions. Additional meeting sessions with the casemanager might have been beneficial. A subsequent process evaluation may provide more insights into the implementation process, as well as the experiences of participants and the casemanagers involved, and possibly clarify the results found (Leontjevas et al., Citation2012). In particular, it would be helpful to examine implementation fidelity in future studies to determine whether the program was administered and implemented as intended.
When controlling for neuropsychiatric symptoms, it seemed that people living with YOD in the intervention group deteriorated more in daily functioning than those in the control group. Although the progressive nature of dementia a deterioration in cognition and performance of daily tasks predicts (Verlinden et al., Citation2016), differences between the groups were not expected. As this score was assessed by the family caregiver, an explanation might be found in family caregivers becoming more aware of the deterioration in daily function of the person living with YOD as a result of the SPAN-intervention. As the SPAN-intervention focuses on current capacities and opportunities for meaningfully spending their days, family caregivers may also become more aware of what is no longer possible. On the other hand, this finding may also indicate that the daily functioning of intervention participants actually deteriorated during the intervention period. If that were the case, it may be plausible that the SPAN-intervention was difficult to use for those participants or may have been less effective than expected. Moreover, due to this period of deterioration, family caregivers may have experienced an increased caregiver burden in this period, thus making it more difficult to support their loved one to use SPAN (Lin et al., Citation2019; Van der Lee et al., Citation2014).
Indeed, family caregivers had an important role in the implementation of the SPAN-intervention. Together with their loved one with dementia, they were supposed to work out a personal action plan tailored to their specific needs and preferences. We expected that involvement in the SPAN-intervention and their supportive role in this would increase feelings of competence and control, but we did not find effects on their feelings of competence, nor their quality of life and emotional distress. Interestingly, we found that caregivers of participants who dropped out of the study experienced a higher caregiver burden at baseline than others. The perceived burden can become so high that people stop participating in research and/or new interventions. These findings may indicate that the SPAN-intervention is less appropriate for family caregivers who experience high levels of caregiver burden.
In addition to caregiver burden, other participant characteristics may have influenced intervention use and thus results found. Our study focused on community-dwelling people living with YOD who received case management, regardless of the type or stage of dementia. In addition to type and stage of dementia, other participant characteristics, such as disease awareness, years since diagnosis and relationship with the caregiver, may also influence the intervention implementation and intervention effects. In this study, group size was too small to conduct quantitative analyses for specific subgroups. Furthermore, qualitative evaluation methods may provide a better understanding of aspects such as experiences, satisfaction and feasibility when evaluating complex psychosocial interventions, and these evaluations may contribute to conclusions about the added value of the SPAN-intervention and potentially identify specific subgroups for whom the intervention may be helpful (Leggett & Kales, Citation2019).
Strengths and limitations
A key strength of this study is that this is one of the few randomized controlled trials on psychosocial interventions for people living with YOD. Furthermore, external validity appears high: people from both rural and urban areas participated, and the heterogeneity of the participants suggests that our study population reflects the wide range of people living with YOD and their family caregivers.
A possible limitation of this study is observer bias, since researchers were aware of the allocation of participants during their assessments. Furthermore, the intervention period of five months may have been insufficient to integrate meaningful activities in daily life and provide opportunities to feel useful. Further, our sample size calculation showed that 60 participants distributed over twenty clusters were needed to detect intervention effects. While a sufficient number of participants started in this study (n = 61), due to drop-out 49 participants participated in the follow-up measurement, which limited the power of the analysis. Finally, follow-up measurements were assessed 5 months after initiation. This interval may have been too long, hiding short-term effects of (parts of) the intervention.
Conclusion
This study evaluated the effects of the SPAN-intervention for people living with YOD and their family caregivers. Although the SPAN-intervention may offer concrete opportunities to engage in activities and stimulate reciprocity, such as social activities, this study did not demonstrate intervention effects. Additional qualitative evaluations are recommended to provide more insight into the implementation process and experiences of people living with YOD and their family caregivers.
Acknowledgements
We would like to thank all of the participants and the healthcare professionals involved for their participation in this study.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets generated and analysed during the current study are not publicly available, but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bakker, C., de Vugt, M. E., van Vliet, D., Verhey, F., Pijnenburg, Y. A., Vernooij-Dassen, M. J., & Koopmans, R. T. (2014a). Unmet needs and health-related quality of life in young-onset dementia. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22(11), 1121–1130. https://doi.org/10.1016/j.jagp.2013.02.006
- Bakker, C., de Vugt, M. E., van Vliet, D., Verhey, F. R., Pijnenburg, Y. A., Vernooij-Dassen, M. J., & Koopmans, R. T. (2013a). Predictors of the time to institutionalization in young- versus late-onset dementia: Results from the Needs in Young Onset Dementia (NeedYD) study. Journal of the American Medical Directors Association, 14(4), 248–253. https://doi.org/10.1016/j.jamda.2012.09.011
- Bakker, C., de Vugt, M. E., van Vliet, D., Verhey, F. R., Pijnenburg, Y. A., Vernooij-Dassen, M. J., & Koopmans, R. T. (2013b). The use of formal and informal care in early onset dementia: Results from the NeedYD study. The American Journal of Geriatric Psychiatry : official Journal of the American Association for Geriatric Psychiatry, 21(1), 37–45. https://doi.org/10.1016/j.jagp.2012.10.004
- Bakker, C., de Vugt, M. E., van Vliet, D., Verhey, F. R., Pijnenburg, Y. A., Vernooij-Dassen, M. J., & Koopmans, R. T. (2014b). The relationship between unmet care needs in young-onset dementia and the course of neuropsychiatric symptoms: A two-year follow-up study. International Psychogeriatrics, 26(12), 1991–2000. https://doi.org/10.1017/s1041610213001476
- Busted, L. M., Nielsen, D. S., & Birkelund, R. (2020). Sometimes it feels like thinking in syrup" - the experience of losing sense of self in those with young onset dementia. International Journal of Qualitative Studies on Health and Well-Being, 15(1), 1734277. https://doi.org/10.1080/17482631.2020.1734277
- Cations, M., Withall, A., Horsfall, R., Denham, N., White, F., Trollor, J., Loy, C., Brodaty, H., Sachdev, P., Gonski, P., Demirkol, A., Cumming, R. G., & Draper, B. (2017). Why aren’t people with young onset dementia and their supporters using formal services? Results from the INSPIRED study. PloS One, 12(7), e0180935. https://doi.org/10.1371/journal.pone.0180935
- Clare, L., Gamble, L. D., Martyr, A., Sabatini, S., Nelis, S. M., Quinn, C., Pentecost, C., Victor, C., Jones, R. W., Jones, I. R., Knapp, M., Litherland, R., Morris, R. G., Rusted, J. M., Thom, J. M., Collins, R., Henderson, C., & Matthews, F. E. (2022). Longitudinal trajectories of quality of life among people with mild-to-moderate dementia: A latent growth model approach with IDEAL cohort study data. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 77(6), 1037–1050. https://doi.org/10.1093/geronb/gbac022
- Cramm, J. M., Hartgerink, J., De Vreede, P., Bakker, T., Steyerberg, E., Mackenbach, J., & Nieboer, A. P. (2012). The relationship between older adults’ self-management abilities, well-being and depression. European Journal of Ageing, 9(4), 353–360. https://doi.org/10.1007/s10433-012-0237-5
- Cramm, J. M., Strating, M. M., de Vreede, P. L., Steverink, N., & Nieboer, A. P. (2012). Validation of the self-management ability scale (SMAS) and development and validation of a shorter scale (SMAS-S) among older patients shortly after hospitalisation. Health and Quality of Life Outcomes, 10(1), 9. https://doi.org/10.1186/1477-7525-10-9
- Csipke, E., Moniz-Cook, E., Leung, P., Yates, L., Birt, L., Walton, H., Hogervorst, E., Mountain, G., Charlesworth, G., & Orrell, M. (2021). Feasibility and acceptability evaluation of the Promoting Independence in Dementia (PRIDE) intervention for living well with dementia. International Psychogeriatrics, 33(6), 601–614. https://doi.org/10.1017/s1041610220001386
- De Jonghe, J., Kat, M. G., Kalisvaart, C., & Boelaarts, L. (2003). Neuropsychiatric inventory questionnaire (NPI-Q): A validity study of the Dutch form. Tijdschr Gerontol Geriatr, 34(2), 74–77.
- de Vugt, M. E., Nicolson, N. A., Aalten, P., Lousberg, R., Jolle, J., & Verhey, F. R. (2005). Behavioral problems in dementia patients and salivary cortisol patterns in caregivers. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(2), 201–207. https://doi.org/10.1176/jnp.17.2.201
- de Vugt, M. E., Stevens, F., Aalten, P., Lousberg, R., Jaspers, N., Winkens, I., Jolles, J., & Verhey, F. R. J. (2004). Do caregiver management strategies influence patient behaviour in dementia? International Journal of Geriatric Psychiatry, 19(1), 85–92. https://doi.org/10.1002/gps.1044
- Dutch Young Onset Dementia Knowledge Centre. (2015). Healthcare standard in young onset dementia (in Dutch).
- Ettema, T. P., Dröes, R.-M., Lange, J. d., Mellenbergh, G. J., & Ribbe, M. W. (2005). A review of quality of life instruments used in dementia. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 14(3), 675–686. https://doi.org/10.1007/s11136-004-1258-0
- Hendriks, S., Peetoom, K., Bakker, C., van der Flier, W. M., Papma, J. M., Koopmans, R., Verhey, F. R. J., de Vugt, M., Köhler, S., Withall, A., Parlevliet, J. L., Uysal-Bozkir, Ö., Gibson, R. C., Neita, S. M., Nielsen, T. R., Salem, L. C., Nyberg, J., Lopes, M. A., Dominguez, J. C., … Ruano, L. (2021). Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurology, 78(9), 1080–1090. https://doi.org/10.1001/jamaneurol.2021.2161
- Herdman, M., Gudex, C., Lloyd, A., Janssen, M., Kind, P., Parkin, D., Bonsel, G., & Badia, X. (2011). Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 20(10), 1727–1736. https://doi.org/10.1007/s11136-011-9903-x
- Hughes, C. P., Berg, L., Danziger, W., Coben, L. A., & Martin, R. L. (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry: The Journal of Mental Science, 140(6), 566–572. https://doi.org/10.1192/bjp.140.6.566
- Hughes, L., Farina, N., Page, T. E., Tabet, N., & Banerjee, S. (2021). Psychometric properties and feasibility of use of dementia specific quality of life instruments for use in care settings: A systematic review. International Psychogeriatrics, 33(9), 917–931. https://doi.org/10.1017/S1041610218002259
- Kat, M. G., De Jonghe, J., Aalten, P., Kalisvaart, C., Dröes, R.-M., & Verhey, F. (2002). Neuropsychiatric symptoms of dementia: Psychometric aspects of the Dutch version of the Neuropsychiatric Inventory (NPI). Tijdschift Voor Gerontologie En Geriatrie, 33(4), 150–155.
- Kaufer, D. I., Cummings, J. L., Ketchel, P., Smith, V., MacMillan, A., Shelley, T., Lopez, O. L., & DeKosky, S. T. (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of Neuropsychiatry and Clinical Neurosciences, 12(2), 233–239. https://doi.org/10.1176/jnp.12.2.233
- Kimura, N. R. S., Neto, J. P. S., Santos, R. L., Baptista, M. A. T., Portugal, G., Johannessen, A., Barca, M. L., Engedal, K., Laks, J., Rodrigues, V. M., & Dourado, M. C. N. (2019). Resilience in carers of people with young-onset Alzheimer disease. Journal of Geriatric Psychiatry and Neurology, 32(2), 59–67. https://doi.org/10.1177/0891988718824039
- Leggett, A. N., & Kales, H. C. (2019). Applying rigor: Intervention studies for behavioral and psychological symptoms of dementia. The American Journal of Geriatric Psychiatry : official Journal of the American Association for Geriatric Psychiatry, 27(6), 590–592. https://doi.org/10.1016/j.jagp.2019.02.004
- Leontjevas, R., Gerritsen, D. L., Koopmans, R. T., Smalbrugge, M., & Vernooij-Dassen, M. J. (2012). Process evaluation to explore internal and external validity of the "Act in Case of Depression" care program in nursing homes. Journal of the American Medical Directors Association, 13(5), 488.e481-488–488.e8. https://doi.org/10.1016/j.jamda.2012.03.006
- Lin, C.-Y., Shih, P.-Y., & Ku, L.-J E. (2019). Activities of daily living function and neuropsychiatric symptoms of people with dementia and caregiver burden: The mediating role of caregiving hours. Archives of Gerontology and Geriatrics, 81, 25–30. https://doi.org/10.1016/j.archger.2018.11.009
- Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L. (1999). Quality of life in Alzheimer’s disease: Patient and caregiver reports. Journal of Mental Health and Aging, 5, 21–32.
- Logsdon, R. G., Gibbons, L. E., McCurry, S. M., & Teri, L. (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine, 64(3), 510–519. https://doi.org/10.1097/00006842-200205000-00016
- Lueken, U., Seidl, U., Völker, L., Schweiger, E., Kruse, A., & Schröder, J. (2007). Development of a short version of the Apathy Evaluation Scale specifically adapted for demented nursing home residents. The American Journal of Geriatric Psychiatry : official Journal of the American Association for Geriatric Psychiatry, 15(5), 376–385. https://doi.org/10.1097/JGP.0b013e3180437db3
- Melis, R. J. F., van Hout, H. P. J., & Metzelthin, S. F. (2019). The older persons and informal caregivers survey minimum dataset (TOPICS-MDS). In D. Gu & M. E. Dupre (Eds.), Encyclopedia of gerontology and population aging (pp. 1–9). Springer International Publishing.
- Pinquart, M., & Sörensen, S. (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18(2), 250–267. https://doi.org/10.1037/0882-7974.18.2.250
- Reisberg, B., Ferris, S. H., de Leon, M. J., & Crook, T. (1982). The Global Deterioration Scale for assessment of primary degenerative dementia. The American Journal of Psychiatry, 139(9), 1136–1139. https://doi.org/10.1176/ajp.139.9.1136
- Roach, P., & Drummond, N. (2014). It’s nice to have something to do’: Early-onset dementia and maintaining purposeful activity. Journal of Psychiatric and Mental Health Nursing, 21(10), 889–895. https://doi.org/10.1111/jpm.12154
- Rogers, E. M., & Singhal, A. (2003). Empowerment and communication: Lessons learned from organizing for social change. Annals of the International Communication Association, 27(1), 67–85. https://doi.org/10.1080/23808985.2003.11679022
- Schuurmans, H., Steverink, N., Frieswijk, N., Buunk, B. P., Slaets, J. P., & Lindenberg, S. (2005). How to measure self-management abilities in older people by self-report. The development of the SMAS-30. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 14(10), 2215–2228. https://doi.org/10.1007/s11136-005-8166-9
- Steverink, N. (2009). Self-Management Ability Scale (SMAS-30), version 2, manual.
- Steverink, N., Lindenberg, S., & Slaets, J. P. J. (2005). How to understand and improve older people’s self-management of wellbeing. European Journal of Ageing, 2(4), 235–244. https://doi.org/10.1007/s10433-005-0012-y
- Stoner, C. R., Orrell, M., Long, M., Csipke, E., & Spector, A. (2017). The development and preliminary psychometric properties of two positive psychology outcome measures for people with dementia: The PPOM and the EID-Q. BMC Geriatrics, 17(1), 72. https://doi.org/10.1186/s12877-017-0468-6
- Stoner, C. R., Orrell, M., & Spector, A. (2018). Psychometric properties and factor analysis of the engagement and independence in dementia questionnaire (EID-Q). Dementia and Geriatric Cognitive Disorders, 46(3-4), 119–127. https://doi.org/10.1159/000488484
- Teerenstra, S., Eldridge, S., Graff, M., de Hoop, E., & Borm, G. F. (2012). A simple sample size formula for analysis of covariance in cluster randomized trials. Statistics in Medicine, 31(20), 2169–2178. https://doi.org/10.1002/sim.5352
- Teunisse, S., & Derix, M. M. (1991). Measurement of activities of daily living in patients with dementia living at home: Development of a questionnaire. Tijdschrift Voor Gerontologie En Geriatrie, 22(2), 53–59.
- Teunisse, S., & Derix, M. M. (1997). The interview for deterioration in daily living activities in dementia: Agreement between primary and secondary caregivers. International Psychogeriatrics, 9 (S1), 155–162. https://doi.org/10.1017/s1041610297004845
- Thorgrimsen, L., Selwood, A., Spector, A., Royan, L., de Madariaga Lopez, M., Woods, R., & Orrell, M. (2003). Whose quality of life is it anyway?: The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Disease and Associated Disorders, 17(4), 201–208. https://doi.org/10.1097/00002093-200310000-00002
- van Corven, C. T. M., Bielderman, A., Wijnen, M., Leontjevas, R., Lucassen, P. L. B. J., Graff, M. J. L., & Gerritsen, D. L. (2021). Defining empowerment for older people living with dementia from multiple perspectives: A qualitative study. International Journal of Nursing Studies, 114, 103823. https://doi.org/10.1016/j.ijnurstu.2020.103823
- van de Veen, D., Bakker, C., Peetoom, K., Pijnenburg, Y., Papma, J. M., de Vugt, M., & Koopmans, R. (2021). An integrative literature review on the nomenclature and definition of dementia at a young age. Journal of Alzheimer’s Disease: JAD, 83(4), 1891–1916. https://doi.org/10.3233/JAD-210458
- Van der Lee, J., Bakker, T. J., Duivenvoorden, H. J., & Dröes, R.-M. (2014). Multivariate models of subjective caregiver burden in dementia: A systematic review. Ageing Research Reviews, 15, 76–93. https://doi.org/10.1016/j.arr.2014.03.003
- van Vliet, D., de Vugt, M. E., Bakker, C., Koopmans, R. T., & Verhey, F. R. (2010). Impact of early onset dementia on caregivers: A review. International Journal of Geriatric Psychiatry, 25(11), 1091–1100. https://doi.org/10.1002/gps.2439
- Van Vliet, D., Persoon, A., Bakker, C., Koopmans, R., de Vugt, M. E., Bielderman, A., & Gerritsen, D. L. (2017). Feeling useful and engaged in daily life: Exploring the experiences of people with young-onset dementia. International Psychogeriatrics, 29(11), 1889–1898. https://doi.org/10.1017/S1041610217001314
- Verlinden, V. J., van der Geest, J. N., de Bruijn, R. F., Hofman, A., Koudstaal, P. J., & Ikram, M. A. (2016). Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 12(2), 144–153. https://doi.org/10.1016/j.jalz.2015.08.001
- Vernooij-Dassen, M. J., Felling, A. J., Brummelkamp, E., Dauzenberg, M. G., van den Bos, G. A., & Grol, R. (1999). Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: A Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. Journal of the American Geriatrics Society, 47(2), 256–257. https://doi.org/10.1111/j.1532-5415.1999.tb04588.x