Abstract
Objectives
To examine how change in benzodiazepine (BZD) use is linked to changes in depressive symptoms intensity, worry intensity, and sleep quality over 16 months.
Method
Data come from a larger randomised controlled trial (RCT) named the ‘Programme d’Aide du Succès au SEvrage (PASSE-60+)’ study (NCT02281175). Seventy-three participants age 60 years and older took part in a 4-month discontinuation programme and were assessed four times over 16 months. Change in BZD use was defined as the difference in reported mg/day between two assessments. Control variables were RCT discontinuation group; BZD use at T1; and either depressive symptoms, worry intensity, or sleep quality at T1. Hierarchical multiple regressions were used to analyse data.
Results
In the short term, right after the discontinuation programme, sleep quality worsened with lower BZD use. This link was no longer significant at the 3- and 12-month follow-up. In the long term, depressive symptoms lowered with lower BZD use. No change was found in worry intensity in relation to BZD use at all measurement times.
Conclusion
Discontinuation may improve depressive symptoms. Our study also questions the long-term effectiveness of BZD use, since long-term discontinuation was not linked with change in worry intensity and sleep quality.
Introduction
Most treatment guidelines recommend that, for treatment of anxiety disorders and insomnia in the general population, benzodiazepines (BZDs) be used only in the short term for a subset of patients with severe distress, selected according to different risk factors (Joint Formulary Committee, Citation2022; Lader, Citation2012; National Institute for Health and Care Excellence, Citation2020; Qaseem et al., Citation2016). Despite these recommendations, in Canada, BZD use remains high and has been relatively stable from 1995 to 2015 (Murphy et al., Citation2016). Moreover, in the United States, BZD and non-benzodiazepine hypnotic prescriptions have increased in the past few decades (Agarwal & Landon, Citation2019; Bachhuber et al., Citation2016; Kaufmann et al., Citation2018; Maust et al., Citation2017, Citation2019), driven by increases in medium- and long-term use. In 2015–2016, an estimated 30.6 million people (12.6% of adults) reported past-year use (Maust et al., Citation2019). This is worrying, because long-term BZD use has been linked to negative effects in a range of neuropsychological functions, and a meta-analysis (Crowe & Stranks, Citation2018) found that these impairments seem to remain after discontinuation, even up to 10 months after withdrawal. BZDs have also been associated with motor vehicle accidents, falls and fractures (American Geriatrics Society Beers Criteria® Update Expert Panel, Citation2019; Brandt & Leong, Citation2017), dependence (Lader, Citation2011; Stahl, Citation2020), an increased risk of mortality (Weich et al., Citation2014), and an increased risk of dementia, although more research is still needed on this last risk to fully establish a causal relation (Brandt & Leong, Citation2017, Lucchetta et al., Citation2018).
With regard to adults age 65 years and older, the American Geriatrics Society, in its Beers criteria, recommends that most older adults avoid BZDs (American Geriatrics Society Beers Criteria® Update Expert Panel, Citation2019). This recommendation is not new; BZDs have been considered inappropriate for this age group for decades (Stuck et al., Citation1994). Despite this, in the United States, BZD use has continued to increase in older adults (Bachhuber et al., Citation2016; Maust et al., Citation2017). In Canada, decreases in BZD use have been measured in older adults, however, this may be related to an increase in Z-drug prescriptions (Alessi-Severini et al., Citation2014; Davies et al., Citation2018; Murphy et al., Citation2016). Z-drugs are non-benzodiazepine hypnotics such as zopiclone, eszopiclone, zaleplon et zolpidem (Geddes & Andreasen, Citation2020; Procyshyn et al., Citation2019). There is no empirical evidence of clinical differences between Z-drugs and short-acting BZDs in terms of efficacy, adverse effects, or dependence/abuse potential (National Institute for Health and Care Excellence, Citation2004). Moreover, even considering the decreases, BZD use in older adults in Canada remains high (Murphy et al., Citation2016). This is particularly worrisome given that older adults have the highest risk of serious adverse effects from these medications (Markota et al., Citation2016; Olfson et al., Citation2015). Furthermore, BZDs are among the most common potentially inappropriate medications (PIMs) among older adults (Malakouti et al., Citation2021). PIMs impose, in addition to the health issues, a significant financial burden (Malakouti et al., Citation2021). It is thus not surprising that different initiatives to facilitate their withdrawal have been studied in recent decades (Dou et al., Citation2019).
Short-term BZD withdrawal symptoms are well known (Ashton, Citation2005; Boland et al., Citation2021), but there are some gaps in the literature around the effects of long-term discontinuation. A significant amount of research has focused on the cognitive impact of long-term BZD discontinuation (Crowe & Stranks, Citation2018), but research on psychological factors is scarce. The few studies available, in the general population, have found that anxiety and depression diminished after long-term withdrawal (Rickels et al., Citation1991; Vikander et al., Citation2010). In older adults, to our knowledge, only two studies measured long-term effects of BZD discontinuation on psychological factors. Curran et al. (Citation2003), in a one-year longitudinal study, found no difference in sleep between withdrawers and continuers and no evidence of emergent depression and anxiety related to discontinuation. However, this study only considered BZD hypnotics. Another study found improvements in some aspects of sleep after BZD discontinuation (Lähteenmäki et al., Citation2019), but the follow-up was too short (6 months) to fully understand the long-term effects.
It is not clear why stopping BZDs may improve some psychological factors. However, tolerance may play a role. The mechanisms explaining tolerance encompass various contributing factors, like uncoupling, intracellular trafficking, post-translational modifications of GABAA receptors (Cheng et al., Citation2018). As tolerance develops, BZDs lose, at least partly, their effectiveness (Bateson, Citation2002; Vinkers & Olivier, Citation2012) but may still have adverse effects (for reviews of adverse effects of BZDs, see Lader (Citation2011) and Brandt & Leong, Citation2017). Thus, when discontinuing BZDs, the loss of positive psychoactive effects may be small but adverse effects may stop, which in turn can have a net positive effect on some psychological factors.
Moreover, withdrawal symptoms from BZDs may resemble the symptoms of anxiety or insomnia for which they are often prescribed (Lader, Citation2011). This can be especially discouraging for long-term users, who typically have started their use during a period of increased anxiety and/or sleeplessness (Lader, Citation2011), and hence may fear a return to their past emotional state. Therefore, information on how those symptoms evolve after discontinuation could inform patients and give a clearer portrait of what they will be facing in the first year after withdrawal. It could also guide practitioners when evaluating the risks and benefits of suggesting BZD discontinuation to a patient.
The objective of the study was thus to identify the psychological consequences of BZD discontinuation on older adults at different points over a 16-month time frame. To date, few studies have evaluated the psychological effect of discontinuing BZDs in older adults, and none have evaluated the effect over such a long follow-up period. On the basis of the existing studies and literature on BZD withdrawal, our hypotheses were that, in the short term, as BZD use decreases, worry intensity and depressive symptoms intensity would increase, while sleep quality would decrease, because withdrawal symptoms may still be affecting participants. In the mid-term and long term, our hypotheses were that a decrease in BZD use should result in a decrease of worry intensity and intensity of depressive symptoms and an increase in sleep quality.
Materials and methods
Participants
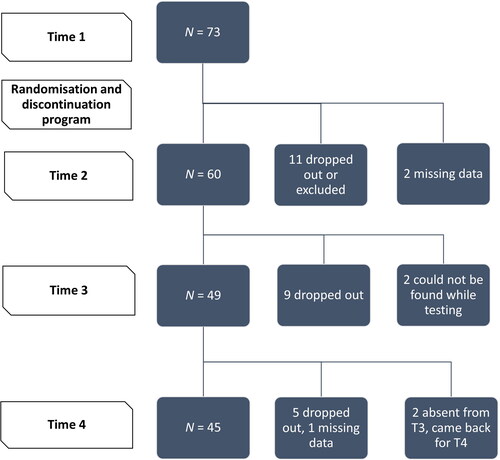
The data analysed in this study come from a larger randomised control trial (RCT) named the ‘Programme d’Aide au Succès du SEvrage (PASSE-60+),’ described below. This study had 73 participants at baseline. A detailed diagram of exclusions and dropouts is shown in .
To obtain a sample as representative as possible of older BZD users, inclusion and exclusion criteria were kept to a minimum. Three inclusion criteria were determined: (1) age 60 years and over, (2) wanting to withdraw from BZD use, and (3) use of a BZD or a Z-drug for at least the past 2 years. The reason for including both types of users (BZD and Z-drug) is, as was said before, the lack of clinical differences in efficacy, adverse effects, or potential for dependence or abuse (National Institute for Health and Care Excellence, Citation2004). To simplify reading, the term BZD will include Z-drugs in this research. Four exclusion criteria were defined: (1) experiencing a crisis (e.g. suicidal ideation), (2) having an alcohol or drug dependence disorder except for BZD dependence, (3) taking BZDs for a medical reason (e.g. epilepsy), and (4) being unable to complete assessment questionnaires or being unable to attend group sessions (e.g. incapacity to travel, cognitive impairment).
Procedure
Advertisements were used to recruit participants. The first contact, when the research coordinator answered participants’ questions, was made by phone. A screening interview was also conducted to verify eligibility. Once the first contact had been made, a letter containing the consent form, the project description, and a withdrawal grid was sent to the participants. A letter was also sent to doctors who prescribed the participants medication to inform them of their patients’ participation in the research project.
The RCT on which we based this article was conducted at the Centre de Recherche de l’Institut Universitaire de Gériatrie de Montréal (CRIUGM) in Montreal, Canada. Participants were assessed four times (Time 1 [T1], Time 2 [T2], Time 3 [T3], Time 4 [T4]). Research assistants carried out the assessments, which lasted around 90 min each. These assistants were trained and supervised by a psychologist (Sébastien Grenier, the Principal Investigator). Afterward, participants were randomly divided into three different withdrawal programmes: 1) self-withdrawal; 2) gradual withdrawal supervised by a physician; 3) gradual withdrawal supervised by a physician combined with group therapy sessions (PASSE-60+). In the current study, these allocation groups were used as a control variable. Participants were first assessed before the beginning of the withdrawal programme (T1). They were assessed a second time (T2) at the end of the withdrawal programme (16 weeks later), then 3 months later (T3) and 12 months after withdrawal (T4). Questionnaires in the different assessments were essentially the same with the only exception of T2, when a programme satisfaction questionnaire was added.
PASSE-60+
The PASSE-60+ study is registered with the U.S. National Library of Medicine ClinicalTrials.gov: NCT02281175. PASSE-60+ is a programme dedicated to increase success of withdrawal from BZDs among people age 60 years and older. It lasts 16 weeks and is based on a programme previously developed and validated for adults (O’Connor et al. Citation2004). The programme is composed of 12 cognitive–behavioural therapy (CBT) sessions. The goal of the programme is to teach anxiety and insomnia management strategies, increase the participants’ confidence to withdraw, increase their sense of self-efficacy, reduce their negative expectations, and teach them how to go on with their everyday life without medication. All participants, before entering the programme, gave their written, free, and informed consent. The PASSE-60+ study has been approved by the Ethics Committee of the CRIUGM (CER IUGM 14-15-012).
Measurements
Sociodemographics (age and sex), BZD dose (both initial and at each assessment), and length of use in years were self-reported by participants. Because participants used different BZDs, each BZD dose was transformed to diazepam-equivalent dose using Heather Ashton’s conversion table (Ashton, Citation1995). The French versions of three questionnaires were completed by the participants at each measurement time (T1, T2, T3, T4). The Beck Depression Inventory–II (BDI–II, Beck et al., Citation1996; Vézina et al., Citation1991) was administered to assess the intensity of depressive symptoms, the Pittsburgh Sleep Quality Index (PSQI; Blais et al., Citation1997; Buysse et al., Citation1989) was used to evaluate the overall quality of sleep, and the Penn State Worry Questionnaire (PSWQ; Gosselin et al., Citation2001; Meyer et al., Citation1990) was used to assess the intensity of worry among participants. All of these questionnaires have shown good psychometric properties. Among older adults, the French version of the BDI–II has a Cronbach’s α of 0.85 and a test–retest reliability of 0.74 (Vézina et al., Citation1991). The PSQI has an internal consistency of 0.83 and a fidelity test–retest value of 0.85 (Buysse et al., Citation1989). One question on the PSQI normally assesses how much medication is used to help people sleep. This question was removed in the analyses because it would be directly correlated with BZD dose at each assessment. The French version of the PSWQ was tested in three different studies and has a good internal consistency that varied between 0.92 and 0.82 and a fidelity test–retest value of 0.86 (Gosselin et al., Citation2001).
Statistical analyses
In the current article, we present secondary analyses based on the RCT data. Hierarchical multiple regressions were used to analyse data. Nine different regressions, one for each dependent variable, were performed. The first three dependent variables were the difference in BDI–II scores between T1 and T2 (first variable), between T1 and T3 (second variable), and T1 and T4 (third variable). Variables 4 to 6 were the difference in PSWQ scores between T1 and T2 (fourth variable), then between T1 and T3 (fifth variable) and T1 and T4 (sixth variable). Variables 7 to 9 were the difference in PSQI scores between T1 and T2 (seventh variable), T1 and T3 (eighth variable), and T1 and T4 (ninth variable).
Independent variables consisted of control variables and the difference between BZD use between T1 and T2, between T1 and T3, and between T1 and T4. Change scores were used instead of direct scores to measure the effect that a change in BZD dose has on changes in psychological factors, rather than the effect of BZD dose on psychological factors. Considering that the 12 CBT sessions of the PASSE-60+ may have an impact on our variables of interest, the first control variable was the RCT allocation group (PASSE-60+ vs. others). Other control variables were the score of the dependent variable at T1 and sociodemographic/BZD intake factors (age, sex, initial dose of BZD, and length of use). Because participants’ sex, age, and length of BZD use were not significantly correlated with any of the dependent variables, they were not further included in multivariable analyses. This exclusion was done to preserve statistical power.
In the first block, we entered the remaining control variables: the allocation group, BZD use at T1, and the score of the dependent variable at T1. In the second block, one of the three independent variables (differences in BZD use) was entered. The independent variable choice was related to the dependent variable; for example, when conducting a regression in which the dependent variable was the difference in BDI–II scores between T1 and T3, the independent variable corresponding to the difference in BZD use between T1 and T3 was used. The goal of the analyses was to assess the effect of BZD discontinuation on several psychological factors. All analyses were tested with an alpha significance level of 0.05 and were carried out with SPSS software (Version 28). Only tables with significant regressions concerning our variables of interest are presented. Nonsignificant regression results are available in tables in the supplementary material.
Linearity, homoscedasticity, and normality were assessed by the visual inspection of a plot of standardised residuals against the standardised predicted values and by using a histogram of standardised residuals (Tabachnick et al., Citation2007). There was no evidence of multicollinearity, as the highest Variance Inflation Factor was 2,664 in our analysis, which is not high enough to be of concern (Laerd Statistics, Citation2015). Some standardised residuals were greater than ±3 SD, but because their leverage and Cook’s distance were within an adequate range (Laerd Statistics, Citation2015; Tabachnick et al. Citation2007), they were not excluded.
Results
Description of the sample
At baseline (T1), on average, depressive symptoms scores were low, specifically in the minimal range of the questionnaire (minimal range = 0–13, mild = 14–19, severe = 29–63; Beck et al. Citation1996), with a substantial standard deviation. Worry intensity scores were higher than community older adult samples, which had a mean of 38.94 (SD = 10.98; for a descriptive values on various samples, see Davey & Wells, Citation2006). However, they were significantly lower than older adult samples with general anxiety disorder, which had a mean of 63.23 (SD = 9.66; Davey & Wells, Citation2006). Two scores were put forward in the tables for sleep quality. The first score corresponds to the score obtained on the PSQI without removing any item. At T1, the majority of our participants had a sleep quality score above 5 (M = 10.03, SD = 3.01), which indicates poor sleep quality (Buysse et al., Citation1989). The second score is the one used in this study, as said earlier, without the question on sleep medication. In terms of the type of drugs used, 41% of our participants used Z-drugs. The remaining participants used a variety of benzodiazepines, the two most common being Lorazepam (23%) and Clonazepam (23%). Moreover, 92% of participants used their medication every day. For more descriptive data on participants at each measurement time, can be consulted. A rapid look at this table suggests that participants get better on most measures; however, this may be due to a multitude of factors. To have a clearer picture whether these improvements are linked to BZD use, regressions were conducted.
Table 1. Participants’ characteristics at each measurement time.
Regression results between BZD use change and intensity of depressive symptoms score change
Change between T1 and T2 and between T1 and T3
Between T1 and T2, no significant association was found between BZD use change and intensity of depressive symptoms change. This was also true for the regression between T1 and T3. Regression coefficients and standard errors can be found in Tables S1 and S2 (in the supplementary material).
Table 2. Hierarchical multiple regression analysis showing the association between BZD use change and intensity of depressive symptoms change between T1 and T4.
Change between T1 and T4
The overall multiple regression model was statistically significant, F = 9,724 p < .001. Alone, control variables (RCT allocation group, intensity of depressive symptoms at T1, and BZD use at T1) explained 36.8% of the variance. Adding the difference between BZD use between T1 and T4 to the model significantly increased the model by 12.5%. BZD use change between T1 and T4 contributed significantly to the overall model (p <.05). The association was positive, meaning that, for example, the more BZD use drops, the more the intensity of depressive symptoms drops, too. Among control variables, the intensity of depressive symptoms at T1 was the only variable that contributed significantly to the model. Regression coefficients and standard errors can be found in .
Regression results between BZD use change and worry intensity change
No significant association was found between BZD use change and worry intensity change. This was the case for all regressions; between T1 and T2, between T1 and T3 and between T1 and T4. Regression coefficients and standard errors can be found in Tables S3, S4 and S5 (in the supplementary material).
Table 3. Hierarchical multiple regression analysis showing the association between BZD use change and PSQI sleep quality score change between T1 and T2.
Regression results between BZD use change and PSQI sleep quality score change
Change between T1 and T2
The overall multiple regression model was statistically significant (F = 4,581 p <.01). Alone, control variables (RCT allocation group, sleep quality scores at T1, and BZD use at T1) explained 16.5% of the variance. Adding the difference between BZD use between T1 and T4 to the model significantly increased the model by 8.5%. BZD use change between T1 and T4 contributed significantly to the overall model (p <.05). The association was negative, meaning that, for example, the more BZD use drops, the more PSQI sleep quality scores increase (an increase in PSQI means sleep quality gets worse). Among control variables, PSQI sleep quality scores at T1 and BZD use at T1 contributed significantly to the model. Regression coefficients and standard errors can be found in .
Change between T1 and T3 and between T1 and T4
Between T1 and T3 and between T1 and T4, no significant association was found between BZD use change and sleep quality change. Regression coefficients and standard errors can be found in Tables S6 and S7 (in the supplementary material).
Discussion
The objective of this study was to identify how BZD discontinuation affects worry intensity, sleep quality, and depressive symptoms at different time points over a 16-month period.
In the short term (T2), right after the withdrawal programme, a lowering in BZD use predicted a lowering in sleep quality at T2; adding BZD use change between T1 and T2 increased the model’s Nagelkerke R2 by 8.5% (p < .05). This was expected because symptoms of BZD withdrawal include insomnia (Procyshyn et al., Citation2019) and, at T2, the end of the discontinuation programme, some symptoms of withdrawal may still have been present. In the short term, no significant association with other variables was found.
In the long term (T4, 12 months after the withdrawal programme), as expected, lowering BZD use was significantly linked to a lowering of depressive symptoms. Adding this variable increased the model’s Nagelkerke R2 by 12.5% (p < .01). However, no significant association with other variables was found in the medium or long term.
It may seem counterintuitive that BZD use lowering was linked to a drop in depressive symptoms. BZDs are taken to treat anxiety and insomnia (Procyshyn et al., Citation2019), and because anxiety and depression are closely linked (Cosci & Fava, Citation2021) one might think that a molecule that improves anxiety would also be beneficial in alleviating depression. Why then, would stopping medication taken to treat anxiety and sleep have beneficial effects on depression?
One of the earliest reports of links between BZD and depression worsening dates back from 1968 (Smith & Salzman, Citation1991). Similar links of BZD causing or worsening depression have been made throughout the literature in the following decades (e.g. Ashton, Citation2002; Guina & Merrill, Citation2018). Psychotropic drug textbooks include treatment-emergent depression in the adverse effects of BZD (Procyshyn et al., Citation2019). However, the causality and explanations for this link are not clear.
Kripke (Citation2007) reviewed parallel randomizing placebo-controlled trials submitted by the industry to the U.S. Food and Drug Administration and collected data for a total of 5,535 participants. In the trials, a higher incidence of depression was found in people who were randomised to a hypnotic versus a placebo. This study, because it is based on RCTs, helps in establishing the causality of the relationship, however, the data were insufficient for a formal meta-analysis, and several limitations impeded generalisation. To explain what underlies the link between BZD and depression, the author indicates that acute sleep deprivation has been found to have antidepressant effects (Wirz-Justice & Van den Hoofdakker, Citation1999) and that it is thus hard to know if the effect BZD have on sleep help or worsen depressive symptoms.
Having a BZD as an add-on to antidepressant treatment in major depressive disorder has also been associated with more severe depressive symptoms and diverse comorbidities in a European cross-sectional multicentre (N = 1,410) study (Dold et al., Citation2020). The explanation put forward is that severe/difficult-to-treat conditions may have prompted the add-on treatment strategy. However, causality is not clear here, and the add-on strategy might also be responsible for a worsening in symptoms. This needs to be explored, because a worsening of symptoms in conditions such as depression are often interpreted as an evolution of the existing disorder, without examining the possible iatrogenic effect of the chronic use of medications (Michelini et al., Citation1996).
Another explanation can stem from the fact that BZDs seem to be problematic in cases of post-traumatic stress disorder, for which they are not recommended and may even prolong the symptoms (Geddes & Andreasen, Citation2020; Guina et al., Citation2015). One of the explanations that has been suggested is that a prognosis is worsened by an avoidant coping style and that BZDs are inherently associated with avoidance by inhibiting cognitive processing. Because avoidance is also linked to depression (Trew, Citation2011), the same factors may be at play here.
In sum, although BZDs and depression appear to be often associated in the literature, further large-scale studies are needed to determine causality and better understand the explanations behind this relationship.
Another question stemmed from our results: Why was no statistically significant change found in worry and sleep quality in the long term? To answer this question, two factors may be considered: tolerance and withdrawal symptoms.
For the hypnotic effect, BZD tolerance develops within days to weeks (Ashton, Citation2005; Bateson, Citation2002; Guina & Merrill, Citation2018; Vinkers & Olivier, Citation2012). For the anxiolytic effect, it is less clear. If tolerance develops, it would be in the longer term, although only few long-term studies on the subject exist (for reviews, see Bateson, Citation2002; Michelini et al., Citation1996; and Vinkers & Olivier, Citation2012).
Withdrawal symptoms appear relatively fast (around 1–2 days for short-acting agents and 5–10 days for long-acting agents) and resemble symptoms for which the patient is treated (anxiety, agitation, insomnia, etc.; Procyshyn et al., Citation2019). As mentioned earlier, those withdrawal symptoms may thus be hard to distinguish from the underlying anxiety symptoms for which they were prescribed (Baldwin et al., Citation2013; Lader, Citation2011). Patients, who often consult at a time of heightened distress (Lader, Citation2011), may fear that they are coming back to their initial symptomatology when experiencing withdrawal. Hence, it may be hard to know, in this context, whether BZDs are pursued for treatment or merely to keep withdrawal symptoms/fear of initial symptoms at bay.
As tolerance for insomnia, and maybe anxiety, grows, BZDs may become a lot less effective but still be used in the long term for the aforementioned factors. Thus, one explanation for our results could be that participants pursued medication that was no longer useful but would not be ceased because of their aversive withdrawal effect. Therefore, when they got through long-term discontinuation, we could not find significant change in their anxiety or insomnia. Again, additional studies are warranted to confirm this explanation.
Strengths and limitations
This study is one of few on the subject, and the only one, to our knowledge, to link continuous BZD use change in withdrawal to psychological factors. It was also conducted with older adults who, as mentioned earlier, are more vulnerable to adverse effects of BZDs (Markota et al., Citation2016; Olfson et al., Citation2015). The length of the study is also a strength; it has multiple data points over 16 months, and no study of that population has followed participants over such a long period. This was essential to our findings, because the link with depressive symptoms decline appeared significant only at T4. This also paints a more detailed picture of long-term discontinuation.
Despite these strengths, there are some limitations to our study. First, due to our sample size, findings of smaller effect sizes may have been prevented by a lack of power. Sample size was lower in part due to high attrition (38.4% of initial participants dropped out before T4), which could also have biased our data. Second, questionnaires in this study were self-report; thus, social desirability and recall bias may have altered the data, although this may have been prevented in part by the overall good validity and reliability of our questionnaires. Another limitation is that most of the participants were women, which may hinder the generalisation of our findings. However, this is also representative of the target population; BZD users are predominantly women (Murphy et al., Citation2016). In addition, as mentioned earlier, the average depressive symptoms scores were low for our participants. Results may have been different in people with higher levels of depressive symptoms. Another limitation is the question of causality: Did the reduction of BZDs lead to a reduction in depressive symptoms, or did the reduction in depressive symptoms facilitate BZD reduction? Because most of the reduction happened between T1 and T2, and the link between BZD use change and depressive symptoms appeared later in time, this seems to hint to an effect with an offset. This would make sense with BZD functioning given that withdrawal symptoms appear in the first weeks of discontinuation and may exacerbate anxiety and sleep problems (Procyshyn et al., Citation2019). The benefits would thus be seen in the follow-ups, when no more withdrawal symptoms are present. However, larger, controlled, randomised experimental studies are needed to examine causal links.
Clinical implications and conclusion
In our research, while looking at the impact of long-term discontinuation, we could not find a change in worry or sleep, and we observed a benefit in depressive symptoms. This, of course, is not systematic, because every patient is unique, and some people worsened as others got better. Nevertheless, this knowledge can help practitioners and patients when tackling the decision of discontinuation.
Our findings also question the effectiveness of long-term BZD use. If BZDs were still an effective treatment for our participants, their discontinuation should have had a negative and significant impact on worry and sleep quality. However, it is hard to know what exactly is happening here. Were small negative impacts unnoticed because of a lack of power? Did participants find other ways to regulate their worries and their sleep while discontinuing? Or did BZDs stop being effective and, past the withdrawal symptoms, their cessation did not have an impact on the participants? Despite decades of BZD use, long-term effectiveness is still misunderstood (Lader, Citation2012). To our knowledge, BZD long-term efficacy studies are scarce, and meta-analyses of the existing studies show no difference from placebo (Shinfuku et al., Citation2019). Much larger studies would be necessary to further understand this phenomenon.
Nevertheless, we know participants in the study used BZDs for a significant amount of time (M use of 12.37 years, SD = 9.24) and, as cited earlier, there are important consequences to long-term BZD use. Thomas R. Insel, former director of the National Institute of Mental Health, has said that one issue with current mental health treatments is that research focuses on short-term effects for long-term illnesses (Insel, Citation2022). BZDs, by offering a short-term fix with no consistent proof of long-term efficacy and various adverse effects, seem to be a good example of the pitfalls of this approach. If BZDs are indeed used past their effective period, this means that our participants may have had unnecessary iatrogenic consequences. This is also without considering the fact that BZDs do not treat underlying symptoms (Ashton, Citation1994) and that treatment alternatives for anxiety and sleep problems do exist. Psychotherapy is as efficacious in the short term, more efficacious on the long term (Nathan & Gorman, Citation2015; Roth & Fonagy, Citation2013; Sudak, Citation2011), and not necessarily more costly (Singh, Citation2013). In this regard, recent Canadian guidelines recommend starting treatment in older adults with non-pharmaceutical options first, except in circumstances dictated by patient preference, symptom severity and risk assessment (Canadian Coalition for Seniors Mental Health, Citation2024). When medication is needed, the guidelines suggest using selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) as effective first-line treatments. It does not recommend BZDs unless other options have failed.
However, as mentioned earlier, despite repeated guidelines that aim to restrain BZD prescriptions, widespread use continues to grow. Further long-term studies, like this one, should be done to fully understand the effects of BZD discontinuation on psychological factors in older adults, to help them make a fair and informed decision regarding their use of this substance. Furthermore, a comprehension of what is still fueling chronic BZD use is essential to take broad, consequential measures. Collin (Citation2015), by dressing the genealogy of the reasoning behind treatments and prescriptions, helped widen our lens around this issue and put it in historical context. Pérodeau et al. (Citation2016) created a systemic model that offers multiple explanations for chronic BZD use, ranging from ageism; to the role of the pharmaceutical industry, health care professionals, and families; to isolation and maintenance dynamics. It also states ways of improving the issue with, for example, interventions geared toward changing public attitudes toward long-term use; better training on geriatric and psychosocial issues to health care professionals and; on a macro level, reconsidering the overall medicalisation of ageing in society. These solutions are broad and overarching because there seems to be no easy fix to this issue; widespread, long-running problems as this one call for widespread, long-lasting actions.
Supplemental Material
Download MS Word (32.1 KB)Acknowledgements
For their work in this research, we thank Michel Préville, Guilhème Pérodeau, Cara Tannenbaum, André Marchand, Fethia Benyebdri, Mélanie Fournel, Mihaela Vitui, Marie Michelle Boudreau, Vanessa Léveillé, Marianne Lemay, Bruno Gunther, Marie-Hélène Gagné, and Josie-Anne Bertrand.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agarwal, S. D., & Landon, B. E. (2019). Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Network Open, 2(1), e187399. https://doi.org/10.1001/jamanetworkopen.2018.7399
- Alessi-Severini, S., Bolton, J. M., Enns, M. W., Dahl, M., Collins, D. M., Chateau, D., & Sareen, J. (2014). Use of benzodiazepines and related drugs in Manitoba: A population-based study. CMAJ Open, 2(4), E208–E216. https://doi.org/10.9778/cmajo.20130076
- American Geriatrics Society Beers Criteria® Update Expert Panel. (2019). American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society, 67(4), 674–694.
- Ashton, C. H. (2002). Benzodiazepines: How they work and how to withdraw. The Ashton Manual, Aug.
- Ashton, H. (1994). Guidelines for the rational use of benzodiazepines: When and what to use. Drugs, 48(1), 25–40. https://doi.org/10.2165/00003495-199448010-00004
- Ashton, H. (2005). The diagnosis and management of benzodiazepine dependence. Current Opinion in Psychiatry, 18(3), 249–255. https://doi.org/10.1097/01.yco.0000165594.60434.84
- Ashton, H. (1995). Toxicity and adverse consequences of benzodiazepine use. Retrieved from https://www.benzo.org.uk/ashtox.htm
- Bachhuber, M. A., Hennessy, S., Cunningham, C. O., & Starrels, J. L. (2016). Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. American Journal of Public Health, 106(4), 686–688. https://doi.org/10.2105/AJPH.2016.303061
- Baldwin, D. S., Aitchison, K., Bateson, A., Curran, H. V., Davies, S., Leonard, B., Nutt, D. J., Stephens, D. N., & Wilson, S. (2013). Benzodiazepines: Risks and benefits. A reconsideration. Journal of Psychopharmacology (Oxford, England), 27(11), 967–971. https://doi.org/10.1177/0269881113503509
- Bateson, A. (2002). Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Current Pharmaceutical Design, 8(1), 5–21. https://doi.org/10.2174/1381612023396681
- Beck, A. T., Steer, R. A., & Brown, G. K. (1996). Beck Depression Inventory–II. Pearson.
- Blais, F. C., Gendron, L., Mimeault, V., & Morin, C. M. (1997). Evaluation de l’insomnie: Validation de trois questionnaires. L’Encéphale: Revue de Psychiatrie Clinique Biologique et Thérapeutique, 23(6), 447–453.
- Boland, R., Verduin, M., & Ruiz, P. (2021). Kaplan & Sadock’s synopsis of psychiatry (12th ed.). Lippincott Williams & Wilkins.
- Brandt, J., & Leong, C. (2017). Benzodiazepines and Z-drugs: An updated review of major adverse outcomes reported on in epidemiologic research. Drugs in R&D, 17(4), 493–507. https://doi.org/10.1007/s40268-017-0207-7
- Buysse, D. J., Reynolds, C. F., III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
- Canadian Coalition for Seniors Mental Health. (2024). Canadian Guidelines for the Assessment and Treatment of Anxiety in Older Adults. Retrieved from https://ccsmh.ca/areas-of-focus/anxiety/clinical-guidelines/
- Cheng, T., Wallace, D. M., Ponteri, B., & Tuli, M. (2018). Valium without dependence? Individual GABAA receptor subtype contribution toward benzodiazepine addiction, tolerance, and therapeutic effects. Neuropsychiatric Disease and Treatment, 14, 1351–1361. https://doi.org/10.2147/NDT.S164307
- Collin, J. (2015). Universal cures for idiosyncratic illnesses: A genealogy of therapeutic reasoning in the mental health field. Health (London, England: 1997), 19(3), 245–262. https://doi.org/10.1177/1363459314545695
- Cosci, F., & Fava, G. A. (2021). When anxiety and depression coexist: The role of differential diagnosis using clinimetric criteria. Psychotherapy and Psychosomatics, 90(5), 308–317. https://doi.org/10.1159/000517518
- Crowe, S. F., & Stranks, E. K. (2018). The residual medium and long-term cognitive effects of benzodiazepine use: An updated meta-analysis. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists, 33(7), 901–911. https://doi.org/10.1093/arclin/acx120
- Curran, H. V., Collins, R., Fletcher, S., Kee, S., Woods, B., & Iliffe, S. (2003). Older adults and withdrawal from benzodiazepine hypnotics in general practice: Effects on cognitive function, sleep, mood and quality of life. Psychological Medicine, 33(7), 1223–1237. https://doi.org/10.1017/s0033291703008213
- Davey, G. C., & Wells, A. (2006). Worry and its psychological disorders: Theory, assessment and treatment. Wiley.
- Davies, S. J., Jacob, B., Rudoler, D., Zaheer, J., de Oliveira, C., & Kurdyak, P. (2018). Benzodiazepine prescription in Ontario residents aged 65 and over: A population-based study from 1998 to 2013. Therapeutic Advances in Psychopharmacology, 8(3), 99–114. https://doi.org/10.1177/2045125317743651
- Dold, M., Bartova, L., Fugger, G., Mitschek, M. M. M., Kautzky, A., Frey, R., Montgomery, S., Zohar, J., Mendlewicz, J., Souery, D., Fabbri, C., Serretti, S., & Kasper, S. (2020). Add-on benzodiazepine treatment in patients with major depressive disorder—Results from a European cross-sectional multicenter study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 41, 70–80. https://doi.org/10.1016/j.euroneuro.2020.09.636
- Dou, C., Rebane, J., & Bardal, S. (2019). Interventions to improve benzodiazepine tapering success in the elderly: A systematic review. Aging & Mental Health, 23(4), 411–416. https://doi.org/10.1080/13607863.2017.1423030
- Geddes, J. R., & Andreasen, N. C. (2020). New Oxford textbook of psychiatry. Oxford University Press.
- Gosselin, P., Dugas, M., Ladouceur, R., & Freeston, M. (2001). Évaluation des inquiétudes: Validation d’une traduction française du Penn State Worry Questionnaire. L’Encéphale, 27(5), 475–484.
- Guina, J., & Merrill, B. (2018). Benzodiazepines I: Upping the care on downers. The evidence of risks, benefits and alternatives. Journal of Clinical Medicine, 7(2), 17. https://doi.org/10.3390/jcm7020017
- Guina, J., Rossetter, S. R., DeRhodes, B. J., Nahhas, R. W., & Welton, R. S. (2015). Benzodiazepines for PTSD: A systematic review and meta-analysis. Journal of Psychiatric Practice, 21(4), 281–303. https://doi.org/10.1097/PRA.0000000000000091
- Insel, T. (2022). Healing: Our path from mental illness to mental health. Penguin.
- Joint Formulary Committee. (2022). British national formulary. (Vol. BNF 83) Pharmaceutical Press.
- Kaufmann, C. N., Spira, A. P., Depp, C. A., & Mojtabai, R. (2018). Long-term use of benzodiazepines and nonbenzodiazepine hypnotics, 1999–2014. Psychiatric Services (Washington, D.C.), 69(2), 235–238. https://doi.org/10.1176/appi.ps.201700095
- Kripke, D. F. (2007). Greater incidence of depression with hypnotic use than with placebo. BMC Psychiatry, 7(1), 42. https://doi.org/10.1186/1471-244X-7-42
- Lader, M. (2011). Benzodiazepines revisited—Will we ever learn? Addiction (Abingdon, England), 106(12), 2086–2109. https://doi.org/10.1111/j.1360-0443.2011.03563.x
- Lader, M. (2012). Benzodiazepine harm: How can it be reduced? British Journal of Clinical Pharmacology, 77(2), 295–301. https://doi.org/10.1111/j.1365-2125.2012.04418.x
- Laerd Statistics. (2015). Multiple regression using SPSS Statistics. https://statistics.laerd.com/premium/spss/mr/multiple-regression-in-spss.php
- Lähteenmäki, R., Neuvonen, P. J., Puustinen, J., Vahlberg, T., Partinen, M., Räihä, I., & Kivelä, S. L. (2019). Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic & Clinical Pharmacology & Toxicology, 124(3), 330–340. https://doi.org/10.1111/bcpt.13144
- Lucchetta, R. C., da Mata, B. P. M., & Mastroianni, P. d C. (2018). Association between development of dementia and use of benzodiazepines: A systematic review and meta-analysis. Pharmacotherapy, 38(10), 1010–1020. https://doi.org/10.1002/phar.2170
- Malakouti, S. K., Javan-Noughabi, J., Yousefzadeh, N., Rezapour, A., Mortazavi, S. S., Jahangiri, R., & Moghri, J. (2021). A systematic review of potentially inappropriate medications use and related costs among the elderly. Value in Health Regional Issues, 25, 172–179. https://doi.org/10.1016/j.vhri.2021.05.003
- Markota, M., Rummans, T. A., Bostwick, J. M., & Lapid, M. I. (2016). Benzodiazepine use in older adults: Dangers, management, and alternative therapies. Mayo Clinic Proceedings, 91(11), 1632–1639. https://doi.org/10.1016/j.mayocp.2016.07.024
- Maust, D. T., Blow, F. C., Wiechers, I. R., Kales, H. C., & Marcus, S. C. (2017). National trends in antidepressant, benzodiazepine, and other sedative–hypnotic treatment of older adults in psychiatric and primary care. The Journal of Clinical Psychiatry, 78(4), e363–e371. https://doi.org/10.4088/JCP.16m10713
- Maust, D. T., Lin, L. A., & Blow, F. C. (2019). Benzodiazepine use and misuse among adults in the United States. Psychiatric Services (Washington, D.C.), 70(2), 97–106. https://doi.org/10.1176/appi.ps.201800321
- Meyer, T. J., Miller, M. L., Metzger, R. L., & Borkovec, T. D. (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28(6), 487–495. https://doi.org/10.1016/0005-7967(90)90135-6
- Michelini, S., Cassano, G., Frare, F., & Perugi, G. (1996). Long-term use of benzodiazepines: Tolerance, dependence and clinical problems in anxiety and mood disorders. Pharmacopsychiatry, 29(4), 127–134. https://doi.org/10.1055/s-2007-979558
- Murphy, Y., Wilson, E., Goldner, E. M., & Fischer, B. (2016). Benzodiazepine use, misuse, and harm at the population level in Canada: A comprehensive narrative review of data and developments since 1995. Clinical Drug Investigation, 36(7), 519–530. https://doi.org/10.1007/s40261-016-0397-8
- Nathan, P. E., & Gorman, J. M. (2015). A guide to treatments that work. Oxford University Press.
- National Institute for Health and Care Excellence. (2004, April 28). Guidance on the use of zaleplon, zolpidem and zopiclone for the short-term management of insomnia. https://www.nice.org.uk/guidance/ta77
- National Institute for Health and Care Excellence. (2020, June 15). Generalised anxiety disorder and panic disorder in adults: Management. https://www.nice.org.uk/guidance/cg113
- Olfson, M., King, M., & Schoenbaum, M. (2015). Benzodiazepine use in the United States. JAMA Psychiatry, 72(2), 136–142. https://doi.org/10.1001/jamapsychiatry.2014.1763
- O’Connor, K. P., Marchand, A., Brousseau, L., Mainguy, N., Landry, P., Savard, P., Turcotte, J., Léveillé, C., Boivin, S., Pitre, D., Robillard, S., & Bouthillier, D. (2004). Évaluation d’un programme d’aide au succès de sevrage des benzodiazépines. Santé Mentale au Québec, 28(2), 121–148. https://doi.org/10.7202/008620ar
- Pérodeau, G., Grenon, É., Grenier, S., & O’Connor, K. (2016). Systemic model of chronic benzodiazepine use among mature adults. Aging & Mental Health, 20(4), 380–390. https://doi.org/10.1080/13607863.2015.1015961
- Procyshyn, R., Bezchlibnyk-Butler, K., & Jeffries, J. (2019). Clinical handbook of psychotropic drugs. (23rd ed.). Hogrefe.
- Qaseem, A., Kansagara, D., Forciea, M. A., Cooke, M., Denberg, T. D., & Clinical Guidelines Committee of the American College of Physicians. (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 165(2), 125–133. https://doi.org/10.7326/M15-2175
- Rickels, K., Case, W. G., Schweizer, E., Garcia-Espana, F., & Fridman, R. (1991). Long-term benzodiazepine users 3 years after participation in a discontinuation program. American Journal of Psychiatry, 148(6), 757–761.
- Roth, A., & Fonagy, P. (2013). What works for whom?. A critical review of psychotherapy research. Guilford Press.
- Shinfuku, M., Kishimoto, T., Uchida, H., Suzuki, T., Mimura, M., & Kikuchi, T. (2019). Effectiveness and safety of long-term benzodiazepine use in anxiety disorders: A systematic review and meta-analysis. International Clinical Psychopharmacology, 34(5), 211–221. https://doi.org/10.1097/YIC.0000000000000276
- Singh, D. (2013). Economic evaluation of benzodiazepines versus cognitive behavioural therapy among older adults with chronic insomnia. [Unpublished doctoral dissertation]. Université de Montréal. https://papyrus.bib.umontreal.ca/xmlui/bitstream/handle/1866/9935/Dharmender_Singh_2012_memoire.pdf?sequence=2&isAllowed=y
- Smith, B. D., & Salzman, C. (1991). Do benzodiazepines cause depression? Hospital & Community Psychiatry, 42(11), 1101–1102. https://doi.org/10.1176/ps.42.11.1101
- Stahl, S. M. (2020). Prescriber’s guide: Stahl’s essential psychopharmacology. Cambridge University Press.
- Stuck, A. E., Beers, M. H., Steiner, A., Aronow, H. U., Rubenstein, L. Z., & Beck, J. C. (1994). Inappropriate medication use in community-residing older persons. Archives of Internal Medicine, 154(19), 2195–2200. https://doi.org/10.1001/archinte.1994.00420190095011
- Sudak, D. M. (2011). Combining CBT and medication: An evidence-based approach. Wiley.
- Tabachnick, B. G., Fidell, L. S., & Ullman, J. B. (2007). Using multivariate statistics. (Vol. 5) Pearson.
- Trew, J. L. (2011). Exploring the roles of approach and avoidance in depression: An integrative model. Clinical Psychology Review, 31(7), 1156–1168. https://doi.org/10.1016/j.cpr.2011.07.007
- Vézina, J., Landreville, P., Bourque, P., & Blanchard, L. (1991). Questionnaire de Dépression de Beck: Étude psychométrique auprès d’une population âgée francophone. Canadian Journal on Aging / La Revue Canadienne du Vieillissement, 10(1), 29–39. https://doi.org/10.1017/S0714980800007236
- Vikander, B., Koechling, U. M., Borg, S., Tönne, U., & Hiltunen, A. J. (2010). Benzodiazepine tapering: A prospective study. Nordic Journal of Psychiatry, 64(4), 273–282. https://doi.org/10.3109/08039481003624173
- Vinkers, C. H., & Olivier, B. (2012). Mechanisms underlying tolerance after long-term benzodiazepine use: A future for subtype-selective GABAA receptor modulators? Advances in Pharmacological Sciences, 2012, 416864–416819. https://doi.org/10.1155/2012/416864
- Weich, S., Pearce, H. L., Croft, P., Singh, S., Crome, I., Bashford, J., & Frisher, M. (2014). Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: Retrospective cohort study. BMJ (Clinical Research ed.), 348(mar19 5), g1996. https://doi.org/10.1136/bmj.g1996
- Wirz-Justice, A., & Van den Hoofdakker, R. H. (1999). Sleep deprivation in depression: What do we know, where do we go? Biological Psychiatry, 46(4), 445–453. https://doi.org/10.1016/s0006-3223(99)00125-0