Abstract
Introduction: Hyperandrogenism affects approximately 10–20% of women of reproductive age. Hyperandrogenic skin symptoms such as hirsutism, acne, seborrhea and alopecia are associated with significant quality of life and psychological impairment. Women with abnormalities in androgen metabolism may have accompanying anovulation and/or polycystic ovary syndrome (PCOS), both of which have reproductive and metabolic implications if left untreated. Cyproterone acetate (CPA), combined with ethinylestradiol (EE), is indicated for the treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhea) and/or hirsutism, in women of reproductive age.
Objective: To review the data on the efficacy and safety of CPA 2 mg/EE 35 μg for the treatment of hyperandrogenic skin symptoms in women.
Methods: A non-systematic narrative review based on a literature search of the PubMed database.
Results: Seventy-eight studies were identified. The majority of sufficiently powered studies show a high efficacy of CPA 2 mg/EE 35 μg in the treatment of severe acne and hirsutism. Studies show that therapeutic response in women with hirsutism requires a long-term approach and that hyperandrogenic skin symptoms in patients with PCOS are efficiently treated. Additional benefits include cycle control and, in some women, improvement in mood and perception of body image. Safety and tolerability data are summarized by the pharmacovigilance risk assessment committee (PRAC) of the European Medicine’s Agency’s (EMA).
Conclusions: This review provides a comprehensive overview about the efficacy of CPA 2 mg/EE 35 μg in the treatment of hyperandrogenic skin symptoms, thus allowing both health care professionals and women to balance the risks and benefits of treatment based on evidence.
Chinese abstract
前言:雄激素过多影响约10-20%的育龄妇女。雄激素过多皮肤症状如多毛症, 痤疮, 皮脂溢和脱发与生活质量和心理障碍相关。 雄激素代谢异常的妇女可能合并无排卵和/或多囊卵巢综合征(PCOS), 如果不治疗这两者都对生殖和代谢有影响。育龄妇女醋酸环丙孕酮(CPA)与炔雌醇(EE)合用治疗雄激素相关的中、重度痤疮(有/无皮脂溢)和/或多毛症。
目的:回顾有关CPA 2 mg/EE 35μg治疗妇女高雄激素性皮肤症状的疗效和安全性的资料。方法:根据PubMed数据库的文献检索, 进行非系统性的综述。结果:确定了78项研究。大多数有效的研究表明CPA 2mg/EE 35μg在治疗严重痤疮和多毛症方面有高效性。研究表明多毛症妇女的治疗是长期过程, PCOS患者的高雄激素性皮肤症状得到有效治疗。其他益处包括调节周期, 可以改善一些女性的心情和体成分。安全和耐药性数据由欧洲药物管理局(EMA)的药物警戒风险评估委员会(PRAC)总结。
结论:本回顾性研究全面概述了CPA 2mg/EE35μg在治疗高雄激素皮肤症状的疗效, 从而使保健专业人员和妇女根据证据平衡治疗的风险和收益。
Introduction
Definition and prevalence of hyperandrogenism and hyperandrogenic skin symptoms
Hyperandrogenism (androgen excess) affects approximately 10–20% of women of reproductive age [Citation1]. It can present as biochemical hyperandrogenism, where there is excessive production and/or secretion of androgens, which may be of ovarian or adrenal origin. It can also present as clinical hyperandrogenism, where the pilosebaceous unit has increased sensitivity to normal serum androgen levels and causes hyperandrogenic skin symptoms [Citation1]. Women with biochemical hyperandrogenism may present solely with clinical symptoms or have accompanying anovulation and/or polycystic ovary syndrome (PCOS).
Hyperandrogenic skin symptoms include acne, hirsutism, seborrhea and alopecia. The majority of data regarding prevalence of these skin symptoms are derived from studies of women with PCOS. Hirsutism is the most sensitive marker for increased levels of androgen (hyperandrogenism), and is present in 70% of women with PCOS [Citation2]. Acne is a less prevalent and less specific marker of elevated androgens (hyperandrogenism) and is present as a symptom in approximately 15% of women with PCOS [Citation3]. Both hirsutism and acne can significantly and negatively impact on quality of life and cause anxiety and depression [Citation4,Citation5]. Female androgenic alopecia affects approximately 35% of women with PCOS and can occur either in isolation (rarely) or in association with other skin symptoms of hyperandrogenism [Citation6]. Seborrhea can also present as a symptom of androgen excess. An accurate figure for prevalence is difficult to determine but it is frequently reported alongside other symptoms of hyperandrogenism. In some cases, women present with all four hyperandrogenic skin symptoms, described as the seborrhea, acne, hirsutism and alopecia (SAHA) syndrome by Orfanos et al. [Citation7]. The SAHA syndrome presents in approximately 20% of women affected by hyperandrogenism and is a useful marker of hormonal disorders of androgen metabolism [Citation8].
Principles of pharmacologic treatment
Pharmacological treatment of hyperandrogenic skin symptoms has two aims: firstly, to reduce the level of circulating androgens and, secondly, to inhibit their effect at tissue level. Unlike the combined oral contraceptives (COCs) commonly used to treat hyperandrogenic symptoms, cyproterone acetate (CPA) 2 mg, combined with ethinylestradiol (EE) 35 μg, is indicated for the treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhea) and/or hirsutism, in women of reproductive age [Citation9]. CPA is a steroidal antiandrogen: it competitively inhibits the binding of both testosterone and its conversion product, 5-α-dihydrotestosterone (DHT) to the androgen receptor; it reduces testosterone and androstenedione production in the ovary; it blocks the conversion of testosterone to DHT by inhibiting the 5-α-reductase [Citation10–16].
The potency of CPA is greater than other antiandrogenic progestogens typically present in COCs [Citation17], resulting in achievement of greater clinical improvement [Citation10]. EE enhances the action of CPA by increasing sex hormone-binding globulin (SHBG) levels, which leads to a reduction in free testosterone and thus adds to the antiandrogenic action of CPA. There is data available regarding the use of CPA/EE in the treatment of hyperandrogenism in women, all of which is underpinned by long-term clinical experience.
The need for an update of evidence and knowledge
Cochrane database reviews describing the efficacy of CPA/EE in hirsutism and acne highlight the heterogeneity of studies and challenges in pooling data to generate robust conclusions about the efficacy of therapeutic interventions in the management of hyperandrogenic skin symptoms [Citation18–20]. Yet, evidence supporting the efficacy of interventions such as CPA/EE remains important. Women with skin symptoms of hyperandrogenism may also have abnormalities in androgen metabolism (biochemical hyperandrogenism) and accompanying anovulation and/or PCOS, both of which have reproductive and metabolic implications [Citation2]. There is growing awareness of the effect of androgen excess on metabolic risk factors and a need to measure the impact of treatment on haemostasis, lipid metabolism and liver function. At the same time, concerns regarding the safety and the risks of antiandrogenic progestogens should be weighed against the benefit for patients. In the wake of these concerns about safety, it seems appropriate to look in detail at the risks and benefits of this important treatment.
This review, developed on behalf of the global appropriate care for women with androgen excess (AWARE) group, sets out to summarize the evidence supporting the efficacy and safety of CPA/EE in the treatment of hyperandrogenic skin symptoms. In addition, it summarizes the effects of CPA/EE on metabolic parameters.
Methods
A limited number of studies involving large study populations, variable time frames and lack of clearly defined assessment of both long- and short-term treatment outcomes in hyperandrogenic skin symptoms [Citation18–20] limits the possibility of systematic review or meta-analysis [Citation21]. As a result, a narrative review (non-systematic approach that borrows from systematic methodologies and employs a bibliographic research strategy) was undertaken by the authors [Citation22,Citation23].
An electronic literature search of the PubMed database was performed. Keywords included (but were not limited to): CPA 2 mg/EE 35 μg; Diane-35; Diane(tte); cyproterone; EE; acne; hirsutism; seborrhea; safety; efficacy; risks/benefits. Inclusion criteria were defined as studies comparing CPA/EE with placebo or other comparator in the treatment of acne, hirsutism, seborrhea, SAHA, hyperandrogenic skin symptoms of PCOS. The search was restricted to English-language abstracts and human studies.
Study abstracts were collated and grouped according to hyperandrogenic skin symptom (acne, hirsutism, seborrhea, SAHA, hyperandrogenic skin symptoms of PCOS). Authors reviewed the search findings and agreed further groupings as follows:
CPA/EE vs placebo and comparator
Large and small studies, where large is defined as ≥100 participants
Treatment duration, where the study period is either ≤ or ≥6 months for acne; ≤ or ≥12 months for hirsutism and seborrhea
Where possible, studies involving validated assessment of improvement in dermatological endpoint were prioritized for review. For acne, outcomes described by the cochrane review were prioritized [Citation19]: facial lesion counts (both total and specific); acne severity grades; global assessment (clinician or participant). For hirsutism, studies using Ferriman–Gallwey (F–G) or modified (m)F–G Clinical Score were prioritized [Citation24,Citation25]. For seborrhea, studies using the seborrhea area and severity index-face were prioritized for review. Safety evaluations included the number and type of adverse drug reactions (ADRs) reported and treatment discontinuation due to ADRs.
Results
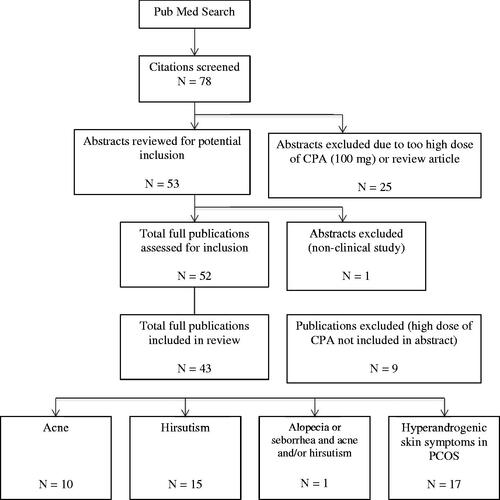
A total of 78 studies were identified as part of the search process (see ). Twenty-five were excluded because the dose of either CPA or EE used in the study was outside of routine clinical practice in the management of hyperandrogenic skin symptoms or the abstract described a review or commentary article rather than a clinical study. A total of 52 abstracts were screened for efficacy and safety findings. Final analysis of the full publications prompted exclusion of a further nine studies due to use of high doses of CPA, either in combination with EE or as part of a higher dose sequential regimen, not disclosed in the abstract. Forty-three studies are therefore included in this narrative review.
CPA/EE in the treatment of acne
Ten studies evaluated the efficacy and safety of CPA/EE in the treatment of acne (see Appendix 1). They dated from 1985 to 2008 and included one placebo-controlled study, five comparator studies, two dosing studies and two combination studies. All of the studies had improvement in acne as a primary outcome measure and assessed changes in the number and severity of acne lesions and/or the number of inflammatory lesions (see Appendix 1). Description of formal evaluation methods was limited to Cook score [Citation26,Citation27] and investigator global assessment (IGA) (1 = clear, 2 = excellent improvement, 3 = good, 4 = moderate, 5 = no and 6 = deterioration) [Citation28]. Secondary outcome measures included laboratory assessment of changes in hormone and lipid levels, bacterial colonization and sebum excretion rate.
Efficacy in acne
The studies reported CPA/EE to be highly effective in improving acne as early as three months, when used alone or in combination, and in some women refractory to other types of treatment [Citation27–30]. Percentage changes in total lesion count with CPA/EE ranged from 53.6 ± 27.5% (n = 528) to 72 ± 27% (n = 44) [Citation28,Citation30]. Greenwood et al. [Citation31] reported an 80% reduction in non-inflammatory and inflammatory lesion counts with CPA/EE (n = 14) at six months. When looking more specifically at types of lesion (comedones, papules, pustules and nodules), statistically significant changes were noted at three months and final numbers of each lesion were reported as 24%, 36%, 17% and 1%, respectively relative to pretreatment (=100%) at six months in the CPA/EE group (n = 88) by Vartiainen et al. [Citation29]. An improvement in acne in 90.2% of patients (n = 480/532), as assessed by IGA, was reported by Palombo-Kinne et al. [Citation28], Dieben et al. [Citation32] reported a reduction in acne grade 4 (nodules present) and 5 (pustules, no nodules) of 50% and 36%, respectively with CPA/EE at six months.
Improvements in acne were seen within two cycles but greatest improvements are seen at six months; two studies evaluated the efficacy of CPA/EE after 12 months and showed a continued improvement [Citation27,Citation33]. In the former study, treatment with CPA/EE improved specific acne lesions (comedones, papules and macules) and overall severity compared to baseline after six months (p < .01). Further significant improvement was achieved in the number of macules and papules after 12 months at which point, over two-thirds of the patients were either free of facial acne or exhibited just a few small, scattered lesions [Citation27]. Efficacy of CPA/EE in acne was consistent, irrespective of whether assessed objectively (by the investigator) or subjectively (by the patient) [Citation29,Citation30].
CPA/EE in the treatment of hirsutism
We identified 15 studies evaluating the efficacy of CPA/EE in women with hirsutism (either idiopathic [IH] or as a skin symptom of PCOS), dating from 1982 to 2012 (see Appendix 2). They included four comparator studies, two dosing studies, nine combination studies and one reverse sequential regimen study.
All of the studies used improvement in hirsutism (the majority assessed by F–G or mF–G) as a primary outcome. Some studies also assessed changes in hair diameter and growth rate; hair distribution (facial, bust and abdomen); and frequency of shaving or hot wax treatment. Secondary outcome measures included laboratory assessment of changes in hormone and lipid levels.
Efficacy in hirsutism
In general, the studies reported CPA/EE to be highly effective in reducing hirsutism scores (either F–G or mF–G), and frequency of shaving or hot wax treatment as early as three months. Statistically significant changes are generally seen between 6 and 12 months after initiation of treatment. Greatest improvements in hirsutism scores with CPA/EE were generally seen at 12 months with mean reductions (±SD) ranging from 24.6 (±1.91)% to 54.31 (±22.1)% [Citation34–37]. One study showed the benefit of continuing treatment with CPA/EE to 24 months, with F–G scores declining from 11.8 ± 0.6 SE to 4.7 ± 0.6, a score almost equal to control (3.6 ± 0.3) [Citation38]. There was some evidence to show that hair in different areas of the body may respond to CPA/EE treatment differently: facial hair appears to respond more quickly [Citation34]. Sert et al. [Citation35] reported a 59% decrease in the need to shave or use hot wax treatment after receiving CPA/EE for 12 months. In studies where CPA/EE was combined with other treatment options, for example, flutamide, finasteride or spironolactone, reductions in hirsutism scores were generally greater with combination treatment than with CPA/EE alone. Ibanez et al. [Citation39] looked at the efficacy of CPA/EE in the treatment of hirsutism and acne in adolescent women and reported a 23.2% reduction in hirsutism score (mF–G) and 36.3% reduction in acne score at six months.
CPA/EE in the treatment of seborrhea or alopecia and/or acne or hirsutism
One study evaluated the efficacy and safety of CPA/EE in the treatment of seborrhea (with or without accompanying symptoms of acne or hirsutism), dated 2002 (see Appendix 3). The study compared drospirenone/EE with CPA/EE. The primary outcomes included sebum production (as measured by photometry), number/diameter of hairs and acne. The study also assessed hormone and lipid profiles. The only study evaluating the efficacy and safety of CPA/EE in alopecia identified as during the search involved use of CPA/EE in combination with CPA and was therefore excluded. CPA/EE is not indicated for the treatment of androgenic alopecia.
Efficacy in seborrhea
In the study looking at the effect of CPA/EE on seborrhea and acne, Van Vloten et al. [Citation40] reported a 39.3% reduction in median sebum production and 58.8% reduction in median acne lesion count at nine months.
CPA/EE in the treatment of hyperandrogenic skin symptoms of PCOS
Seventeen studies evaluated the efficacy and safety of CPA/EE in the treatment of hyperandrogenic symptoms of PCOS, dating from 1986 to 2014 (see Appendix 4). They included 13 comparator studies (of which five involved an insulin sensitizer as the comparator), two combination studies and one open-label study. All studies assessed the clinical and biochemical features of hyperandrogenism. Improvement in hirsutism score featured as an outcome measure in 12 studies, hirsutism and acne in three studies and hirsutism and seborrhea in one study.
Efficacy in hyperandrogenic skin symptoms of PCOS
In general, the studies described significant improvements in serum androgen levels and hyperandrogenic skin symptoms of PCOS with CPA/EE as early as six months. The significant reductions in hirsutism scores (F–G and mF–G) at six months ranged from 17.7% to 38.2% [Citation41–43]. When treatment effect was assessed at 9 or 12 months, changes in hirsutism score ranged from 5% to 35% [Citation44,Citation45].
Creatsas et al. [Citation46], Luque-Ramierz et al. [Citation47], Lemay et al. [Citation48], Falsetti et al. [Citation49] described consistent reduction in hirsutism scores (F–G and mF–G) in all areas of the body. Kahraman et al. [Citation46] describe more prominent changes in specific body areas, for example, the abdomen. In an open-label study, Golland and Elstein [Citation50] reported a healing/improvement rate of 54.6% (reduction in mean F–G score from 14.3 to 5.7) at 12 months with CPA/EE in 22 women with severe facial hirsutism. Seven women experienced complete resolution of symptoms and a further six women experienced significant symptom improvement with CPA/EE after 12 months. Two studies described the return of hyperandrogenic skin symptoms following cessation of treatment with CPA/EE treatment by six months [Citation33,Citation51].
When looking at other parameters, CPA/EE showed significant reduction in hair diameter in all areas evaluated (chin, abdomen, mid-thigh, forearm and combined arms) at 12 months [Citation45]. Greatest changes were seen in the abdomen (25%) and mid-thigh (20%) and objective results correlated with patients’ own qualitative assessment of improvement in hirsutism at 12 months [Citation45]. Golland and Elstein [Citation51] also reported correlation between objective and patient assessment.
Cyproterone acetate/ethinylestradiol was shown to improve coexisting acne and seborrhea in women with PCOS. In the study by Erkkola et al. [Citation52], CPA/EE normalised seborrhea in 59.2% of patients within nine months and also led to an improvement of facial acne in 80.7% and complete healing of acne in 59.7% of women. Golland and Elstein [Citation51] also described improvements in coexisting acne and seborrhea with CPA/EE.
Four of the studies compared the effects of CPA/EE on the hyperandrogenic skin symptoms of PCOS with COCs [Citation10,Citation46,Citation47,Citation53]. Kahraman et al. [Citation45] compared CPA/EE with drosperinone (DRSP)/EE and reported a significant difference in reduction in hirsutism score between the two, changes in mF–G score of −35% (−71 to 10) and −18% (−18 to 30) in women treated with CPA/EE and DRSP/EE, respectively.
Mastorakos et al. [Citation54] and Creatsas et al. [Citation46] compared the effects of CPA/EE with desogestrel (DSG)/EE and described similar and significant declines in F–G score for both formulations (compared to baseline) at six months and a continued, but not significant decline until 12 months. Bhattarcharya and Jha [Citation10] compared the effects of CPA/EE on hirsutism score with both DSG/EE and DRSP/EE over 12 months. There was a significant decrease in mF–G score with CPA/EE (−5.29) when compared with DSG (−1.69) and DRSP (−2.12) [Citation10].
CPA/EE as combination treatment
Studies involving CPA/EE combined with other agents showed mixed results. For example, when combined with antibiotics (tetracycline) in the treatment of acne, or with antiandrogens (finasteride or spironolactone) in the treatment of hirsutism, the potential for enhanced efficacy was suggested [Citation31,Citation36,Citation37,Citation55]. Studies where CPA/EE was added to gonadotropin releasing hormone agonist (GnRHa) to evaluate potential for amplification of treatment response in hyperandrogenic symptoms of PCOS described significant improvement in hirsutism symptoms overall (reductions in hirsutism score were between 24% and 47.4%) but no significant difference between the two groups [Citation50,Citation52,Citation56]. Sequential use of CPA/EE with rosiglitazone did not generate any greater symptom improvement than use of CPA/EE alone in women with PCOS [Citation49].
Additional benefits of CPA/EE
Cyproterone acetate/ethinylestradiol offers additional benefits when used in the treatment of hyperandrogenic skin symptoms: improvements in menstrual cycle regularity, potential quality of life and psychological benefit and effective contraception in those women wishing to avoid pregnancy. CPA/EE induced regular withdrawal bleeds and reduced ovary size during treatment in women with hyperandrogenic skin symptoms associated with PCOS [Citation47,Citation54,Citation62]. There was a trend towards improvements in quality of life with CPA/EE in adolescents [Citation57]. CPA/EE demonstrated improvement in subjective perception of psychiatric status in women with hyperandrogenic skin symptoms associated with PCOS [Citation57].
Safety of CPA/EE
Concerns have been raised recently about the safety of CPA/EE, particularly the risk of thromboembolic events. A review conducted by the European Medicine’s Agency’s (EMA) pharmacovigilance risk assessment committee (PRAC) concluded that the benefits of CPA/EE outweighed the risks when used to treat moderate to severe acne related to androgen excess and/or hirsutism in women of reproductive age provided that several measures were undertaken to minimize the risk of thromboembolism [Citation58]. The PRAC review confirmed the rare and known risk of thromboembolism with CPA/EE and highlighted the importance of discussing with patients the increase in risk associated with age, smoking, obesity and prolonged immobility [Citation59]. The studies included within this review show CPA/EE to be generally well tolerated with a side effect profile similar to that seen with EE-containing COCs. Reported side effects include headaches, nausea, weight gain, breast tenderness, loss of libido and, rarely, hepatotoxicity effects, with low rates of discontinuation. Breast tension persisted through six months of treatment in one study [Citation59]. No thromboembolic events are reported in any of the 46 reviewed studies involving treatment for up to 24 months.
Metabolic effects of CPA/EE
The metabolic effects of CPA/EE and their implications are not discussed in detail. Observations were mainly limited to lipid metabolism when CPA/EE use was evaluated in the treatment of hyperandrogenic skin symptoms. The conflicting findings regarding metabolic effects of CPA/EE in the treatment of skin symptoms in women with PCOS are discussed in Ruan et al. [Citation60].
Lipid metabolism
In the studies involving CPA/EE in the treatment of acne, generally, any changes in liver function or lipid levels were reported to be within normal limits [Citation60]. Some changes in lipid metabolism (elevated triglycerides and changes in high-density lipoprotein (HDL) cholesterol/low-density lipoprotein (LDL) cholesterol were seen but these remained within normal limits and declined in clinical relevance beyond six months [Citation60].
Weight and other vital signs
When studied in the treatment of acne, no effect was seen on BMI or vital signs with CPA/EE [Citation28]. There were no significant changes in clinical markers of obesity and blood pressure reported with 12-month treatment of acne in women with PCOS [Citation10,Citation61]. BMI and waist to height ratio (WHR) remained stable with CPA/EE throughout six months treatment [Citation62]. However, CPA/EE was shown to increase arterial stiffness as a predictor of CV risk [Citation62].
Discussion
Findings and interpretation
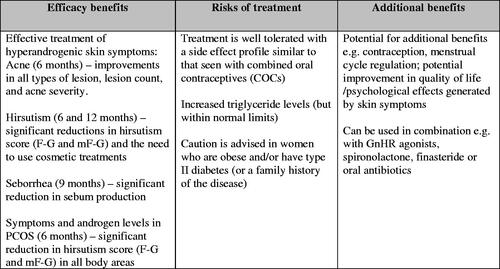
Critical analysis of efficacy and safety findings in the studies included in the review confirms the role of CPA/EE in the treatment of hyperandrogenic skin symptoms. Although some improvement in both hirsutism and acne is seen within six months, maximum effect is generally achieved over a longer period. However, the return of hyperandrogenic skin symptoms within six months of cessation of treatment indicates the need for long-term therapy. In general, improvement is described as a quantifiable reduction of symptom scores (either objective or subjective). It does not indicate ‘healing of the disease’ but amelioration of symptoms with a positive effect on quality of life.
Published clinical trials show that CPA/EE is generally well tolerated with a side effect profile similar to that seen with COCs. No thromboembolic events were reported in any of the 46 studies included in this review, some of which involved treatment for up to 24 months. However, the number of patients included does not allow the authors to make a general statement on cardiovascular safety.
Cyproterone acetate/ethinylestradiol offers some additional benefits when used in the treatment of hyperandrogenic skin symptoms: improvements in menstrual cycle regularity, potential quality of life and psychological benefit and effective contraception in those women wishing to avoid pregnancy.
Strength and weaknesses of the review and comparison with previous reviews
Data describing the efficacy of CPA/EE in hirsutism and acne has been included in three cochrane database reviews [Citation18–20]. Conclusions from these reviews highlight the heterogeneity of studies and challenges in pooling data to generate robust conclusions about the efficacy of therapeutic interventions in the management of hyperandrogenic skin symptoms as clinical entity.
Based on the cochrane reviews, we have performed a narrative review focusing on clinically relevant studies and using transparent selection criteria. The conclusions and themes drawn here are limited to the studies included in the review and are influenced by a number of factors. These include small patient populations, lack of validated tools to assess changes in skin symptoms (with the exception of hirsutism), inconsistencies in the reporting of results leading to difficulties when comparing study outcomes and summarising overall effects, and the limited long-term safety assessment due to the short-term duration of studies. We believe, however, that the overarching results are clinically meaningful, especially given that these conditions are chronic in nature and, although symptoms can be improved, they rarely disappear completely. Compared to what has been shown in the Cochrane reviews, the present narrative review, which included newer studies, confirms and strengthens the evidence supporting the efficacy of the combination of CPA 2 mg and EE 35 μcg, not only in acne but also in hirsutism and it underlines the importance of long term treatment of these chronic skin diseases.
Unanswered questions and future research
The findings of this review highlight a number of areas where further study would be useful. Clinical studies confirmed an effect of CPA/EE on lipid metabolism but generally regarded changes to be within normal limits and of little clinical relevance. Effects of CPA/EE on insulin resistance remain inconsistent and prompt the need for more research into metabolic changes in women affected by hyperandrogenism. There is potential for the efficacy of CPA/EE in the treatment of hyperandrogenic skin symptoms to be enhanced by using it in combination with other treatments but further research is needed.
The specific role of CPA/EE in the management of hyperandrogenic symptoms in women with PCOS is discussed in an accompanying review by Mueck et al. [Citation61].
Implications for clinical practice
Hyperandrogenic skin symptoms such as acne and hirsutism are associated with considerable impairment of quality of life and adverse psychological impact [Citation4,Citation5]. Pharmacological treatment of these symptoms aims to reduce the level of circulating androgens and inhibit their effect at tissue level. CPA/EE has been available as a treatment option for hyperandrogenic skin symptoms for approximately three decades. It is indicated for the treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhea) and/or hirsutism, in women of reproductive age and has been described as the treatment of choice for hyperandrogenism [Citation62,Citation63].
Critical analysis of the role of CPA/EE in the treatment of hyperandrogenic skin symptoms identified five key themes relevant to best practice (see ):
A high efficacy in the treatment of moderate to severe acne;
A high efficacy in the treatment of hirsutism;
Treatment is well tolerated with a side effect profile similar to that seen with COCs;
A definite effect on lipid metabolism but within normal limits and of little clinical relevance; however, caution is advised when considering women who are obese and/or have type II diabetes;
Potential for the efficacy of CPA/EE in the treatment of hyperandrogenic skin symptoms to be enhanced by using it in combination;
Potential for additional benefits when used in the treatment of hyperandrogenic skin symptoms.
Given the characteristics of the combination treatment of CPA/EE and the fact that hyperandrogenic skin disorders such as acne and hirsutism are chronic diseases, the implications for clinical practice are:
Treatment success depends on long term adherence of the patients;
Repetitive treatment episodes may be necessary;
Chronic treatment needs a continuous re-evaluation of the benefit/risk ratio, especially in patients in whom additional risk factors for cardiovascular complications may occur over time, such as obesity or smoking;
Age itself is an independent risk factor and should always be considered.
Conclusions
Clinical trials included in this review show a high efficacy of CPA/EE in the treatment of hyperandrogenic skin symptoms, irrespective of whether they occur independently of or secondary to PCOS. Resolution is gradual and governed by symptom severity and duration of treatment. The review also shows that CPA/EE is generally well tolerated with a side effect profile similar to that seen with COCs.
This review does not set out to compare the effects of CPA/EE across the different studies or effect comparisons with other hormonal treatments; it focuses on summarizing the main effects of treatment in order to inform clinical practice to provide evidence-based information to assist in balancing the benefits and risks of this treatment. It also highlights areas to consider when individualizing treatment and where further study would add to that knowledge. These include the safety of long-term use of CPA/EE and the associated metabolic implications, given the complex interaction between hyperandrogenism and insulin sensitivity, particularly in PCOS.
Disclosures statement
Johannes Bitzer has worked as an Advisor for and received honoraria by Bayer Health Care, Merck, Teva, Exeltis, Lilly, Boehringer-Ingelheim, Vifor, Gedeon Richter. He has also given invited lectures and received honoraria by Bayer AG, Merck, Johnson and Johnson, Teva, Mylan, Allergan, Abbott, Lilly, Pfizer, Gedeon Richter.
Thomas Römer has received honorarium and travel cost for lectures and advisory boards from Bayer AG, MSD and Gedeon Richter.
Agnaldo Lopes da Silva Filho has received honoraria for lecturing and acting in an advisory capacity for a number of pharmaceutical companies including Bayer AG, Roche, MSD, TEVA and Grünenthal.
Acknowledgements
The authors are members of the AWARE group, an independent panel of physicians with expert interest in the treatment of androgen excess in women. Other members of the group include Angela Aguilar, Deng ChengYan, Ali Kubba, Alfred Mueck and Christos Zouboulis. Formation of the AWARE group and the group’s meetings were supported by Bayer AG. Members received honoraria for attendance at meetings but no honoraria were paid for contributions to this manuscript. This publication and its content are solely the responsibility of the authors. Medical writing assistance was provided by Clark Health Communications under the direction of the authors and paid for by Bayer AG.
References
- Redmond GP. Androgens and women’s health. Int J Fertil Womens Med. 1998;43:91–97.
- Fauser BCJM, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.
- Azziz R, Sanchez L, Knochenhauer ES, et al. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89:453–462.
- Ekbäck MP, Lindberg M, Benzein E, et al. Health-related quality of life, depression and anxiety correlate with the degree of hirsutism. Dermatology (Basel). 2013;227:278–284.
- Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol. 1998;139:846–850.
- Ozdemir S, Ozdemir M, Görkemli H, et al. Specific dermatologic features of the polycystic ovary syndrome and its association with biochemical markers of the metabolic syndrome and hyperandrogenism. Acta Obstet Gynecol Scand. 2010;89:199–204.
- Orfanos CE. Antiandrógenos en Dermatologí. Arch Arg Derm. 1982;32:51–55.
- Orfanos CE, Adler YID, Zouboulis CC. The SAHA syndrome. Horm Res. 2000;54:251–258.
- Dianette Summary of Product Characteristics; 2017. Available from: http://www.medicines.org.uk/emc/medicine/1814/SPC/dianette/.
- Bhattacharya SM, Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil Steril. 2012;98:1053–1059.
- Neumann F. The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research. Exp Clin Endocrinol. 1994;102:1–32.
- Spona J, Huber J. Efficacy of low-dose oral contraceptives containing levonorgestrel, gestoden and cyproterone acetate. Gynecol Obstet Invest. 1987;23:184–193.
- Fang S, Liao S. Antagonistic action of anti-androgens on the formation of a specific dihydrotestosterone-receptor protein complex in rat ventral prostate. Mol Pharmacol. 1969;5:428–431.
- Fedele L, Marchini M, Cavalli G, et al. Marked deciliation and insufficient secretory modification of endometrial surface during treatment with a new progestogen–estrogen combination. Contraception. 1987;35:497–505.
- Neumann F. The physiological action of progesterone and the pharmacological effects of progestogens – a short review. Postgrad Med J. 1978;54:11–24.
- Aydinlik S, Kaufman J, Lachnit-Fixson U, et al. Long-term therapy of signs of androgenisation with a low-dosed antiandrogen-oestrogen combination. Clin Trials J. 1990;27:392–402.
- Elger W, Beier S, Pollow K, et al. Conception and pharmacodynamic profile of drospirenone. Steroids. 2003;68:891–905.
- Van der Spuy ZM, le Roux PA. Cyproterone acetate for hirsutism. Cochrane Database Syst Rev. 2003;4:CD001125.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi: 10.1002/14651858.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for hirsutism (excluding laser and photoepilation therapy alone). Cochrane Database Syst Rev. 2015;4:CD010334. doi: 10.1002/14651858.CD010334.pub2.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9.
- Collins JA, Fauser CJMB. Balancing the strengths of systematic and narrative reviews. Hum Reprod Update. 2005;11:103–104.
- Ferrari R. Writing narrative style literature reviews. Medical Writing. 2015;24:230–235.
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447.
- Hatch R, Rosenfield RL, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815–830.
- Carmina E, Lobo RA. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin Endocrinol (Oxf). 2002;57:231–234.
- Fugère P, Percival-Smith RK, Lussier-Cacan S, et al. Cyproterone acetate/ethinyl estradiol in the treatment of acne. A comparative dose–response study of the estrogen component. Contraception. 1990;42:225–234.
- Palombo-Kinne E, Schellschmidt I, Schumacher U, et al. Efficacy of a combined oral contraceptive containing 0.030 mg ethinylestradiol/2 mg dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg ethinylestradiol/2 mg cyproterone acetate. Contraception. 2009;79:282–289.
- Vartiainen M, de Gezelle H, Broekmeulen CJ. Comparison of the effect on acne with a combiphasic desogestrel-containing oral contraceptive and a preparation containing cyproterone acetate. Eur J Contracept Reprod Health Care. 2001;6:46–53.
- Carlborg L. Cyproterone acetate versus levonorgestrel combined with ethinyl estradiol in the treatment of acne. Results of a multicenter study. Acta Obstet Gynecol Scand Suppl. 1986;134:29–32.
- Greenwood R, Brummitt L, Burke B, et al. Acne: double blind clinical and laboratory trial of tetracycline, oestrogen–cyproterone acetate, and combined treatment. Br Med J (Clin Res Ed). 1985;291:1231–1235.
- Dieben TO, Vromans L, Theeuwes A, et al. The effects of CTR-24, a biphasic oral contraceptive combination, compared to Diane-35 in women with acne. Contraception. 1994;50:373–382.
- Carmina E, Lobo RA. Gonadotrophin-releasing hormone agonist therapy for hirsutism is as effective as high dose cyproterone acetate but results in a longer remission. Hum Reprod. 1997;12:663–666.
- Belisle S, Love EJ. Clinical efficacy and safety of cyproterone acetate in severe hirsutism: results of a multicentered Canadian study. Fertil Steril. 1986;46:1015–1020.
- Sert M, Tetiker T, Kirim S. Comparison of the efficiency of anti-androgenic regimens consisting of spironolactone, Diane 35, and cyproterone acetate in hirsutism. Acta Med Okayama. 2003;57:73–76.
- Sahin Y, Dilber S, Keleştimur F. Comparison of Diane 35 and Diane 35 plus finasteride in the treatment of hirsutism. Fertil Steril. 2001;75:496–500.
- Keleştimur F, Sahin Y. Comparison of Diane 35 and Diane 35 plus spironolactone in the treatment of hirsutism. Fertil Steril. 1998;69:66–69.
- Porcile A, Gallardo E. Long-term treatment of hirsutism: desogestrel compared with cyproterone acetate in oral contraceptives. Fertil Steril. 1991;55:877–881.
- Ibanez L, Diaz M, Sebastiani G, et al. Treatment of androgen excess in adolescent girls: ethinylestradiol–cyproteroneacetate versus loq-does pioglitazone-flutamide-metformin. J Clin Endocrinol Metab. 2011;96:3361–3366.
- van Vloten WA, van Haselen CW, van Zuuren EJ, et al. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis. 2002;69:2–15.
- Morin-Papunen LC, Vauhkonen I, Koivunen RM, et al. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: a randomized study. J Clin Endocrinol Metab. 2000;85:3161–3168.
- Dahlgren E, Landin K, Krotkiewski M, et al. Effects of two antiandrogen treatments on hirsutism and insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod. 1998;13:2706–2711.
- Gokmen O, Senoz S, Gulekli B, et al. Comparison of four different treatment regimes in hirsutism related to polycystic ovary syndrome. Gynecol Endocrinol. 1996;10:249–255.
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4116–4123.
- Kahraman K, Sükür YE, Atabekoğlu CS, et al. Comparison of two oral contraceptive forms containing cyproterone acetate and drospirenone in the treatment of patients with polycystic ovary syndrome: a randomized clinical trial. Arch Gynecol Obstet. 2014;290:321–328.
- Creatsas G, Koliopoulos C, Mastorakos G. Combined oral contraceptive treatment of adolescent girls with polycystic ovary syndrome. Lipid profile. Ann N Y Acad Sci. 2000;900:245–252.
- Luque-Ramírez M, Alvarez-Blasco F, Botella-Carretero JI, et al. Comparison of ethinyl-estradiol plus cyproterone acetate versus metformin effects on classic metabolic cardiovascular risk factors in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2453–2461.
- Lemay A, Dodin S, Turcot L, et al. Rosiglitazone and ethinyl estradiol/cyproterone acetate as single and combined treatment of overweight women with polycystic ovary syndrome and insulin resistance. Hum Reprod. 2006;21:121–128.
- Falsetti L, Pasinetti E, Ceruti D. Gonadotropin-releasing hormone agonist (GnRH-A) in hirsutism. Acta Eur Fertil. 1994;25:303–306.
- Golland IM, Elstein ME. Results of an open one-year study with Diane-35 in women with polycystic ovarian syndrome. Ann N Y Acad Sci. 1993;687:263–271.
- De Leo V, Fulghesu AM, la Marca A, et al. Hormonal and clinical effects of GnRH agonist alone, or in combination with a combined oral contraceptive or flutamide in women with severe hirsutism. Gynecol Endocrinol. 2000;14:411–416.
- Erkkola R, Hirvonen E, Luikku J, et al. Ovulation inhibitors containing cyproterone acetate or desogestrel in the treatment of hyperandrogenic symptoms. Acta Obstet Gynecol Scand. 1990;69:61–65.
- Mastorakos G, Koliopoulos C, Creatsas G. Androgen and lipid profiles in adolescents with polycystic ovary syndrome who were treated with two forms of combined oral contraceptives. Fertil Steril. 2002;85:420–427.
- Mastorakos G, Koliopoulos C, Deligeoroglou E, et al. Effects of two forms of combined oral contraceptives on carbohydrate metabolism in adolescents with polycystic ovary syndrome. Fertil Steril. 2006;77:919–927.
- Tartagni M, Schonauer LM, De Salvia MA, et al. Comparison of Diane 35 and Diane 35 plus finasteride in the treatment of hirsutism. Fertil Steril. 2000;73:718–723.
- Acién P, Mauri M, Gutierrez M. Clinical and hormonal effects of the combination gonadotrophin-releasing hormone agonist plus oral contraceptive pills containing ethinyl-oestradiol (EE) and cyproterone acetate (CPA) versus the EE-CPA pill alone on polycystic ovarian disease-related hyperandrogenisms. Hum Reprod. 1997;12:423–429.
- Chung JP, Yiu AK, Chung TK, et al. A randomized crossover study of medroxyprogesterone acetate and Diane-35 in adolescent girls with polycystic ovarian syndrome. J Pediatr Adolesc Gynecol. 2014;27:166–171.
- European Medicines Agency. Benefits of Diane 35 and its generics outweigh risks in certain patient groups – PRAC recommendation endorsed by CMDh. EMA/31830/2013; 2013.
- Fugere P, Percival-Smith RKL, Cacan SL, et al. Cyproterone acetate/ethinyl estradiol in the treatment of acne. Comparative dose-response study of the estrogen component. Contraception. 1990;40:225–234.
- Ruan X, Mueck AO, Aguilar A, Kubba A. Use of cyproterone acetate/ethinyl estradiol in polycystic ovary syndrome: rationale and practical aspects. 2016, in press.
- Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care. 2007;30:471–478.
- Venturoli S, Marescalchi O, Colombo FM, et al. A prospective randomized trial comparing low dose flutamide, finasteride, ketoconazole, and cyproterone acetate-estrogen regimens in the treatment of hirsutism. J Clin Endocrinol Metab. 1999;84:1304–1310.
- Inal MM, Yildirim Y, Taner CE. Comparison of the clinical efficacy of flutamide and spironolactone plus Diane 35 in the treatment of idiopathic hirsutism: a randomized controlled study. Fertil Steril. 2005;84:1693–1697.
- Charoenvisal C, Thaipisuttikul Y, Pinjaroen S, et al. Effects on acne of two oral contraceptives containing desogestrel and cyproterone acetate. Int J Fertil. 1996;41:423–429.
- Miller JA, Wojnarowska FT, Dowd PM, et al. Anti-androgen treatment in women with acne: a controlled trial. Br J Dermatol. 1986;114:705–716.
- Colver GB, Mortimer PS, Dawber RPR. Cyproterone acetate and two doses of oestrogen in female acne; a double-blind comparison. Br J Dermatol. 1988;118:95–99.
- Batukan C, Muderris II, Ozcelik B, Ozturk A. Comparison of two oral contraceptives containing either drospirenone or cyproterone acetate in the treatment of hirsutism. Gynecol Endocrinol. 2007;23:38–44.
- Barth JH, Cherry CA, Wojnarowska F, Dawber RPR. Cyproterone acetate for severe hirsutism: results of a double-blind dose-ranging study. Clin Endocrinol. 1991;35:5–10.
- Taner C, Inal M, Basogul Ö, et al. Comparison of the clinical efficacy and safety of flutamide versus flutamide plus an oral contraceptive in the treatment of hirsutism. Gynecol Obstet Invest. 2002;54:105–108.
- Vegetti W, Testa G, Maggioni P, et al. An open randomized comparative study of an oral contraceptive containing ethinyl estradiol and cyproterone acetate with and without the GnRH analogue goserelin in the long-term treatment of hirsutism. Gynecol Obstet Invest. 1996;41:260–268.
- Karakurt F. Comparison of the clinical efficacy of flutamide and spironolactone plus ethinyloestradiol/cyproterone acetate in the treatment of hirsutism: a randomised controlled study. Adv Ther. 2008;25:321–328.
- Couzinet B, Le Strat N, Brailly S, Schaison G. Comparative effects of cyproterone acetate or a long-acting gonadotropin-releasing hormone agonist in polycystic ovarian disease. J Clin Endocrinol Metab. 1986;63:1031–1035.