Abstract
Objectives: This study evaluated acceptability, user satisfaction, body weight control and general well-being of estetrol (E4) combined with either drospirenone (DRSP) or levonorgestrel (LNG).
Methods: In this open-label, multi-centre, dose-finding, 6-cycle study, 396 healthy women of reproductive age were randomised into five treatment groups in a 24/4-day regimen: 15 mg or 20 mg E4 combined with either 3 mg DRSP or 150 μg LNG, and as reference estradiol valerate (E2V) combined with dienogest (DNG). Data on acceptability, user well-being, satisfaction and body weight were collected.
Results: The number of completers was the highest in the 15 mg E4/DRSP group (91.1%), and the lowest for 20 mg E4/LNG (70.1%). The largest proportion of treatment satisfaction was reported for 15 mg E4/DRSP (73.1%), and the lowest for 15 mg E4/LNG (50.6%). The number of women willing to continue with the assigned study treatment was the highest in the 15 mg E4/DRSP group (82.1%) and the lowest for 20 mg E4/LNG (58.3%). Well-being with E4/DRSP combinations was statistically significantly better than with E4/LNG combinations: OR (95% CI) 2.00 (1.13; 3.53) and 1.93 (1.06; 3.56) for 15 and 20 mg E4, respectively, and comparable to E2V/DNG. Proportion of women with a 2 kg or more weight loss after 3 and 6 cycles was the highest in the 15 mg E4/DRSP group (30.7 and 36.7%, respectively).
Conclusions: The present study shows that 15 mg estetrol combined with 3 mg DRSP is associated with a high-user acceptability and satisfaction, and with a favourable body weight control.
Chinese abstract
目的:本研究评估了屈螺酮(DRSP)或左炔诺孕酮(LNG)联合雌四醇(E4)治疗的可接受性, 患者满意度, 及其对患者一般健康状况及体重控制的影响。
方法:在这项开放性多中心的为期6个周期的剂量调查研究中, 396名健康的育龄妇女随机分为5个治疗组, 进行24/4天方案:15mg或20mg E4联合3mg DRSP或150μg LNG, 以戊酸雌二醇(E2V)联合地诺孕素(DNG)作为参考。收集有关可接受性, 一般健康状况, 满意度和体重的数据。
结果:15mg E4/DRSP组中完成者最多(91.1%), 20mg E4/LNG的完成者最低(70.1%)。结果显示治疗满意度最大的为15mg E4/DRSP组(73.1%), 而15mg E4/LNG满意度最低(50.6%)。愿意继续接受指定研究治疗的妇女人数在15mg E4/DRSP组中最高(82.1%), 而20mg E4/LNG组最低(58.3%)。 E4/DRSP组的健康状况在统计学上显着优于E4/LNG组:15和20mg E4的OR(95%CI)分别为2.00(1.13; 3.53)和1.93(1.06; 3.56), 与 E2V/DNG组可比。 3个周期和6个周期后减重2kg及以上的妇女比例在15mg E4/DRSP组中最高(分别为30.7%和36.7%)。
结论:本研究显示, 15mg雌四醇联合3mg DRSP治疗的患者有较高的可接受性及满意度, 并体重控制良好。
Introduction
Combined oral contraceptives (COCs), which contain a synthetic estrogen and progestin, are highly effective; pregnancy rates range from 0.1 to 0.3% among perfect users [Citation1]. However, failure rates up to 8% in the first year have been reported, most importantly because of lack of compliance [Citation2]. Noncompliance and discontinuation of contraception is often a result of side effects, weight gain and sub-optimal cycle control [Citation3,Citation4]. Meanwhile, it has been shown that (sexual) well-being and user satisfaction can improve treatment compliance [Citation5]. Therefore, there is still a medical need to develop a COC with both an optimal cycle control and good user acceptability.
Estetrol (E4) is a natural estrogen, synthesized by the human fetal liver and is present only during human pregnancy. A summary of E4 research data until 2015 is available [Citation6]. The combination of E4 at doses of 5 or 10 mg with 3 mg drospirenone (DRSP) has been shown to suppress ovulation, as well as the combination of E4 at doses of 5, 10 or 20 mg with 150 μg levonorgestrel (LNG) [Citation7]. These combinations also have a limited impact on the synthesis of liver proteins such as sex hormone-binding globulin, and triglyceride levels, which may indicate a potential reduction of both the risk of venous thromboembolism and cardiovascular disease [Citation8]. Compared to COCs containing ethinylestradiol (EE) and DRSP, the E4/DRSP combinations led to reduction in coagulation markers and reduced haemostatic effects [Citation9].
An open-label, multi-centre, randomized, dose-finding study (FIESTA) was performed to assess bleeding pattern and cycle control of E4 combined with either DRSP or LNG. The 15 mg E4/DRSP combination proved to be the most efficacious with respect to bleeding and cycle control, and was subsequently selected for further phase III clinical development [Citation10]. The FIESTA study also evaluated user acceptability, satisfaction, body weight control and general well-being. The results of this evaluation are presented here.
Methods
This was an open-label, multi-centre, randomized, dose-finding study in healthy women of reproductive age. The study was conducted between September 2010 and September 2011 in 10 centres in Finland (ClinicalTrials.gov identifier NCT01221831). Approval was obtained by the regional independent ethics committee of the Hospital District of Helsinki and Uusimaa (HUS), and the Finnish Medicines Agency (FIMEA). The study was conducted in accordance with the ethical principles established by the Declaration of Helsinki and the International Conference on Harmonization – Good Clinical Practice Guidelines. Written informed consent was obtained from all participants prior to any study related procedures, including screening evaluations and an appropriate wash-out period for medication that participants were using prior to study entry.
Participants
Healthy women, aged 18–35 years with a BMI between 18 and 30 kg/m2 and a regular menstrual cycle (24–35 days), were eligible for inclusion. Women who were already using hormonal contraceptives and hormonal contraceptive-naïve women (switchers and starters, respectively) could participate. Exclusion criteria were in line with the World Health Organization’s medical eligibility criteria for COC use [Citation11], and described previously in more detail [Citation10].
Treatment
Participants were randomly allocated to one of the five treatment groups: (i) 15 mg E4 combined with 3 mg DRSP (15E4/DRSP); (ii) 20 mg E4 combined with 3 mg DRSP (20E4/DRSP); (iii) 15 mg E4 combined with 150 μg LNG (15E4/LNG); (iv) 20 mg E4 combined with 150 μg LNG (20E4/LNG); (v) and as a reference estradiol valerate (E2V) combined with dienogest (DNG) (E2V/DNG), the commercial packaging of 4-phasic Qlaira® (Bayer HealthCare, Berlin, Germany). To achieve equal distributions across the groups, randomization was stratified by switchers, starters, and sites.
Participants were to complete six treatment cycles of 28 days. For the E4 groups one cycle comprised of 24 study medication days, followed by four placebo days. For the E2V/DNG group one cycle comprised of 26 days of active pills followed by two placebo days, as indicated in the labelling of Qlaira®.
Assessments and outcome variables
The primary objective of this study was to assess vaginal bleeding patterns and cycle control, and its results are published elsewhere [Citation10].
The secondary study objectives included the evaluation of acceptability of the study medication, user satisfaction and general well-being by completing a Subject Satisfaction and Health-Related Questionnaire, and recording of body weight and return of menstruation. The results of this evaluation are presented in this article.
Acceptability of the study medication was assessed by recording discontinuation rates, reasons for discontinuations and compliance with the study medication. At each visit during the treatment period (scheduled at the end of cycles 1, 2, 3, 4 and 6), women were asked to return the blisters of study medication in order to evaluate treatment compliance. They were also asked to record their daily intake of the study medication in a diary. This information was used as a measure for the extent of exposure and compliance.
User satisfaction and well-being were evaluated at each visit using a self-reported Subject Satisfaction and Health-Related Questionnaire. The questionnaire assesses user satisfaction with the study medication (domain 1) and its effects on mood (domain 2a), sexual life (domain 2b), pre-menstrual symptoms (domain 2c), and its overall effect (domain 2d) (). The domains and questions are similar to those used in validated measures like the Treatment Satisfaction Questionnaire for Medication (TSQM), the Spanish society of Contraception Quality-of-Life (SEC-QOL), or the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) [Citation12–14]. Possible responses varied from (much) better, no change, and (much) worse compared to that recorded at the previous visit.
Table 1. Subject Satisfaction and Health-Related Questionnaire.
Body weight was measured using standardized equipment at each study visit. The proportions of women with either a weight gain of ≥2 kg or a weight loss of ≥2 kg were calculated for each treatment group at the end of cycles 3 and 6 (or end of study), according to the method described by Foidart et al. [Citation15]. In addition, the resulting ratio (weight loss ≥2 kg/weight gain ≥2 kg) was calculated.
After study completion (or premature discontinuation), women were followed for up to one year until return of spontaneous menstruation or pregnancy occurred. Women starting other forms of hormonal contraception were not followed-up.
Statistical analysis
Data analyses for treatment acceptability, user satisfaction and health-related questionnaire were performed for the intent-to-treat (ITT) population, comprising all-subjects-treated (AST) with data for any non-bleeding parameter and for dosing compliance.
A mixed-effects proportional odds regression model was used for a post hoc multifunctional analysis of the domains 1 and 2a–d () [Citation16], including the treatment, the question, the subgroup (starter or switcher), the visit, and all double interactions between these factors as fixed effects, and a random subject-specific intercept. For this purpose, the change from baseline for each of the five visits was longitudinally converted to a three-level ordinal outcome: −1 = worse or much worse, 0 = no change; 1 = better or much better. The results were summarized in a mosaic plot and odds ratios (OR) with 95% confidence intervals (CI) were calculated for pairwise treatment differences of interest. The analyses on the domains satisfaction and future use were only descriptive, and presented by means and frequencies.
Results
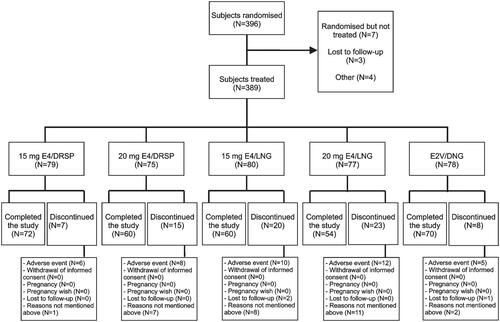
A total of 396 women were randomized (), of whom 389 (98.2%) received study medication. The proportion of switchers (66.8%) and starters (33.2%) was generally similar across treatment groups. Demographic and baseline characteristics were similar across the treatment groups, except for smoking which varied between 10.7% for 20E4/DRSP and 24.7% for 20E4/LNG ().
Table 2. Main demographics and baseline characteristics (all-subjects-treated population).
Acceptability of study medication
Of the 389 women receiving study medication, 73 (18.8%) discontinued prematurely (). The number of completers was the highest for 15E4/DRSP (72/79; 91.1%) and E2V/DNG (70/78; 89.7%), and the lowest for 20E4/LNG (54/77; 70.1%) ().
Table 3. Reasons for discontinuation of study treatment, n (%) (all-subjects-treated population).
Reasons for discontinuation are displayed in the . Treatment-emergent adverse events (TE-AEs) were the most common reason for discontinuation (n = 39; 10.0%) (). In addition, two women (one assigned to 15E4/DRSP and one assigned to E2V/DNG) discontinued because of an AE that started before taking study medication. No women in the 15E4/DRSP and E2V/DNG groups discontinued because of vaginal bleeding, in contrast to a total of three to four (3.9–5.0%) in the other groups (). Compliance was generally good, with 97.6% of tablets taken per cycle based on diary card entries. In line with the discontinuations across treatment groups, the mean extent of exposure varied between 144.1 days for 20E4/LNG and 159.5 days for 15E4/DRSP. Accordingly, the mean total number of cycles varied between 5.2 for 20E4/LNG and 5.7 for 15E4/DRSDP and E2V/DNG (data not shown).
Subject Satisfaction and Health-Related Questionnaire
At randomisation, the majority of women (>90%) reported a very good to average general feeling. In contraceptives users the domains ‘Mood’ and ‘Overall effect’ were scored high (91–98%), but ‘Sexual life’ and ‘Premenstrual complaints’ scored relatively low (58–68%) (data not shown).
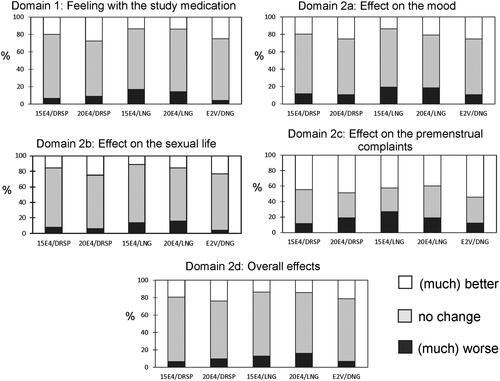
The changes at Cycle 6 compared to baseline, expressed as ‘Better’ (white bar), ‘No change’ (grey bar) or ‘Worse’ (black bar) for the domains 1 and 2a–d, are presented in . Scoring of better ‘General feeling’ was 19.9 and 27.6% in the 15E4/DRSP and 20 E4/DRSP groups, respectively, which was comparable for E2V/DNG (25.0%), but higher than observed for 15E4/LNG and 20E4/LNG groups (13.7%). Similar results were observed for the domains ‘Mood’, ‘Sexual life’ and ‘Overall effect’, with better scores in the E4/DRSP groups compared to the E4/LNG groups. Less subjects reported worsening of their premenstrual complaints in the 15E4/DRSP (11.7%) and E2V/DNG (12.2%) groups, compared to 19.2% for 20E4/DRSP. The highest proportion of women reporting a worsening of their premenstrual complaints was observed in the 15E4/LNG (27.1%) and 20E4/LNG groups (19.0%).
Figure 2. Mosaic plots of the change from baseline score recorded at cycle 6 (or end-of-study) stratified by domains (general feeling, mood, sexual life, premenstrual complaints and overall effect); white: better; grey: no change; black: worse.
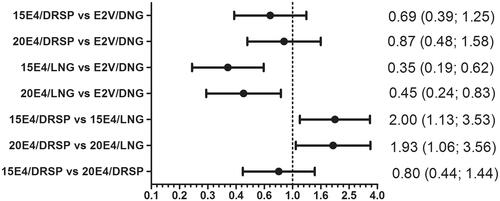
Odds ratio’s comparing overall treatment differences () showed that well-being scores were significantly worse for 15E4/LNG and 20E4/LNG compared to E2V/DNG [OR 0.35 (95% CI: 0.19; 0.62) and OR 0.45 (95% CI: 0.24; 0.83), respectively]. Comparison of DRSP groups versus LNG groups showed that DRSP treatment was significantly better with an OR 2.00 (95% CI: 1.13; 3.53) for the 15E4 treatment groups and an OR 1.93 (95% CI: 1.06; 3.56) for the 20E4 treatment groups (). The differences between the two E4/DRSP groups and between E4/DRSP groups and E2V/DNG were not statistically significant.
Figure 3. OR (95% CI) comparing the overall outcome* of different treatments (x-axis in log10-scale). *Domains: general feeling, mood, sexual life, premenstrual complaints and overall effect; combined recordings at cycles 1, 2, 3, 4, and 6. NB. Values <1 are indicative of a worse well-being outcome, and values >1 are indicative of a better well-being outcome.
Regarding user satisfaction with study medication, >50% of women in any treatment group reported being satisfied or very satisfied at cycle 6, with the largest proportion in the 15E4/DRSP group (73.1%), and the lowest in the 15E4/LNG group (50.6%). E2V/DNG showed a satisfaction rate of 67.6%. Reporting to be (very) dissatisfied was the highest in the E4/LNG groups (18.1–22.6%) while fewer women were (very) dissatisfied in the other groups (between 12.2 and 14.1%) (, ). The response ‘yes’ or ‘may be’, to consider future using of the assigned study medication, was the highest for 15E4/DRSP (82.1%) and the lowest for 20E4/LNG (58.3%) (, ).
Figure 4. Frequencies (%) of a satisfaction [(very) satisfied] response or willingness of future use (yes and maybe), recorded at cycle 6 (or end-of-study) on the Subject Satisfaction and Health-Related Questionnaire.
![Figure 4. Frequencies (%) of a satisfaction [(very) satisfied] response or willingness of future use (yes and maybe), recorded at cycle 6 (or end-of-study) on the Subject Satisfaction and Health-Related Questionnaire.](/cms/asset/f66d79ad-5c8f-46d2-bc1f-765521d5f419/iejc_a_1336532_f0004_b.jpg)
Table 4. Subject satisfaction and health-related questionnaire; responses [n (%)] at cycle 6 for the items Satisfaction and Future use.
Body weight
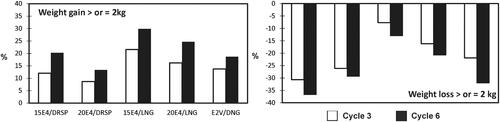
The proportion of women with a weight gain of ≥2 kg was the lowest for 20E4/DRSP (8.7 and 13.3% at cycles 3 and 6, respectively), and the highest for 15E4/LNG (21.5 and 29.9%, respectively). The proportion of women with a weight loss of ≥2 kg was the highest for 15E4/DRSP (30.7 and 36.7% at cycles 3 and 6, respectively), and the lowest for 15E4/LNG (7.7 and 13.0%, respectively) (). The resulting ratio in the combined E4/DRSP groups to have a weight loss ≥2 kg compared to a weight gain ≥2 kg was 2.8:1 at cycle 3 and 2.0:1 at cycle 6. The corresponding ratios were 1.6:1 and 1.7:1, respectively, in the E2V/DNG group, and 0.7:1 and 0.6:1, respectively, in the combined E4/LNG groups.
Return of menstruation
Of 36 women who did not start with a new hormonal contraceptive method after study completion (or end of treatment), 32 (89%) had a return of menstruation within the first 2 months, and two had (both in the 15E4/LNG group) menstruation returned in 2–4 months. The remaining two women (both in the 15E4/LNG group) became pregnant in the first cycle after stopping study treatment, with an estimated date of conception of more than 14 days after last active tablet.
Discussion
Findings and interpretation
User satisfaction and health-related outcomes (well-being), together with acceptability of the study medication, are the basis of the present evaluation. The responses on the Subject Satisfaction and Health-Related Questionnaire revealed that at treatment cycle 6, using 15 mg E4/DRSP was accompanied by the highest proportion of satisfaction (73.1%) and using 15 mg E4/LNG by the lowest (50.6%). This is in line with the current finding based on ORs for the post hoc multifunctional statistical analysis that well-being with E4/DRSP combinations is significantly better than with E4/LNG combinations. In general, the scores in the E4/DRSP groups were in the same range as those in the E2V/DNG group.
Since E4 is a natural estrogen, E2V/DNG was chosen as reference COC in the present study, because it was the only marketed COC also containing a natural estrogen. E2V/DNG has shown to improve quality of life (QoL), together with a positive effect on sexuality, although DNG is a progestin with anti-androgenic properties [Citation17–19]. This was recently confirmed in an observational study for women who switched to a COC based on a natural estrogen [Citation20]. For this reason, it was also relevant to use E2V/DNG in the present study as a reference to assess user satisfaction and health-related quality of life with E4-containing COCs.
The EE/DRSP combination has shown to provide added benefits to the user by improving general well-being and subsequent better compliance [Citation5,Citation21,Citation22]. Compared to EE/LNG, the EE/DRSP combination showed a higher self-rated improvement on the Clinical Global Impression Scale (66 versus 59%) [Citation23]. Documented evidence suggests that EE/DRSP has a negative influence on female sexual function [Citation24], a finding however not observed in the current study with E4/DRSP.
In the present study, compliance of study medication was similar across treatment groups. The number of women who completed the six cycles ranged between 91.1% for 15 mg E4/DRSP and 70.1% for 20 mg E4/LNG (). The high acceptability in the E2V/DNG group (89.7%), despite much heavier and persistent unscheduled bleeding than in the E4-groups [Citation10], may be due to the open-label design of our study. Both because of the small group sizes (77–80 subjects per group) and the open-label design of the study, no post hoc statistical analysis was performed on discontinuation rates. Participants allocated to the E4 combinations were aware that they were using a new and experimental COC, whereas those allocated to E2V/DNG knew that they received a commercial product (Qlaira®). Nevertheless, 29.7% of women assigned to the E2V/DNG treatment answered “No or Uncertain” to the question to consider future use of this combination. This negative response was lower in the 15 mg E4/DRSP (18.0%) and 20 mg E4/DRSP (21.9%) groups (, ). In line with this finding, a high proportion of women was satisfied or very satisfied at cycle 6 in the 15 mg E4/DRSP group (73.1%) versus 68.6% for 20 mg E4/DRSP and 67.6% for E2V/DNG.
Weight gain is considered as an important and common reason for discontinuation of COCs, despite the continued need for fertility control [Citation5]. Therefore, the higher proportion of women with weight loss in the E4/DRSP treatment groups () is an important advantage and may play a role in treatment compliance and continuation. An explanation for the weight loss with the DRSP COCs may be a decrease of water-retention, associated with the anti-mineralocorticoid activity of DRSP [Citation5]. Of note, is the weight loss seen in this study with E2V/DNG at cycle 6, with a similar magnitude to the weight loss seen with E4/DRSP. This is in contrast to previous studies with E2V/DNG, where weight gain was reported as one of the most common adverse events and reason for treatment discontinuation [Citation25].
Apart from a few women receiving 15 mg E4/LNG, menstruation returned within 2 months in those who did not start with a new hormonal contraceptive method. The absence of persistent ovarian suppression is considered relevant for future fertility of women who are ready to become pregnant [Citation26]. Previous experience has shown that after cessation of COCs, a delay of 3–6 months in conception may occur. The results of the current study are consistent with previous data on return to fertility [Citation26,Citation27]. Moreover, none of the subjects experienced post-pill amenorrhea.
Strengths and weaknesses of the study
Strengths of this study are the favourable effect on body weight and the statistically significant beneficial health-related outcomes of the E4/DRSP combinations.
In this study, a non-validated questionnaire was used; however, since the outcome in the E2V/DNG group was similar to those based on validated questionnaires [Citation17,Citation28], it suggests that the questionnaire used in the present study can be considered reliable for its purpose. Other weaknesses are the absence of blinding of the comparator and the relatively low number of participants in each treatment group, which did not allow to differentiate between starters and switchers of the COCs investigated.
The populations in the treatment groups were balanced for age, BMI, switchers and starters, but not for smoking (). Current smoking varied between 10.7% for 20 mg E4/DRSP and 24.7% for 20 mg E4/LNG. This difference, probably due to chance, is unlikely to have an effect on the well-being outcome.
Differences in results and conclusions in relation to other studies
The present study was the first investigating the effect of E4 combinations on general well-being and body weight.
Relevance of the findings: implications for clinicians and policymakers
The primary aim of the present study was to assess vaginal bleeding patterns and cycle control of 15 and 20 mg E4 combined with either DRSP or LNG, administered during six treatment cycles in a 24:4 day regimen. It was concluded that the 15 mg E4/DRSP combination had the best properties and was superior to the E2V/DNG contraceptive [Citation10]. The secondary objectives – user satisfaction and health-related outcomes – together with acceptability of the study medication revealed that E4/DRSP combinations have a more favourable user satisfaction outcome than the E4/LNG combinations. In addition and contrary to E4 combined with LNG, the E4/DRSP combinations had a favourable effect on body weight.
In an observational study in 39 subjects, aged <35 years, the effects of E2V/DNG on quality of life and sexual function have been assessed by using the Short Form-36 (SF-36) and Female Sexual Function Index questionnaires [Citation28]. General Health was improved after 6 months (p < .01), but overall sexual function remained unchanged. In subjects <48 years, all SF-36 scales improved after 6 months (p < .01), and improvement of sexuality was observed based on the Short Personal Experience questionnaire (p < .05) [Citation17]. Direct comparison of 15 mg E4/DRSP and E2V/DNG in the present study showed a comparable favourable overall outcome (including sexual function), satisfaction, and weight control ().
Altogether, the current findings support the recommendation of 15 mg E4/DRSP as a promising new COC for further phase III clinical development [Citation10].
Unanswered questions and future research
The current findings of a favourable effect on well-being and body weight of the E4/DRSP combinations, have to be confirmed in larger numbers of women and over longer treatment periods.
Conclusions
The present study shows that 15 mg estetrol combined with 3 mg DRSP is associated with a high user acceptability and satisfaction, and with a favourable body weight control.
Acknowledgements
The authors gratefully acknowledge the healthcare centres and their staff who conducted this study, and the women who participated in the study.
The study was performed in Finland at Väestöliitto Helsinki, Mehiläinen Helsinki, YTHS Jyvaskyla, YTHS Kuopio, Terveystalo Kuopio, Laboratorio Simpanen, Väestöliitto Oulu, YTHS Tampere, Tampereen Lääkärikeskus Oy, and Väestöliitto Turku. Site monitoring was done by TFS OY, Finland.
Categorical data analyses on Satisfaction and Health-Related Questionnaire outcomes were performed by Marco Munda and Fabrice Nollevaux (Arlenda S.A., Saint-Georges-sur-Meuse, Belgium).
The authors wish to thank Jan Egberts and Mireille Gerrits (Terminal 4 Communications, Hilversum, the Netherlands) for providing support in manuscript preparation.
Disclosure statement
The authors alone are responsible for the content and writing of this article. M.M. and C.M. are employees; J.-M.F. and H.C.B. are Strategic Scientific Advisors at Mithra Pharmaceuticals. Y.Z. and H.C.B. are employees, and L.B. a former employee, of Pantarhei Bioscience BV. D.A. does not report any conflicts of interest.
Additional information
Funding
References
- Kiley J, Hammond C. Combined oral contraceptives: a comprehensive review. Clin Obstet Gynecol. 2007;50:868–877.
- Kost K, Singh S, Vaughan B, et al. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21.
- Huber LR, Hogue CJ, Stein AD, et al. Contraceptive use and discontinuation: findings from the contraceptive history, initiation, and choice study. Am J Obstet Gynecol. 2006;194:1290–1295.
- Skouby SO. Contraceptive use and behavior in the 21st century: a comprehensive study across five European countries. Eur J Contracept Reprod Health Care. 2004;9:57–68.
- Bitzer J, Paoletti AM. Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: results of three multicentre trials. Clin Drug Investig. 2009;29:73–78.
- Coelingh Bennink HJT, Foidart JM. Estetrol, a fetal steroid for the treatment of adults. J Reprod Med Endokrinol Online. 2015;12:399–403.
- Duijkers IJ, Klipping C, Zimmerman Y, et al. Inhibition of ovulation by administration of estetrol in combination with drospirenone or levonorgestrel: results of a phase II dose-finding pilot study. Eur J Contracept Reprod Health Care. 2015;20:476–489.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463–475.
- Kluft C, Zimmerman Y, Mawet M, et al. Reduced haemostatic effects with drospirenone-based oral contraceptives containing estetrol versus ethinyl estradiol. Contraception. 2017;95:140–147.
- Apter D, Zimmerman Y, Beekman L, et al. Bleeding pattern and cycle control with estetrol-containing combined oral contraceptives: results from a phase II, randomised, dose-finding study (FIESTA). Contraception. 2016;94:366–373.
- World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. Geneva, Switzerland: WHO Press; 2009. p. 15–44.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
- Perez-Campos E, Duenas JL, de la Viuda E, et al. Development and validation of the SEC-QOL questionnaire in women using contraceptive methods. Value Health. 2011;14:892–899.
- Egarter C, Topcuoglu MA, Imhof M, et al. Low dose oral contraceptives and quality of life. Contraception. 1999;59:287–291.
- Foidart JM, Wuttke W, Bouw GM, et al. A comparative investigation of contraceptive reliability, cycle control and tolerance of two monophasic oral contraceptives containing either drospirenone or desogestrel. Eur J Contracept Reprod Health Care. 2000;5:124–134.
- Agresti A. An introduction to categorical data analysis. 2nd ed. Wiley-Interscience; 2007.
- Caruso S, Agnello C, Romano M, et al. Preliminary study on the effect of four-phasic estradiol valerate and dienogest (E2V/DNG) oral contraceptive on the quality of sexual life. J Sex Med. 2011;8:2841–2850.
- Davis SR, Bitzer J, Giraldi A, et al. Change to either a nonandrogenic or androgenic progestin-containing oral contraceptive preparation is associated with improved sexual function in women with oral contraceptive-associated sexual dysfunction. J Sex Med. 2013;10:3069–3079.
- Nappi RE, Serrani M, Jensen JT. Noncontraceptive benefits of the estradiol valerate/dienogest combined oral contraceptive: a review of the literature. Int J Womens Health. 2014;6:711–718.
- Lete I, de la Viuda E, Perez-Campos E, et al. Effect on quality of life of switching to combined oral contraception based on natural estrogen: an observational, multicentre, prospective phase IV study (ZOCAL Study). Eur J Contracept Reprod Health Care. 2016;21:276–284.
- Apter D, Borsos A, Baumgartner W, et al. Effect of an oral contraceptive containing drospirenone and ethinylestradiol on general well-being and fluid-related symptoms. Eur J Contracept Reprod Health Care. 2003;8:37–51.
- Foidart JM. Added benefits of drospirenone for compliance. Climacteric. 2005;8(Suppl. 3):28–34.
- Kelly S, Davies E, Fearns S, et al. Effects of oral contraceptives containing ethinylestradiol with either drospirenone or levonorgestrel on various parameters associated with well-being in healthy women: a randomized, single-blind, parallel-group, multicentre study. Clin Drug Investig. 2010;30:325–336.
- Ciaplinskiene L, Zilaitiene B, Verkauskiene R, et al. The effect of a drospirenone-containing combined oral contraceptive on female sexual function: a prospective randomised study. Eur J Contracept Reprod Health Care. 2016;21:395–400.
- Palacios S, Wildt L, Parke S, et al. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a phase III trial. Eur J Obstet Gynecol Reprod Biol. 2010;149:57–62.
- Bagwell MA, Thompson SJ, Addy CL, et al. Primary infertility and oral contraceptive steroid use. Fertil Steril. 1995;63:1161–1166.
- Chasan-Taber L, Willett WC, Stampfer MJ, et al. Oral contraceptives and ovulatory causes of delayed fertility. Am J Epidemiol. 1997;146:258–265.
- Di Carlo C, Gargano V, De Rosa N, et al. Effects of estradiol valerate and dienogest on quality of life and sexual function according to age. Gynecol Endocrinol. 2014;30:925–928.