Abstract
Purpose: Evaluate bleeding patterns for the Liletta® levonorgestrel 52 mg intrauterine system (IUS) using the World Health Organization Belsey definitions.
Material and methods: This prospective multicenter trial evaluates the efficacy and safety of Liletta® (Clinicaltrials.gov NCT00995150). We evaluated bleeding patterns for 1700 nulliparous and multiparous women using a daily diary completed by participants for the first 2 years and by questionnaire every 3 months thereafter. We assessed amenorrhea rates over 3 years and the proportion of subjects with infrequent, frequent, prolonged and irregular bleeding per 90-day reference period over 2 years for the entire study population as well as comparing nulliparous and parous women and obese and non-obese women.
Results: Amenorrhea rates at 1 and 3 years in levonorgestrel 52 mg IUS users were 19 and 37%, respectively. The infrequent bleeding rate increased from 14% in the first 90 days to 30% at the end of Year 1, and was maintained at the same rate through Year 2. Frequent, prolonged and irregular bleeding declined to low levels by the end of the first year. Discontinuation for bleeding-related complaints occurred in 35 (2.1%, 95% CI 1.3–2.7%) women during the first 36 months; only one subject discontinued for amenorrhea (in Year 2). Outcomes did not vary for nulliparous versus parous or obese versus non-obese women.
Conclusions: Among Liletta users, amenorrhea and infrequent bleeding become more prevalent over time and amenorrhea rates continue to increase after the first year of use. Bleeding patterns do not differ significantly by parity or by obesity-status. Discontinuation for bleeding concerns is uncommon with this product.
Chinese abstract
目的:根据世界卫生组织的Belsey定义, 评估Liletta 52mg左炔诺孕酮宫内缓释系统(IUS))的出血模式。
材料与方法:此前瞻性多中心试验评估了Liletta的疗效和安全性(Clinicaltrials.gov NCT00995150)。我们采用受试者前两年记日记和此后每3个月进行一次问卷调查的方法, 对1700名未生育过的妇女和多胎妇女的出血模式进行评估。评估整个研究人群的3年以上闭经比例, 以及在2年以上的每90天参照期中, 出血过稀、出血过频、出血期过长和不规则出血的受试者比例, 并比对未生育过的妇女和多胎妇女及肥胖和非肥胖妇女进行了比较。
结果:52mg左炔诺孕酮IUS应用者的第1年和第3年闭经比例分别为19%和37%, 月经稀发比例从前90天的14%上升到第1年末的30%, 并且在第2年保持同样的比例。第1年末, 出血过频、出血期过长和不规则出血的比例降到低水平。在前36个月中, 有35名妇女因出血相关不适而停止治疗(2.1%, 95% CI 1.3-2.7%), 只有1名受试者因闭经而停止治疗(第2年)。经过对比发现, 未生育过的妇女和多胎妇女之间及肥胖和非肥胖妇女之间的结果并无差异。
结论:在Liletta的应用者中, 闭经和月经稀发在用Liletta一段时间后更常见, 且闭经比例在Liletta用一年后持续增加。不同产次和肥胖状态下的出血模式无显著差异。因担忧出血问题而停用本产品的情况不常见。
Introduction
The World Health Organization (WHO) recommends reporting hormonal contraception bleeding patterns using the Belsey criteria [Citation1]. These descriptors present a global picture of bleeding patterns over 90-day intervals (). For levonorgestrel intrauterine system (IUS) products, the Belsey method has been used to describe bleeding patterns for the lower dose products, but not for the levonorgestrel 52 mg IUS. Moreover, the limited bleeding data available for levonorgestrel 52 mg IUS users were primarily derived from Scandinavian multiparous women, 75% of whom had used intrauterine contraception previously [Citation2]. The recent development of a newer levonorgestrel 52 mg IUS has provided the opportunity to obtain a detailed collection of bleeding data from a very large and diverse population. In this report, we describe the bleeding patterns with the Liletta® levonorgestrel 52 mg IUS using the WHO Belsey criteria. Providing this information can allow a more standardised understanding of potential differences in bleeding patterns between different levonorgestrel IUS products.
Table 1. World Health Organization Belsey definitions of bleeding patterns with contraceptive use [Citation1].
Materials and methods
We present a secondary analysis of data from the ACCESS IUS multicenter, Phase 3, open-label clinical trial of the Liletta levonorgestrel 52 mg IUS [Medicines360, San Francisco, CA and Allergan, Irvine, CA; Liletta® is a registered trademark of Odyssea Pharma SPRL (Belgium), an Allergan affiliate]. Liletta is marketed for contraception as Levosert™ and Donasert™ in Europe using this same data. The methods of this study have been reported previously [Citation3]. A central or local Institutional Review Board for each center approved the study. All women signed written informed consent before study participation.
Briefly, investigators at 29 clinical sites in the USA enrolled healthy, non-pregnant, sexually active, nulliparous and parous women aged 16–45 years (inclusive) who desired a hormonal IUS for contraception. Participants had to report regular menstrual cycles every 21–35 days with a variation of typical cycle length of no more than 5 days. Those women currently using hormonal contraception could not be using it for cycle control and reported a typical history of regular cycles prior to their most recent hormonal contraception initiation. Women recently using progestin injectable contraception could not enter the study if the prior injection was within the preceding 9 months, or 6 months if the subject had two spontaneous menstrual cycles (minimum of three menses) that met criteria for normal menstrual cycles.
After subjects completed screening and enrollment (IUS placement), follow-up included visits at 1, 3, 6 and 12 months and then every 6 months thereafter. Telephone contact was initiated at Month 9 and occurred at the 3-month interval between scheduled visits. Subjects completed a daily diary for the first 2 years to indicate the greatest amount of bleeding that day as none, spotting, light flow, normal flow, or heavy flow based on their own subjective impression. After 2 years, subjects were asked at each visit or telephone contact to describe their bleeding pattern over the preceding 3 months.
For the analysis in this report, we only included women with successful IUS placement who provided bleeding data through at least the first 90 days or who discontinued for bleeding-related complaints in the first 90 days. We limited the description of prolonged, frequent, infrequent and irregular bleeding with the levonorgestrel 52 mg IUS to 2 years because we only had daily diary data for the first 2 years of use. The bleeding data collected after 2 years was sufficient to precisely evaluate amenorrhea rates, which we did through 3 years. We assessed all bleeding patterns over 90-day intervals using the WHO Belsey criteria () [Citation1]. We also compared the patterns between nulliparous and parous women as well as non-obese and obese women, with obesity defined as a body mass index ≥30 kg/m2. We used Fisher’s exact testing for comparisons of proportions with p values ≤.05 considered significant.
Results
Of the 1751 women enrolled, 1714 (97.9%) had successful placement: 14 (0.8%) of these women discontinued prior to 90 days for non-bleeding-related complaints leaving 1700 women in the analysis. Participant characteristics are presented in . Overall, bleeding data was available at the end of 1, 2 and 3 years for 1448 (85.2%), 1178 (69.3%) and 935 (55.0%) women, respectively.
Table 2. Demographics and contraceptive method at enrollment for women in a phase 3 study who had successful placement of a Liletta levonorgestrel 52 mg IUS and at least 90 days of follow-up (N = 1700).
Discontinuation for bleeding complaints occurred in 35 (2.1%, 95% CI 1.3–2.7%) levonorgestrel 52 mg IUS users cumulatively during the first 36 months, most commonly during Months 6–18 (). Reasons provided by women who primarily discontinued for bleeding-related complaints included heavy flow (12 women) and pattern-related issues including irregular bleeding (12 women), prolonged flow (7 women) and increased frequency (3 women); only one subject discontinued for amenorrhea (in year 2). Few women were lost to follow-up with 68 (4.0%), 55 (3.2%) and 21 (1.2%) in years 1, 2 and 3, respectively.
Table 3. Discontinuation for bleeding-related complaints over 3 years for women using a Liletta levonorgestrel 52 mg IUS.
Bleeding patterns
Amenorrhea rates in levonorgestrel 52 mg IUS users increased over 3 years ( and ). Infrequent bleeding was reported by 14% of levonorgestrel 52 mg IUS users in the first 90 days, increased to 30% at the end of Year 1, and was maintained at the same rate through Year 2. Frequent bleeding occurred in 26% of levonorgestrel 52 mg IUS users in the first 90 days and quickly declined to fewer than 10% in the second 90-day reference period. Prolonged bleeding declined in a similar manner from 51% in the first 90 days to 10% in the second 90-day reference period. Irregular bleeding was reported by 38% of women in the first 90 days, declining to 14% in the second 90 days and 6% by the end of the first year ( and ).
Figure 1. Amenorrhea, infrequent bleeding, frequent bleeding, prolonged bleeding and irregular bleeding rates over 2 years of levonorgestrel 52 mg use.
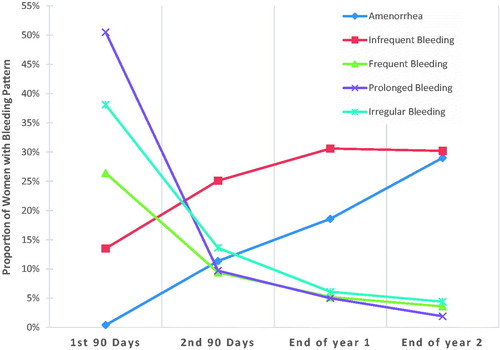
Table 4. Amenorrhea rate over 3 years for women using levonorgestrel 52 mg IUS overall and by parity and obesity-status.
Table 5. Infrequent, frequent and prolonged bleeding rates over 2 years for women using levonorgestrel 52 mg IUS overall and by parity and obesity-status.
Bleeding patterns for nulliparous versus parous women and obese versus non-obese women are presented in and . We found sporadic and inconsistent differences related to frequency of bleeding. Although nulliparous women had lower rates of infrequent bleeding in the first 90 days as compared to parous women, the opposite is true in the 90 days at the end of Year 1 and no differences were present in the second 90 days or at the end of Year 2. Lower rates of frequent bleeding are found in parous women during the first and second 90 day periods but no differences are present at 1 or 2 years. Overall, no trends are present which suggest that parity or obesity status affects bleeding pattern in any consistent pattern.
Discussion
Our findings demonstrate that amenorrhea rates increase each year with continued use of the Liletta levonorgestrel 52 mg IUS. Rates of frequent and prolonged bleeding decline rapidly in the first few months of levonorgestrel IUS use, with rates of 10% or less during months four through six of use. The patterns overall demonstrate that levonorgestrel 52 mg IUS users are likely to experience frequent, irregular and/or prolonged bleeding episodes in the first 90 days that quickly diminish to light and less prolonged bleeding for most women. The bleeding patterns do not vary significantly or consistently based on parity or obesity status. Removal rates for bleeding-related complaints are very low over 3 years (2.1%) with about one-third of such discontinuations due to heavy flow, one-third due to irregular bleeding and one-third related to prolonged or frequent bleeding.
This study is the first to use the WHO Belsey criteria to describe bleeding patterns with a levonorgestrel 52 mg IUS in a large, prospectively collected dataset. Lower dose levonorgestrel IUS products introduced to the market within the past decade have reported bleeding patterns using these criteria [Citation4,Citation5]. Standardised reporting of bleeding with contraceptive use has been endorsed for decades [Citation1,Citation6]. Yet, the current prescribing information for the three different doses of levonorgestrel IUS products (13.5, 19.5 and 52 mg) provides information in different ways, even among dose-equivalent products [Citation4–8]. As such, clinically relevant differences in bleeding patters are difficult to distill. By presenting our results using the Belsey criteria, we can begin to assess potential differences between the products, and provide information that is easily conveyed to patients.
A comparison of our results with previously published data indicates that women using lower dose levonorgestrel 13.5 [Citation5] and 19.5 mg [Citation4] IUS products have lower rates of amenorrhea and infrequent bleeding and have higher rates of irregular bleeding than women using the Liletta levonorgestrel 52 mg IUS; rates of frequent and prolonged bleeding are similar for all three doses. Women using levonorgestrel 13.5, 19.5 and 52 mg IUS products have very similar frequent and prolonged bleeding pattern rates over the first few years. Such a comparison is limited by differences in study participant populations. The lower dose levonorgestrel IUS studies included a mix of women from 11 countries (Argentina, Canada, Chile, Finland, France, Hungary, Mexico, the Netherlands, Norway, Sweden, and USA) whereas this current study was performed exclusively in the USA [Citation9]. Additionally, the lower dose levonorgestrel IUS studies had 39.2% nulliparous women with a mean BMI of 25.3 kg/m2 [Citation9] whereas this current study had 57.5% nulliparous women with a mean BMI of 27.3 kg/m2. These characteristics, as well as other differences, could account for some variations in bleeding patterns and limit comparisons among these studies.
To truly understand general similarities and differences in bleeding patterns between levonorgestrel IUS products with different doses, a randomised study would be required for a more precise comparison. Although a Phase 2 randomised trial comparing the levonorgestrel 13.5, 19.5 and 52 mg IUS (Mirena®, Bayer HealthCare Pharmaceuticals, Whippany, NJ) has been published, the report only included amenorrhea rates at the end of 1 year [Citation10]. The study included 239, 245, and 254 European women in the three groups, respectively with 216 (90.4%), 213 (86.9%) and 218 (85.8%) women, respectively completing 1 year of use. The authors report amenorrhea rates of 12.7, 18.9, and 23.6% for 13.5, 19.5 and 52 mg devices, respectively (p = .012 for levonorgestrel 13.5 mg versus 52 mg and p = .30 for levonorgestrel 19.5 mg versus 52 mg). However, this study had limited statistical power to fully compare amenorrhea rates.
Conclusions
This study represents the largest dataset to systematically evaluate bleeding patterns among levonorgestrel 52 mg IUS users over 2 years. Women who use the Liletta levonorgestrel 52 mg IUS commonly experience amenorrhea or infrequent bleeding with continued duration of use; frequent and irregular bleeding decrease significantly after the first 90 days of use. Bleeding patterns do not vary consistently based on parity or between non-obese and obese women. The information can be helpful when providing counseling to women about what bleeding patterns to expect with levonorgestrel 52 mg IUS products.
Acknowledgements
The authors thank the participating investigators and coordinators at the 29 study centers for conduct of the clinical trial and submission of data (investigators funded by Medicines360 to conduct the study).
Disclosure statement
This research is sponsored by Medicines360. The universities for C.A.S., S.B.T., P.D.B., L.M.K. and M.D.C. receive funding from Medicines360 to conduct this research. The university for C.A.S. receives contraceptive research funding from Bayer Healthcare and NIH/NICHD. S.B.T. has served as a consultant for Bayer Healthcare, Allergan, and Merck & Co. The university for S.B.T receives contraceptive research funding from Bayer Healthcare, Contramed, Medicines360, Merck & Co., NIH/NICHD and the Society of Family Planning; the university for L.M.K receives contraceptive research funding from Agile Therapeutics, Contramed and PRA International. A.I.O. is an employee of Medicines360. M.D.C. receives speaking honoraria from Allergan and Gedeon Richter, serves on an Advisory Board for Merck & Co. and is a consultant for Estetra, Gedeon Richter, Icebreaker Health and Medicines360; the university for M.D.C. receives contraceptive research funding from Contramed, Medicines360, Merck & Co., NIH/NICHD and the Society of Family Planning.
Additional information
Funding
References
- Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253–260.
- US Food and Drug Administration [Internet]. Washington (DC): Centre for Drug Evaluation and Research. Mirena. FDA drug approval package. Clinical pharmacology biopharmaceutics review(s); [cited 2017 Aug 8]. Availabe from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-225.pdf_Mirena_BioPharmr.pdf
- Eisenberg DL, Schreiber CA, Turok DK, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10–16.
- Kyleena. Package insert. Kyleena prescribing information. Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ. 2016.
- Skyla. Package insert. Skyla prescribing information Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ. 2017.
- Mishell DR Jr, Guillebaud J, Westhoff C, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception. 2007;75:11–15.
- Liletta package insert: Allergan USA Inc. and Medicines360. Liletta prescribing information, 2016.
- Mirena. Package insert. Mirena prescribing information. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc. 2017.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205–1213.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97:616–622.
