Abstract
Objective: Medical termination of pregnancy (MToP, or medical abortion) is a highly effective method with a reported efficacy of 95–98%. However, different criteria are currently used to define success, and there are different recommendations for the treatment of what is considered a failure of MToP. This work was undertaken to develop a consensus around a set of well-defined MToP outcomes, as recommended by the Core Outcomes in Women’s and Newborn Health initiative.
Methods: A literature search was made of national and international guidelines and of recommendations of expert groups for various outcomes of MToP and subsequent management. Based on a review of the findings, a group of European experts in MToP undertook a consensus process to agree on a set of core MToP outcomes.
Results: The following core MToP outcomes were defined: success, failure (ongoing pregnancy), need for additional treatment (medical or surgical) to complete MToP (missed abortion, incomplete abortion), complications and the woman’s request for additional treatment (medical or surgical). Recommendations for the management of unsuccessful outcomes were also formulated.
Conclusion: New definitions of MToP outcomes that are more focused on objective criteria and consequently less dependent on provider interpretation are proposed. This should allow better comparison of the efficacy of different regimens and improve the management of failed or incomplete abortion.
摘要
目的:药物终止妊娠(MToP或药物流产)是一种非常有效的方法, 报道的有效率为95-98%。然而目前用于定义药物终止妊娠成功的标准不同, 对于MToP治疗失败也有不同的建议。开展这项工作是为了按照妇女和新生儿健康倡议核心成果的建议, 就一系列明确定义MToP达成共识。
方法:查阅国内外关于MToP结局及后续管理的各种指南及专家小组的建议。在对调查结果进行查阅的基础上, 欧洲专家进行了MToP的一组进行了协商一致, 对MToP的一系列结局达成一致。
结果:MToP的核心结局被定义为:成功、失败(持续妊娠)、需要额外治疗(药物或手术)完成MToP(稽留流产、不全流产), 并发症及妇女要求额外治疗(药物或手术)。对不成功的结果管理也提出了建议。
结论:提出了MToP结局的新定义, 更侧重与客观标准, 因此更少地依赖患者的解释。这样可以更好地比较不同方案的疗效, 并改善失败或不完全流产的管理。
Introduction
Medical termination of pregnancy (MToP, or medical abortion) is a highly effective method with a reported efficacy of 95–98% [Citation1]. However, different definitions of treatment success are used in various publications and clinical guidelines [Citation2]. Most include provider-dependent criteria. The absence of a standard, clear and consistent definition makes it difficult to compare the outcome of different studies or different regimens of mifepristone and misoprostol for MToP. Also, an unambiguous definition of failed or incomplete abortion is needed to give clear guidelines for managing these outcomes.
Most international guidelines, expert groups and clinical trials define the success of MToP as complete termination of pregnancy without recourse to a surgical procedure. This is also true for the recent Medical Abortion Reporting of Efficacy (MARE) guidelines [Citation3]. However, other definitions of MToP outcome may be found in clinical guidelines, as well as different recommendations for managing incomplete or failed MToP: surgery, additional medical treatment or expectant management.
The frequency of surgical intervention following MToP is provider-dependent, especially for incomplete abortion diagnosed via ultrasound, which carries the risk of misinterpreting a thick and heterogeneous appearance of the endometrium as an incomplete abortion [Citation4]. Using the rate of surgical intervention as an indicator for success is misleading for another reason: some providers treat unsuccessful outcomes medically instead of surgically, by repeating the combined treatment or giving an extra dose of misoprostol [Citation5]. Ongoing pregnancy after MToP can also be treated by repeating the treatment regimen and thus avoiding surgical intervention.
Guidelines and clinical practice differ in the number of additional misoprostol doses and the indications for which they are given. Providers also vary considerably in their threshold for administering additional medical treatment to complete the expulsion of the uterine cavity contents (gestational sac, blood clots or residua, of various diameters), especially when retained products of conception are diagnosed by ultrasound [Citation1]. Another difficulty is that the rate of surgical intervention may be biased, since some clinical trials classify surgical evacuation at the woman’s request as a failure, while others only do so if it has been carried out for medical reasons [Citation2].
All these factors make the rate of surgical intervention highly variable, provider-dependent and open to bias, and therefore unsuitable to define success of MToP.
An evidence-based and objective definition of MToP outcome is urgently needed, especially as MToP is used increasingly around the world. Therefore, this study endeavours to develop a consensus around a set of relevant and well-defined first trimester MToP outcomes, as recommended by the Core Outcomes in Women’s and Newborn Health initiative [Citation6]. It may be considered a first step for the Standardizing Abortion Research Outcomes project, dedicated to producing, disseminating and implementing a core outcome dataset for medical and surgical abortion research [Citation7].
Methods
Literature review
We conducted a literature review of international guidelines published from 2007 to 2017 for success and failure definitions, as well as for failure management. The following international guidelines on MToP, including national guidelines from European countries, were searched: World Health Organization (WHO) 2008 [Citation8], 2012 [Citation9] and 2014 [Citation10]; International Planned Parenthood Federation (IPPF) 2008 [Citation11]; International Federation of Gynecology and Obstetrics (FIGO) 2011 [Citation12]; Gynuity Health Projects 2009 [Citation13] and European guidelines: France (Haute Autorité de Santé [HAS] 2010 [Citation14,Citation15]); UK (Royal College of Obstetricians and Gynaecologists [RCOG] 2011 [Citation16] and 2015 [Citation17]); and Swedish Society of Obstetrics and Gynaecology (SFOG) guidelines 2018 [Citation18]. Recent MARE guidelines aiming to improve the reporting of MToP efficacy were also included [Citation3].
These national and international guidelines were also searched to find information on managing different types of MToP outcome [Citation2–4].
Role of the expert group
The expert group included clinicians, researchers and members of the pharmaceutical industry involved in MToP. Drawing on the literature search, the group established consensus definitions of MToP outcome, as well as consensus proposals based on the evidence for management of non-successful outcomes. Any disagreement between members of the expert group was discussed in depth during face-to-face meetings involving all experts. They agreed on the final definitions proposed below.
Results
Below is a summary of the available guidelines pertaining to MToP definitions and outcome management.
Definitions of success and failure
No clearly specified definition of success or failure was found in WHO [Citation8–10], FIGO [Citation12] or RCOG [Citation16,Citation17] sources. Definitions were available from IPPF [Citation11], Gynuity Health Projects [Citation13] and HAS [Citation14,Citation15], but they varied considerably ().
Table 1. MToP outcome definitions.
Management of abortion outcome
WHO guidelines suggest offering vacuum aspiration or repeat administration of misoprostol every 3 h in up to five doses to complete the procedure for a woman reporting ongoing symptoms of pregnancy and/or who has only minimal bleeding after taking the abortifacient medications as directed [Citation9]. HAS suggests the administration of additional doses of misoprostol (400 µg, usually via the oral route) following first misoprostol intake in most first trimester MToP studies [Citation15]. FIGO recommends additional doses of 600–800 µg via the sublingual, vaginal or buccal route [Citation5].
A follow-up visit is not necessary from a clinical point of view if expulsion has been confirmed at the time of the procedure [9,16]. It is still recommended by IPPF 14 days after the procedure, to initiate contraception [Citation11]. It is also legally mandatory in some countries (e.g. France) and must be held 2 weeks after misoprostol administration [Citation14].
MToP outcome at a follow-up visit may be complete abortion, incomplete abortion (which can be difficult to differentiate from intrauterine blood clots on ultrasound), missed abortion (persistent but non-developing pregnancy) or ongoing pregnancy [Citation4].
Incomplete abortion
No clear and uniform definition of incomplete abortion exists, nor are there any criteria on how to diagnose it: with or without ultrasound, with or without human chorionic gonadotropin (hCG) testing, with or without gynaecological examination, etc. (). WHO and FIGO recommend either vacuum aspiration or treatment with misoprostol for incomplete abortion if uterine size is equivalent to a pregnancy of gestational age ≤13 weeks [Citation9,Citation12]. The recommended regimen of misoprostol is a single dose given either sublingually (400 µg) or orally (400–800 µg). Misoprostol may also be given vaginally, but this route of administration should be avoided in women with a level of bleeding that might affect absorption of misoprostol [Citation10–12,Citation17,Citation19–21]. Expectant management may also be considered [Citation9,Citation10,Citation20]. Surgical evacuation of the uterus may be carried out at the woman’s request or in case of a clinical indication (e.g. haemodynamically unstable situation, heavy or prolonged bleeding, anaemia, suspicion of infection) [Citation10].
Table 2. Management of abortion outcome up to 13 weeks at follow-up: guidelines.
Missed abortion
For management of missed abortion independently of prior treatment for MToP, FIGO’s 2017 guidelines recommend misoprostol 800 µg vaginally every 3 h (maximum two doses) or 600 µg sublingually every 3 h (maximum two doses) [Citation5]. SFOG guidelines recommend either misoprostol or surgery [Citation18].
Ongoing pregnancy
For ongoing pregnancy (), WHO, IPPF and FIGO recommend that women be offered a uterine evacuation procedure as quickly as possible [Citation9,Citation11,Citation12]. This is usually done by vacuum aspiration [Citation9,Citation11,Citation12]; however, a second identical course of MToP may also be given if the woman prefers and if the gestational age is still within the approved gestational limit for the drug [Citation18].
Discussion
During the first 20 years of MToP, success was defined as expulsion of the pregnancy without surgical intervention, because most complications (heavy bleeding) or undesired outcomes (ongoing pregnancy, missed or incomplete abortion) were treated surgically. However, the frequency of a surgical intervention in these cases not only depends on the diagnosis of the outcome but to a large extent on the provider (experience and motivation to perform or avoid a surgical intervention) [Citation22] and the woman being treated. Moreover, this definition is no longer applicable for two reasons:
Except for ongoing pregnancy, undesired outcomes are currently defined by widely varying criteria. Therefore, the frequency varies considerably depending on the provider.
Recent years have seen a tendency towards fewer surgical interventions to treat undesired outcomes and complications of MToP, because clinical experience has shown that most situations may be handled with medical treatment: additional doses of misoprostol, a repeat course of the combination of mifepristone and misoprostol, or expectant management.
New definitions of MToP outcomes are necessary. However, it will not be possible to compare the rates of success and failure presented in previous literature with the rates found using the new definitions. This should be kept in mind for future meta-analyses.
Diagnosing and managing different outcomes of MToP
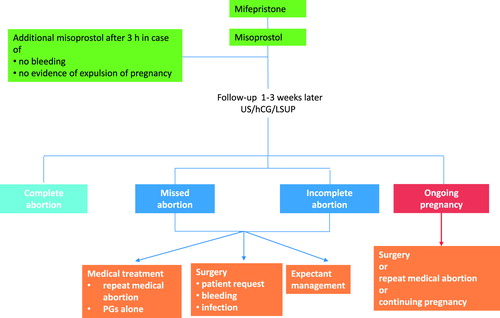
The outcome of MToP may be one of the following three situations ():
Success: expulsion without the need for additional intervention.
Failure: ongoing viable pregnancy.
Need for additional treatment or expectant management (because of incomplete or missed abortion, a complication, or at the woman’s request).
Follow-up assessment
The outcome of MToP can be diagnosed during the procedure and/or during the follow-up visit. The purpose of the visit is to confirm expulsion of the pregnancy and enable the woman to make an early decision on how to proceed in the rare case of an ongoing pregnancy. The delay between misoprostol intake and the follow-up visit is a trade-off between the woman’s interest in knowing the treatment outcome as soon as possible and the reliability of the examination (ultrasound or hCG), which may be inconclusive if done too early but becomes highly reliable with increasing time.
Early identification of the outcome is important to avoid delay in potentially necessary additional treatment, especially in the case of ongoing pregnancy. Diagnosis of ongoing pregnancy can be done by ultrasound; by interpreting a woman’s report of signs and symptoms of ongoing pregnancy; by a specially designed, self-performed, low-sensitivity urinary pregnancy (LSUP) test; by serial serum hCG testing; or by serial use of a multilevel pregnancy test [Citation23]. Follow-up can be organised by self-assessment [Citation24], telephone follow-up, or by systematic clinic visits and ultrasound [Citation25].
Ongoing pregnancy
Ongoing pregnancy is arguably the only true failure of treatment. It is easy and unambiguous to diagnose and is a reliable criterion to compare the results from different studies. The rate of ongoing pregnancy is, however, very low following MToP carried out according to recommended guidelines [Citation26]. Treatment of an ongoing pregnancy can be by surgical evacuation, as recommended by most guidelines, or repeat MToP [Citation24]. However, few published data give evidence-based recommendations for the best treatment in such circumstances.
Missed and incomplete abortion
Missed abortion (persistent non-viable pregnancy) and incomplete abortion (remaining residue) are clinical situations that may also need additional treatment. Different recommendations exist for this additional treatment [Citation10]. However, the diagnosis is usually unclear in the published literature and treatment varies widely in clinical practice. Treatment can be surgical (aspiration), medical (additional misoprostol or the repeated combination of mifepristone and misoprostol) or expectant management. These differences make it almost impossible to compare results from different studies, especially since some interventions are classified as failures (e.g. surgical intervention), while other interventions for the same undesired outcome are classified as successes (e.g. additional medical treatment).
The diagnosis of missed abortion uses clinical symptoms in combination with either a post-treatment LSUP test, confirmed by ultrasound or ultrasound alone.
The diagnosis of incomplete abortion is usually based on clinical signs and symptoms (no expulsion; nausea; breast tenderness; enlarged, soft uterus; dilated cervix; prolonged bleeding), and/or elevated hCG levels, and/or ultrasound that may find an echogenic structure in the uterine cavity. However, blood clots frequently look identical to pregnancy residue on ultrasound, and intrauterine blood clots are frequently found in a complete abortion [Citation27]. These two situations may be indistinguishable without histological examination of the tissue ( and ). Therefore, the diagnosis of incomplete abortion should not be based on ultrasound criteria alone but should include hCG testing and/or clinical evaluation [Citation16,Citation28,Citation29]. Also, interventions based on ultrasound diagnosis or a single hCG test alone might be unnecessary.
Figure 2. Vaginal ultrasound. (a) Uterine cavity immediately after aspiration of an 8-week pregnancy. (b) Presence of blood clots in the same woman 10 days after surgical abortion.
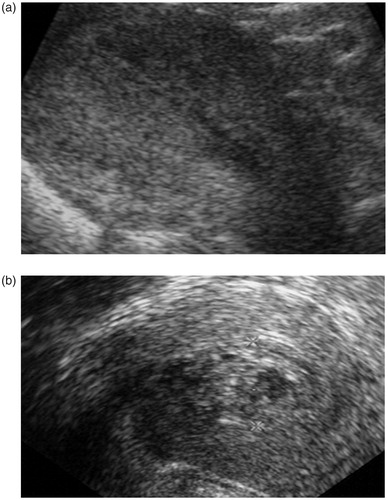
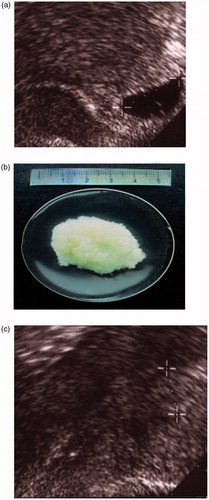
Figure 3. Vaginal ultrasound. (a) Uterus with an intrauterine pregnancy corresponding to 6 weeks after the last menstrual period, with yolk sac visible and serum hCG 32,000 IU/l. (b) Gestational sac of the same woman expelled on day 3. (c) Uterine cavity diameter 12 mm in the same woman 8 days after intake of mifepristone in an MToP procedure. Serum hCG had dropped to 837 IU/l. The gestational sac that was visible in the uterine cavity prior to the procedure is gone.
New definition for outcome of MToP
Based on the above analysis, the expert group suggests the following classification for MToP outcome, as assessed 1–3 weeks after the procedure ():
Table 3. Proposed definitions of outcome of MToP (or medical abortion).
Success: expulsion of the gestational sac with no need for additional treatment (repeat MToP, misoprostol alone, or surgical vacuum aspiration).
Failure: ongoing pregnancy that can be unambiguously diagnosed.
Need for additional treatment:
To complete MToP: additional treatment can be medical (repeat MToP or misoprostol alone) or surgical (vacuum aspiration). However, a surgical intervention is invasive, which can greatly impact treatment satisfaction, since a woman who chooses medical treatment often wants to avoid surgery [Citation23].
To treat a complication: this would include surgery or medical treatment (antibiotics, misoprostol or blood transfusion) for adverse outcomes such as heavy or prolonged bleeding, infection or persistent significant pain, if these outcomes are not associated with missed or incomplete abortion.
At the woman’s request.
Future research
Thirty years after MToP was first marketed in France in 1988, many questions about it have been answered in numerous clinical studies. Its high efficacy and safety have been demonstrated [Citation30], along with the immediate return of ovulation [Citation31] and no negative impact on future pregnancies [Citation32,Citation33]. However, future research is needed on aspects such as improving pain management and reducing the length and intensity of bleeding.
Conclusion
The currently used definitions of success of MToP are inconsistent and provider-dependent. The increasing use of medical treatments in place of surgery makes it necessary to find new definitions for MToP outcomes where the only true failure is verifiable and unambiguous: ongoing viable pregnancy. The new definitions recommended in this review should allow for easier and more reliable comparison of the efficacy of different regimens in the future and should help providers manage MToP outcomes appropriately.
Acknowledgements
The authors would like to thank J. Arthur for her help in medical writing.
Disclosure statement
All authors, except for Sharon Cameron, are members of the external scientific advisory board of Exelgyn. Sharon Cameron was a member until 2016 and ceased thereafter; she has no conflict of interest. Christian Fiala has served on an ad hoc basis as an invited lecturer for Exelgyn and Teva. Teresa Bombas is a member of the advisory board of MSD and HRA and a speaker in conferences/symposia organised by Bayer, MSD, HRA, Gedeon and Exelgyn. Mirella Parachini has served on an ad hoc basis as a consultant for Nordic Pharma. Aubert Agostini is a member of the board at MSD and has served as an investigator for a Nordic Pharma study. Roberto Lertxundi has financial relationships (member of advisory boards, lecturer and/or consultant) with Nordic Pharma, Exeltis, Bayer and Teva. Marek Lubusky has no conflict of interest. Laurence Saya is an employee of Altius Pharma, a consultancy and medical writing company, which received funding from Exelgyn for help with this work. Kristina Gemzell-Danielsson serves or has served on an ad hoc basis as an invited lecturer for Exelgyn, Linepharma and Gynuity, and as an investigator in clinical trials conducted by Concept Foundation/SunPharma.
Additional information
Funding
References
- Fiala C, Gemzell-Danielson K. Review of medical abortion using mifepristone in combination with a prostaglandin analogue. Contraception 2006;74:66–86.
- Raymond EG, Shannon C, Weaver MA. First-trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception 2013;87:26–37.
- Creinin MD, Chen MJ. Medical abortion reporting of efficacy: the MARE guidelines. Contraception 2016;94:97–103.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190–195.
- International Federation of Gynecology and Obstetrics. Misoprostol-only recommended regimens 2017. [cited 2018 Jan 9]. Available from: www.figo.org/sites/default/files/uploads/project-publications/Miso/FIGO_Dosage_Chart%20EN_0.pdf.
- Khan K. The CROWN initiative: journal editors invite researchers to develop core outcomes in women’s health. J Ovarian Res 2015;8:6.
- Whitehouse KC, Kim CR, Ganatra B, et al. Standardizing abortion research outcomes (STAR): a protocol for developing, disseminating and implementing a core outcome set for medical and surgical abortion. Contraception. 2017;95:437–441.
- World Health Organization. Managing incomplete abortion. Education material for teachers of midwifery. Midwifery education modules. 2nd ed. Geneva: WHO; 2008.
- World Health Organization. Safe abortion: technical and policy guidance for health systems. 2nd ed. Geneva: WHO; 2012.
- World Health Organization. Clinical practice handbook for safe abortion. Geneva: WHO; 2014.
- International Planned Parenthood Federation. First trimester abortion guidelines and protocols. Surgical and medical procedures. 2008 [cited 2017 Nov 29]. Available from: www.ippf.org/resource/First-Trimester-Abortion-Guidelines-and-Protocols.
- FIGO Working Group on Prevention of Unsafe Abortion and its Consequences; International Federation of Gynecology and Obstetrics. The combination of mifepristone and misoprostol for the termination of pregnancy. Int J Gynaecol Obstet. 2011;115:1–4.
- Gynuity Health Projects. Medical abortion guidebook. 2009 [cited 2017 Nov 29]. Available from: http://gynuity.org/resources/info/medical-abortion-guidebook.
- Haute Autorité de Santé. Interruption volontaire de grossesse par méthode médicamenteuse. Recommandations. 2010 [cited 2017 Nov 29]. Available from: www.has-sante.fr/portail/upload/docs/application/pdf/2011-04/ivg_methode_medicamenteuse_-_argumentaire_-_mel_2011-04-28_11-39-33_198.pdf.
- Haute Autorité de Santé. Interruption volontaire de grossesse par méthode médicamenteuse. Argumentaire. 2010 [cited 2017 Nov 29]. Available from: www.has-sante.fr/portail/upload/docs/application/pdf/2011-04/ivg_methode_medicamenteuse_-_recommandations_-_mel_2011-04-28_11-39-11_882.pdf.
- Royal College of Obstetricians and Gynaecologists. The care of women requesting induced abortion. Evidence-based clinical guideline number 7. 2011 [cited 2017 Nov 29]. Available from: www.rcog.org.uk/globalassets/documents/guidelines/abortion-guideline_web_1.pdf.
- Royal College of Obstetricians and Gynaecologists. Best practice in comprehensive abortion care. Best practice paper no. 2. 2015 [cited 2017 Nov 29]. Available from: www.rcog.org.uk/globalassets/documents/guidelines/best-practice-papers/best-practice-paper-2.pdf.
- Work and Reference Group for Family Planning, Swedish Society of Obstetrics and Gynaecology. Induced abortion. Guideline no. 78 [in Swedish]. 2018 [2018 Oct 4]. Available from: www.sfog.se/natupplaga/ARGrappor9792c7d5-5648-475e-bee6-81478b0d9323.pdf.
- Tang J, Kapp N, Dragoman M, et al. WHO recommendations for misoprostol use for obstetric and gynecologic indications. Int J Gynaecol Obstet. 2013;121:186–189.
- Kapp N, Whyte P, Tang J, et al. A review of evidence for safe abortion care. Contraception. 2013;88:350–363.
- Tang OS, Schweer H, Lee SWH, et al. Pharmacokinetics of repeated doses of misoprostol. Hum Reprod. 2009;24:1862–1869.
- Gomperts R, Petow S, Jelinska K, et al. Regional differences in surgical intervention following medical termination of pregnancy provided by telemedicine. Acta Obstet Gynecol Scand. 2012;91:226–231.
- Raymond EG, Shochet T, Blum J, et al. Serial multilevel urine pregnancy testing to assess medical abortion outcome: a meta-analysis. Contraception 2017;95:442–448.
- Cameron ST, Glasier A, Johnstone A, et al. Can women determine the success of early medical termination of pregnancy themselves? Contraception. 2015;91:6–11.
- Oppegaard KS, Qvigstad E, Fiala C, et al. Clinical follow-up compared with self-assessment of outcome after medical abortion: a multicenter, non-inferiority, randomized, controlled trial. Lancet. 2015;385:698–704.
- Lièvre M, Sitruk-Ware R. Meta-analysis of 200 or 600 mg mifepristone in association with two prostaglandins for termination of early pregnancy. Contraception. 2009;80:95–100.
- Laifer-Narin SL, Kwak E, Kim H, et al. Multimodality imaging of the postpartum or posttermination uterus: evaluation using ultrasound, computed tomography and magnetic resonance imaging. Curr Probl Diagn Radiol. 2014;43:374–385.
- Acharya G, Haugen M, Brathen A, et al. Role of routine ultrasonography in monitoring the outcome of medical abortion in a clinical setting. Acta Obstet Gynecol Scand. 2004;83:390–394.
- Kaneshiro B, Edelman A, Sneeringer RK, et al. Expanding medical abortion: can medical abortion be effectively provided without the routine use of ultrasound. Contraception. 2011;83:194–201.
- Kulier R, Kapp N, Gülmezoglu AM, et al. Medical methods for first trimester abortion. Cochrane Database Syst Rev. 2011;11:CD002855.
- Schreiber CA, Sober S, Ratcliffe S, et al. Ovulation resumption after medical abortion with mifepristone and misoprostol. Contraception. 2011;84:230–233.
- Saccone G, Perriera L, Berghella V. Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214:572–591.
- Woolner A, Bhattacharya S, Bhattacharya S. The effect of method and gestational age at termination of pregnancy on future obstetric and perinatal outcomes: a register-based cohort study in Aberdeen, Scotland. BJOG. 2014;121:308–318.