Abstract
Objectives: The aims of this study were: (1) to identify which measurement instruments are used in practice to assess the quality of life or well-being of individuals with and without (sub)fertility; (2) to describe the design and outcomes of studies comparing quality of life or well-being of individuals with and without fertility problems; and (3) to determine which of the outcomes of the identified studies could be used in cost-utility studies.Methods: A systematic literature review was performed of studies published before July 2018, using multiple databases. Included studies investigated (health-related) quality of life or well-being of individuals with fertility problems. The applied instruments were assessed, as were the outcomes and suitability for use in cost-utility studies.Results: Twenty-six studies met the inclusion criteria. Twelve distinct instruments of measurement were applied: two generic quality-of-life instruments, five generic well-being instruments and five disease-specific instruments. Most studies found negative associations in one or more domains assessing fertility problems and quality of life or well-being. However, two studies found the opposite. None of the studies reported outcomes relevant for cost-utility studies.Conclusion: Quality of life and well-being related to having fertility problems are regularly studied. However, the reported information is not suitable for use in cost-utility studies. There is a clear need for studies investigating the impact of fertility problems on quality of life in a way that outcomes can be compared across studies and disease areas.
摘要
目的:本研究的目的是:(1)为确定在实践中使用哪些测量工具来评估有生育能力问题和无生育能力问题或生育能力降低的患者的生活质量或幸福感;(2)描述比较有和无生育能力问题的患者的生活质量或幸福感的研究设计和结局;(3)确认哪些已确定研究的结果可以用于成本效用研究。
方法:使用多个数据库对2018年7月之前发表的研究进行了系统性文献回顾。包括调查(健康相关)有生育问题的患者的生活质量或健康状况的研究。评估了应用的工具, 以及在成本效用研究中的结果和适宜性。
结果:纳入了26项符合标准的研究。有12种不同的测量工具被使用:2种测量生活质量通用工具, 5种一般健康的测量工具, 5种特殊疾病的测量工具。大多数研究发现, 有一个或多个方面的生育问题与生活质量或健康状况呈负相关。然而, 有两项研究发现与之相反。所有研究均未报告与成本效用研究相关的结果。
结论:要定期研究与生活质量和健康状况相关的生育问题。但是, 已有的报道信息不适合用于成本效用研究。显然研究生育问题对生活质量的影响是有必要的, 以便将研究和疾病领域的结果进行比较。
Introduction
Up to 15% of reproductive-aged couples worldwide experience infertility [Citation1]. In absolute numbers, 48.5 million couples are unable to fulfil their desire for a child (defined as not having been able to conceive over a period of 5 years). Of these, 19.2 million couples do not succeed in having a first child and 29.3 million do not succeed in having a second child [Citation2]. About half of couples with infertility seek medical help [Citation3]. Fertility problems affect individuals in high-income countries, as well as those in middle- and low-income countries [Citation2].
A study among the general Dutch population showed that as many as 91% of those questioned considered having mild fertility problems to be an unacceptable health condition for women aged 30, and 93% felt that infertility was unacceptable [Citation4]. In other words, fertility seems an important aspect of a normal healthy life. Be that as it may, not being fertile, or being less fertile, may not result in directly visible health problems. Fertility care may therefore sometimes be seen as unnecessary or of low priority. In practice, in many countries this has resulted in policies limiting access to fertility care in health insurance schemes or in national health service systems. In the Netherlands, couples currently get a maximum of three in vitro fertilisation (IVF) or intra-cytoplasmic sperm injection attempts reimbursed through the basic benefits package of the mandatory health insurance system. In Austria, 70% of treatment and drug costs are reimbursed under certain circumstances, while in the USA a large proportion of women pay for their own treatment [Citation5]. These limitations in access to fertility-related care may contribute to the burden of disease of women with fertility problems.
Although, obviously, the most important outcome of fertility treatment may be considered the birth of a baby, this outcome may not be the primary outcome considered in policy-makers’ reimbursement decisions. In many countries, cost-effectiveness outcomes play an important role in these decisions. In other words, does a treatment (e.g. IVF) offer value for money? Effectiveness in cost-effectiveness studies is preferentially expressed in costs per quality-adjusted life year (QALY). Cost-effectiveness studies expressing outcomes in QALYs are commonly referred to as cost-utility studies. The QALY is a composite measure of length of life and quality of life of the individual. However, most health economic studies with regard to fertility treatment examine the costs of fertility treatment per live birth, rather than the costs per QALY gained. The difficulty for policy-makers is that costs per live birth cannot be compared with cost-effectiveness outcomes of other medical interventions treating other diseases. Consequently, it is not possible to determine whether fertility treatments offer value for money compared with treatments for other diseases. In order to be able to conduct cost-utility studies it is necessary to have insights into the effects of subfertility and infertility on quality of life.
The effect of fertility problems on the quality of life of individuals has often been studied. Outcomes show that infertility can cause tremendous psychological distress in women and men. Disease severity may sometimes be as profound as that of life-threatening diseases such as cancer and heart disease [Citation6]. Still, such evidence may not always reach policy-makers, or be adequately included in reimbursement decisions, since the quality-of-life studies were not designed to feed cost-utility studies. Most available evidence is collected in studies within social sciences covering a single domain of health or well-being rather than general quality of life. In order for evidence to be able to be included in cost-utility studies, quality of life needs to be measured and valued in such a way that the outcomes are comparable across diseases. Commonly, this implies using preference-based generic quality-of-life or well-being instruments.
The extent to which evidence is available regarding the effects of fertility problems on quality of life suitable for use in cost-utility studies is unclear. To gain insights into the impact of infertility on quality of life, it is especially interesting to examine studies comparing the quality of life or well-being of women with fertility problems with that of people without fertility problems.
The aims of this study were: (1) to identify which measurement instruments are used in practice to assess the quality of life or well-being of individuals with and without (sub)fertility; (2) to describe the design and outcomes of studies comparing quality of life or well-being of individuals with and without fertility problems; and (3) to determine which of the outcomes of the identified studies could be used in cost-utility studies.
In order to meet these objectives an extensive systematic review was conducted using multiple databases.
Methods
The systematic review was set up to identify a wide scope of scientific studies investigating either disease-specific or generic quality of life and/or well-being of individuals with fertility problems now or in the past, with or without (ever) receiving treatment, with or without children.
The review included the following databases: Medline (OvidSP), Web of Science, PsycINFO (OvidSP), Embase, Cochrane, PubMed and Google Scholar. The searches were conducted to include all studies published before 9 July 2018. Words included in the search strategy were related to: (1) fertility (such as ‘fertility’, ‘subfertility’ and ‘infertility’); (2) quality-of-life aspects (including ‘well-being’, ‘quality of life’ and ‘distress’); and (3) measurement instruments (including ‘questionnaire’, ‘scale’ and ‘score’). Different search strategies were explored to determine the most eligible one, thus including the most relevant studies. The specific search queries may be found in the appendix.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were as follows: (1) studies had to specifically assess generic or disease-specific quality of life or well-being of humans with (past) infertility/subfertility; (2) studies had to be of a quantitative nature (e.g. studies applying open interviews were excluded); (3) studies had to be original scientific research (i.e. comments, editorials and reviews were excluded); (4) studies were excluded if they were primarily aimed at the evaluation of a specific fertility-related treatment; (5) studies were excluded when they focussed on fertility-related problems or causes of infertility, when they studied the relationship between quality of life or well-being and factors other than fertility, or when they investigated an indirect effect of fertility on quality of life or well-being (e.g. the role of coping styles in the effect of infertility on quality of life); (6) studies were excluded when they focused on many conditions of which subfertility or infertility was one; (7) studies had to compare quality of life or well-being of individuals with and without fertility problems; and (8) instrument development studies were excluded.
Identification of published studies and instruments
To identify the relevant published papers from the initial search, the following procedure was undertaken. First, titles and abstracts were examined and independently coded by two researchers (KH and MK) as irrelevant, likely to be relevant, or unclear. Differences were discussed until consensus was reached. In case of doubt, studies were included in the full-text investigation. Studies that were coded as irrelevant received an additional code to describe which of the inclusion criteria were not met or which exclusion criteria applied.
After the title and abstract search, full-text papers were examined to determine whether all inclusion criteria were indeed met and the exclusion criteria did not apply. Full-text examinations were conducted in close collaboration between KH and MK.
After relevant studies had been identified, it was assessed what the studies aimed to measure specifically and what type of instrument was used to measure quality of life or well-being: generic, disease or domain specific. Generic instruments are designed to measure quality of life over the complete spectrum of diseases in various populations and can be used to compare changes across different patient groups. Disease-specific instruments are designed to measure the most relevant domains of quality of life specific to a particular disease, while domain-specific instruments focus on a single domain of health (e.g. social well-being). After identifying the instruments, the content of the applied instruments was examined and summarised.
An overview table was constructed summarising the main characteristics of the studies comparing quality of life or well-being of people with and without fertility problems, such as the study aim, the sample size, the instruments used and the main outcomes. Finally, it was investigated whether the applied instruments and the presented outcomes in the studies were suitable for use in cost-utility studies.
Compliance with ethical standards
As this article does not contain any studies with human participants or animals performed by any of the authors, no ethics approval was required.
Results
Review process
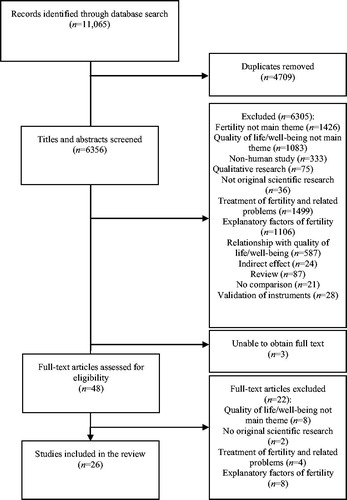
As shown in , the initial database searches identified 11,065 potentially relevant papers, of which 4695 duplicate studies were detected and excluded by means of EndNote X7 (https://endnote.com). A further 14 duplicates were detected and manually removed. Title and abstract searches resulted in the exclusion of 6305 studies: 1426 were not primarily aimed at fertility, 1083 did not consider quality of life or well-being as a main theme, 333 explored non-human subjects, 75 were qualitative studies, 36 did not report original scientific research, 1499 were about treatment of fertility and fertility-related problems, 1106 investigated causes of infertility, 587 were about the relationship between quality of life or well-being and other factors than fertility, 24 studied an indirect effect of fertility on quality of life or well-being, 87 were reviews of previous literature, 21 did not compare quality of life/well-being of individuals with and without fertility problems, and 28 aimed to validate rather than apply instruments. The initial similarity percentage between the coding of the researchers in the title and abstract examination was high (91.8%).
A total of 51 studies were intended to be included in the full-text examinations. However, we were not able to obtain the full-text paper of three studies; 22 studies were excluded based on the full-text examination. This left a total of 26 studies included in the analyses.
A short description of the design of the studies, an overview of the quality-of-life and/or well-being instruments used and a summary of the studies’ outcomes are presented in . In most countries only one or two studies were conducted, with the exception of Iran (six studies) [Citation8–10,Citation13,Citation23,Citation27], Germany (three studies) [Citation22,Citation28,Citation31] and Turkey (three studies) [Citation25,Citation26,Citation29]. Most studies had similar aims and objectives (to compare quality of life/well-being of individuals with fertility problems with quality of life/well-being of individuals without fertility problems). However, study populations differed. For instance, some studies investigated quality of life of individuals during IVF treatment and some of individuals after IVF treatment. Some only studied women’s quality of life, while others investigated quality of life in couples. Diverse quality-of-life and well-being instruments were used.
Table 1. Summary of identified studies’ designs and outcomes.
Quality-of-life and well-being instruments
We identified 12 instruments used in the 26 studies, of which three were generic instruments measuring quality of life, five were generic instrument measuring well-being and four were disease-specific instruments. A short description of the applied instruments is provided in . The study outcomes are discussed below. When a difference in outcomes is described as significantly different, it is statistically different according to the outcomes reported in the specific study. Note that the distinction between quality-of-life instruments and well-being instruments was not always evident. We categorised the instruments based on labelling of the instrument in the instruments’ modes of instruction.
Table 2. Applied quality-of-life and well-being instruments.
Generic quality-of-life instruments and their outcomes
Sixteen of the 26 identified studies applied a generic health status or quality-of-life instrument. Three different (types of) generic instruments were used: the Short Form 36 (SF-36), two versions of World Health Organization (WHO) quality-of-life measures – the abbreviated version, WHOQOL-BREF, and the WHOQOL-100 – and one self-constructed rating scale.
The SF-36 was used in nine of the included empirical studies [Citation8–10,Citation12–16,Citation29]. De Pascalis et al. [Citation12] found that couples who underwent successful assisted reproductive technology (ART) reported lower quality of life in the SF-36 physical summary score. Ashraf et al. [Citation10] reported significantly worse scores in quality of life of infertile or subfertile women compared with fertile women in the domains of physical functioning, role limitations due to physical problems, general health, vitality, social functioning, role limitations due to emotional problems and mental health. Ahmadi et al. [Citation8] reported that during pregnancy, women who conceived naturally had better physical functioning and less role limitation due to physical problems, less bodily pain and better social functioning, while women who conceived by ART reported better general health, vitality, role limitation due to emotional problems and mental health. After childbirth, women who had conceived by ART scored better compared with the natural conception group on all but one dimension. Drosdzol and Skrzypulec [Citation14] showed significantly worse scores on the SF-36 among infertile women compared with fertile controls in five out of nine domains, while the other indices were slightly but insignificantly better. Infertile males were found to have no significantly different values compared with fertile controls, although vitality was slightly decreased. El Kissi et al. [Citation15] found a lower summary score in the mental dimension of the SF-36 among infertile men compared with controls. Infertile women compared with controls scored worse on both summary scores. Amiri et al. [Citation9] found that the total quality-of-life score was not significantly different between fertile and infertile women. Direkvand-Moghadam et al. [Citation13] found that infertile women scored worse in the domains of physical function, role limitations due to physical problems, general health, vitality, social functioning, role limitations due to emotional problems and mental health. Graham [Citation16] found that childless women experienced significantly better scores on the mental and physical component summary measures compared with mothers. Sezgin et al. [Citation29] found that infertile women reported poorer quality of life compared with fertile control women.
The WHOQOL-BREF was used in five studies [Citation7,Citation23,Citation25–27]. Masoumi et al. [Citation23] found that fertile couples had significantly higher quality of life compared with infertile couples. Sani and Tamannaeifar [Citation27] found the quality of life of infertile women to be lower than that of fertile women. Onat and Beji [Citation25] reported a significantly higher quality-of-life score among the infertile group compared with the fertile group in all subdomains of the WHOQOL-BREF. By contrast, Pinar and Zeyneloglu [Citation26] reported scores in all subdomains of quality of life to be significantly lower in the infertile group. Aduloju et al. [Citation7] found that quality of life was significantly lower among infertile women compared with fertile women. The WHOQOL-100 was used in one study, by Xiaoli et al. [Citation32], who concluded that infertile women had significantly lower overall quality of life compared with fertile women.
Klemetti et al. [Citation21] measured quality of life on a single-item scale from 0 to 10 and concluded that quality of life was significantly lower among infertile childless men compared with fertile men, but no difference was found among women.
Generic well-being instruments and their outcomes
Six of the 26 identified studies applied five different well-being instruments: the Psychological General Well-Being Index (PGWB), the Scales of Psychological Well-Being–Short Form (SPWB-SF), the Von Zerssen symptom checklist, a self-constructed rating scale on global well-being and the Quality of Well-Being Scale (QWB). A description of the instruments is provided in .
The PGWB was applied in two separate studies [Citation19,Citation20]. Johansson et al. [Citation20] found that total PGWB scores and domain scores, except anxiety, were worse among men with unsuccessful IVF than among men with successful IVF. Additionally, compared with controls, men with unsuccessful IVF reported worse scores in the domains of depression and positive well-being. Women who had undergone unsuccessful IVF scored worse on depression compared with a control group. However, no difference in total PGWB scores was found when comparing successful IVF treatments in men or women with a control group. Johansson et al. [Citation19] found that compared with control groups, men with unsuccessful IVF scored worse in the PGWB domains of depression and positive well-being, men with successful IVF had better vitality, and women with unsuccessful IVF had significantly worse well-being scores in anxiety and depression.
The SPWB-SF was used in one cross-sectional study by Jeffries and Konnert [Citation18] which reported no significant differences in overall psychological well-being between involuntarily childless women, voluntarily childless women and mothers.
Kowalcek et al. [Citation22] applied the generic Von Zerssen symptom checklist to investigate the well-being of infertile couples. These authors found no notable difference between the symptom scores of infertile couples and the applied reference data of healthy comparators.
In a study by Callan [Citation11] a self-constructed rating scale was used to measure global well-being. Well-being was assessed by posing a question on life satisfaction on a 1–9 scale, where 1 indicated being not very satisfied and 9 very satisfied. Comparing mothers, infertile women and voluntarily childless women, infertile women were less satisfied with their life compared with voluntarily childless women.
The QWB was used in one study. Monga et al. [Citation24] found that women in couples seeking infertility treatment showed lower health-related quality of life compared with women in couples seeking elective sterilisation. Additionally, no difference was found in men in the same couples.
Disease-specific quality-of-life and well-being instruments and their outcomes
Four of the 26 studies included in our review applied disease-specific instruments. Four different instruments were applied: the Fertility Quality-of-Life Questionnaire (FertiQoL), the Tübinger Lebensqualitätsfragebogen für Männer mit Kinderwunsch (TLMK), the Quality-of-Life Questionnaire C30 (QLQ-C30) and one self-constructed rating scale.
Valsangkar et al. [Citation30] applied the FertiQoL instrument and found that infertile women had a lower fertility-related quality of life compared with reference data.
Applying the TLMK, Schanz et al. [Citation28] found that the quality-of-life scores over 5 years of involuntarily childless men had improved in the domains of desire for a child and gender identity, indicating that the negative impact of these domains had decreased. However, no differences in quality of life were found among those who had become fathers and those who remained childless.
The QLQ-C30 was used in a case–control study by Hassanin et al. [Citation17], who reported that infertile women had a significantly lower quality of life compared with fertile women.
Wischmann et al. [Citation31] applied a generic rating system comparing subjective change in quality of life on a single scale of 1–5 (1 indicating being much worse after termination of infertility treatment and 5 being much better). They found that childless men and women and parents reported increased quality of life after termination of infertility treatment, but this increase was not significantly different between childless partners and parents.
Quality-of-life and well-being outcomes
The majority of the included studies reported decreased quality of life or well-being in one or more domains due to infertility/subfertility. Sixteen out of the 26 studies showed evidence of significantly deprived health status in men, women and couples in one or more domains [Citation7,Citation10–15,Citation17,Citation21,Citation23,Citation24,Citation26,Citation27,Citation29,Citation30,Citation32]. Two studies found the opposite for either women or couples [Citation16,Citation25]. Eight studies found no overall evident relationship between infertility/subfertility and quality of life or well-being [Citation8,Citation9,Citation18–20,Citation22,Citation28,Citation31].
Suitability for use in cost-utility studies
Preference-based utility weights were available for three of the 12 applied instruments in the studies included in this review. Such utility weights are important since these can be used to construct QALYs to allow for comparison of outcomes across diseases. The three instruments for which preference-based utility weights were available (and therefore suitable for use in cost-utility studies) are the SF-36, the QWB and the QLQ-C30. As shown in , these instruments were applied in 11 studies. This means that in those studies it would have been possible to report utilities that could be applied in cost-utility studies. However, none of the studies reported outcomes in utilities.
Discussion
The impact of having fertility problems on quality of life and well-being of individuals is regularly studied. Our study reviewed the available evidence regarding the impact of having fertility problems on quality of life and well-being.
Findings and interpretation
The outcomes of this systematic review indicated that the relationship between fertility and quality of life is not always clear. Although in most studies people with fertility problems scored lower in one or more domains of quality of life/well-being, not all did. Moreover, the association between having fertility problems and different domains of quality of life was not consistent between studies. There can be various reasons for these differences. For instance, the 26 studies were conducted in 15 different countries; cultural differences in these countries, such as social acceptability of infertility, are likely to have influenced the association between fertility problems and quality of life. Moreover, the differences in reported outcomes may be related to other aspects of the study design, such as the sample size and, obviously, the applied instruments. The 26 studies reported a total of 12 distinct instruments, making comparisons between studies difficult. In addition, the study population of interest differed between studies. Of course, having insight into the effect of fertility problems on quality of life in different subgroups is in itself important.
Almost half of the studies applied instruments that enable the outcomes to be presented in terms of utilities. Remarkably, however, none of the studies reported quality-of-life outcomes expressed in utilities (which is necessary to be able to include outcomes in cost-utility studies). This implies that in order to be able to properly include the available quality-of-life evidence related to (in)fertility in comparative health economic analyses it is necessary to reanalyse the available data to calculate the outcomes in terms of utilities. This could also allow interesting comparisons of outcomes between the available studies.
Limitations and strengths of the study
A potential limitation of this review deserves attention. Although the search strategy was quite extensive, we might have missed some relevant quality-of-life evidence as a result of methodological choices made, such as excluding multi-disease studies and studies primarily aimed at the evaluation of a specific treatment.
Despite this limitation, some findings are important. Although outcomes were not consistent, evidence seems to indicate that there is a negative association between having fertility problems and quality of life/well-being.
Unanswered questions and future research
How the impact of fertility problems on quality of life relates to other health problems is far from clear. Therefore, it is necessary to better quantify the impact of fertility problems on quality of life and well-being to determine the relative impact compared with that of having other health problems. It is advised that future studies (additionally) include generic quality-of-life/well-being instruments and subsequently report the outcomes in terms of comparable measures. Suitable instruments are, for instance, the EuroQoL 5D, the Health Utility Index and the Short Form 6D. Disease-specific instruments such as FertiQoL may be used to provide additional relevant disease-specific information.
Note that using cost-utility studies to determine the value for money of treating fertility problems is not without dispute. Fertility treatments may be argued to be one of the very few parts of health care not primarily aimed at increasing or maintaining people’s health or welfare. For instance, the value of creating new life may be difficult to grasp in our current economic evaluations, as the future QALYs gained (and the economic value) of the baby born as a result of fertility treatment are commonly not included in cost-effectiveness studies. Another challenging factor is that parenthood (the result of successful fertility treatment) may also have negative effects on quality of life [Citation36]. These aspects are important to consider in fertility-related quality-of-life research.
Conclusions
Quality of life and well-being related to having fertility problems is regularly studied. However, the reported quality-of-life information is not suitable for use in cost-utility studies. There is a clear need for studies investigating the impact of fertility problems on quality of life in a way that outcomes can be compared across studies and disease areas.
Acknowledgments
The authors would like to thank Wichor Bramer for his help in designing the systematic literature search strategy and Job van Exel for his advice with regard to the study design. The authors would also like to Christiaan Veraart for his useful comments and suggestions to earlier drafts of this manuscript.
Disclosure statement
KH and MK report personal fees from Merck BV during the conduct of the study and outside the submitted work. AMMA reports personal fees from Merck BV during the conduct of the study.
Additional information
Funding
References
- Cui W. Mother or nothing: the agony of infertility. Bull World Health Organ. 2010;88:881–882.
- Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356.
- Schmidt L. Psychosocial burden of infertility and assisted reproduction. Lancet. 2006;367:379–380.
- Brouwer WBF, Van Exel NJA, Stolk EA. Acceptability of less than perfect health states. Soc Sci Med. 2005;60:237–246.
- IVF Worldwide (2015). Policy of reimbursement. Available from: www.ivf-worldwide.com/education/introduction/ivf-costs-worldwide/policy-of-reimbursement.html (accessed 15 November 2015).
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynecol. 1993;14:45–52.
- Aduloju OP, Olaogun OD, Aduloju T. Quality of life in women of reproductive age: a comparative study of infertile and fertile women in a Nigerian tertiary centre. J Obstet Gynaecol. 2018;38:247–251.
- Ahmadi SE, Montazeri A, Mozafari R, et al. Health-related quality of life and primi-gravid: a comparative study of natural conception and conception by assisted reproduction technologies (ARTs). Int J Fertil Steril. 2014;8:167–174.
- Amiri M, Chaman R, Sadeghi Z, et al. Quality of life among fertile and infertile women. Iran J Psychiatry Behav Sci. 2017;11:e5641.
- Ashraf DM, Ali D, Azadeh DM. Effect of infertility on the quality of life, a cross- sectional study. J Clin Diagn Res. 2014;8:OC13–OC15.
- Callan VJ. The personal and marital adjustment of mothers and of voluntarily and involuntarily childless wives. J Marriage Fam. 1987;49:847–856.
- De Pascalis L, Agostini F, Monti F, et al. A comparison of quality of life following spontaneous conception and assisted reproduction. Int J Gynaecol Obstet. 2012;118:216–219.
- Direkvand-Moghadam A, Delpisheh A, Montazeri A, et al. Quality of life among Iranian infertile women in postmenopausal period: a cross-sectional study. J Menopausal Med. 2016;22:108–113.
- Drosdzol A, Skrzypulec V. Quality of life and sexual functioning of Polish infertile couples. Eur J Contracept Reprod Health Care. 2008;13:271–281.
- El Kissi Y, Amamou B, Hidar S, et al. Quality of life of infertile Tunisian couples and differences according to gender. Int J Gynaecol Obstet. 2014;125:134–137.
- Graham M. Is being childless detrimental to a woman’s health and well-being across her life course? Women’s. Womens Health Issues. 2015;25:176–184.
- Hassanin IMA, Abd-El-Raheem T, Shahin AY. Primary infertility and health-related quality of life in Upper Egypt. Int J Gynaecol Obstet. 2010;110:118–121.
- Jeffries S, Konnert C. Regret and psychological well-being among voluntarily and involuntarily childless women and mothers. Int J Aging Hum Dev. 2002;54:89–106.
- Johansson M, Adolfsson A, Berg M, et al. Quality of life for couples 4-5.5 years after unsuccessful IVF treatment. Acta Obstet Gynecol Scand. 2009;88:291–300.
- Johansson M, Adolfsson A, Berg M, et al. Gender perspective on quality of life, comparisons between groups 4-5.5 years after unsuccessful or successful IVF treatment. Acta Obstet Gynecol Scand. 2010;89:683–691.
- Klemetti R, Raitanen J, Sihvo S, et al. Infertility, mental disorders and well-being-a nationwide survey. Acta Obstet Gynecol Scand. 2010;89:677–682.
- Kowalcek I, Wihstutz N, Buhrow G, et al. Subjective well-being in infertile couples. J Psychosom Obstet Gynaecol. 2001;22:143–148.
- Masoumi SZ, Garousian M, Khani S, et al. Comparison of quality of life, sexual satisfaction and marital satisfaction between fertile and infertile couples. Int J Fertil Steril. 2016;10:290–296.
- Monga M, Alexandrescu B, Katz SE, et al. Impact of infertility on quality of life, marital adjustment, and sexual function. Urology. 2004;63:126–130.
- Onat G, Kizilkaya Beji N. Effects of infertility on gender differences in marital relationship and quality of life: a case-control study of Turkish couples. Eur J Obstet Gynecol Reprod Biol. 2012;165:243–248.
- Pinar G, Zeyneloglu HB. Quality of life, anxiety and depression in Turkish women prior to receiving assisted reproductive techniques. Int J Fertil Steril. 2012;6:1–12.
- Sani MS, Tamannaeifar M. The comparison of quality of life, self-efficacy and resiliency in infertile and fertile women. Middle East J Fam Med. 2017;15:111–118.
- Schanz S, Hafner HM, Ulmer A, et al. Quality of life in men with involuntary childlessness: long-term follow-up. Andrologia. 2014;46:731–737.
- Sezgin H, Hocaoglu C, Guvendag-Guven ES. Disability, psychiatric symptoms, and quality of life in infertile women: a cross-sectional study in Turkey. Shanghai Arch Psychiatry. 2016;28:86–94.
- Valsangkar S, Bodhare T, Bele S, et al. An evaluation of the effect of infertility on marital, sexual satisfaction indices and health-related quality of life in women. J Hum Reprod Sci. 2011;4:80–85.
- Wischmann T, Korge K, Scherg H, et al. A 10-year follow-up study of psychosocial factors affecting couples after infertility treatment. Hum Reprod. 2012;27:3226–3232.
- Xiaoli S, Mei L, Junjun B, et al. Assessing the quality of life of infertile Chinese women: a cross-sectional study. Taiwan J Obstet Gynecol. 2016;55:244–250.
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483.
- Hsu PY, Lin MW, Hwang JL, et al. The fertility quality of life (FertiQoL) questionnaire in Taiwanese infertile couples. Taiwan. J Obstet Gynecol. 2013;52:204–209.
- Ried K, Alfred A. Quality of life, coping strategies and support needs of women seeking Traditional Chinese Medicine for infertility and viable pregnancy in Australia: a mixed methods approach. BMC Women’s Health. 2013;13:17.
- Kohler H, Mencarini L. The parenthood happiness puzzle: an introduction to special issue. Eur J Population. 2016;32:327–338.