Abstract
Objective: The aim of the study was to examine treatment continuation and satisfaction over 1 year among women receiving nomegestrol acetate (NOMAC)/oestradiol (E2) combined oral contraception (COC) in real-world clinical practice.
Methods: The 17β-Estradiol and Nomegestrol Acetate (BOLERO) Study is an observational, non-interventional, prospective, multicentre cohort study of premenopausal women (aged 18–50 years) who received prescription NOMAC/E2 (2.5 mg/1.5 mg) for contraception during routine clinical practice. Assessments were carried out at enrolment and at 3, 6 and 12 months. Probability of treatment continuation through 12 months (primary outcome) was examined using Kaplan–Meier survival analysis. Secondary outcomes included treatment satisfaction, menstrual cycle-related symptoms, libido and adverse events (AEs).
Results: Of 298 enrolled women, 292 were evaluable. The probability of NOMAC/E2 continuation through 12 months was 73.7% (95% confidence interval [CI] 68.0%, 78.5%). Satisfaction with NOMAC/E2 increased from 56.9% (37/65) of women at initial evaluation to 89.2% (58/65) of women at 12 months. Physician ratings at 12 months showed satisfactory to very satisfactory in 84.0% (168/200) of women. Libido was not affected. Menstrual cycle-related symptoms significantly declined from enrolment (6.04 ± 4.32) to 3 months (3.25 ± 3.05) and 12 months (2.62 ± 2.74; p < .0001). Treatment-related AEs were reported by 38.7% (113/292) of women.
Conclusion: The real-world experience of women receiving NOMAC/E2 indicated very good treatment continuation, high satisfaction and significantly improved menstrual cycle-related symptoms.
摘要
目的:本研究的目的是在真实世界的临床实践中, 研究服用醋酸诺美孕酮(NOMAC)/雌二醇(E2)联合的口服避孕药(COC)的妇女治疗时间在1年以上的持续性和满意度。
方法:17β-雌二醇和醋酸诺美孕酮(BOLERO)研究是一项观察性、非介入性、前瞻性、多中心队列研究, 研究对象为绝经前妇女(18-50岁), 她们在常规临床诊疗实践中接受醋酸诺美孕酮/E2(2.5 mg/1.5 mg)联合治疗进行避孕。分别在入组时及3、6和12个月时进行评估。应用Kaplan-Meier生存分析, 观察治疗持续12个月(主要结局)的可能性。次要结局包括治疗满意度、月经周期相关症状、性欲和不良事件(AEs)。
结果:在298名入组女性中, 292名是可评估的。NOMAC/E2持续12个月的概率为73.7%(95%置信区间[CI] 68.0%, 78.5%)。女性对NOMAC/E2的满意度从最初评估的56.9%(37/65)上升到12个月时的89.2%(58/65)。治疗12个月时的医生评分显示, 84.0%(168/200)的女性对医生的评分从满意变为非常满意, 且性欲没有受到影响。月经周期相关症状从入组(6.04 ± 4.32)明显下降至3个月(3.25 ± 3.05), 直至12个月时为2.62 ± 2.74;p < .0001)。38.7%(113/292)的妇女报告了治疗相关的AEs。
结论:真实世界的接受NOMAC/E2治疗的女性的实际经验表明, 她们的治疗持续性很好, 满意度很高, 并且明显改善了与月经周期相关的症状。
Introduction
Combined oral contraception (COC) continues to be a frequently selected contraceptive method among European women [Citation1]. The evolution in COC formulations to improve safety and tolerability has included ethinylestradiol (EE) and progestin dose reductions, development of new progestins and varied dosing regimens [Citation2]. More recently, COC based on oestradiol (E2), structurally identical to endogenous 17β-E2, has become available [Citation3]. Nomegestrol acetate (NOMAC)/E2 (Zoely; Teva Italia, Milan, Italy), approved by the European Medicines Agency in 2011, is the first monophasic 24/4 day COC regimen to use 17β-E2. It has been shown that E2, micronised to improve bioavailability, has weak estrogenic effects and a mild metabolic impact on estrogen-sensitive hepatic proteins [Citation3]. NOMAC, derived from 19-norprogesterone with almost exclusive binding to the progesterone receptor, has been shown to be metabolically neutral with no androgenic, estrogenic or glucocorticoid activity and with moderate antiandrogenic activity [Citation3].
The efficacy of NOMAC/E2, as well as good menstrual cycle control, with shorter and lighter withdrawal bleedings and absence of withdrawal bleeding for some women, has been demonstrated in randomised, open-label, multicentre trials that compared NOMAC/E2 with a drospirenone/EE 21/7 day regimen [Citation4,Citation5]. NOMAC/E2 has also been associated with reduced premenstrual and menstrual symptoms and menstrual cramping [Citation6,Citation7], as well as improved quality of life [Citation7,Citation8]. NOMAC/E2 contraceptive efficacy was maintained with less stringent back-up requirements following missed pills, likely related to the long half-life of NOMAC (46 h) and the 24/4 day regimen of NOMAC/E2 [Citation4,Citation5]. Consistent with this finding, contraceptive protection is not reduced if the missed NOMAC/E2 active pill is taken <24 h late [Citation9]. Further, compared with levonorgestrel/EE, NOMAC/E2 has demonstrated significantly less haemostatic impact (assessed via evaluation of coagulation and fibrinolysis markers), no alteration of carbohydrate metabolism (assessed using glucose tolerance and insulin resistance) and a neutral effect on lipid metabolism (assessed through levels of triglycerides and low- and high-density lipoprotein cholesterol) [Citation10]. The potential for reduced haemostatic impact is noteworthy given the continued efforts to reduce the risk of venous thromboembolism that is moderately associated with COC treatment [Citation10].
NOMAC/E2 may help address an ongoing need for COC formulations that support treatment continuation, given that concerns about the use of synthetic hormones and associated side effects are frequently reported by women as reasons for COC discontinuation [Citation11,Citation12]. Women have expressed a preference for COC based on natural estrogen, following contraceptive counselling, primarily because of fear of synthetic hormones or a desire for decreased bleeding associated with E2-based COC [Citation13]. To address whether NOMAC/E2 meets these characteristics, the current study examined treatment continuation and satisfaction over 1 year among women receiving NOMAC/E2 for contraception in real-world clinical practice.
Methods
Study design and population
The 17β-Estradiol and Nomegestrol Acetate (BOLERO) Study is an observational, non-interventional, prospective, multicentre cohort study conducted in 17 centres in Italy. Eligible participants were premenopausal women aged 18–50 years, with or without prior COC use, who were prescribed NOMAC 2.5 mg/E2 1.5 mg for contraception during routine clinical practice. Contraceptive treatment selection and consent to study participation were independent decisions. Women received their prescription for NOMAC/E2 ≥ 1 month and <3 months prior to study enrolment. Exclusion criteria included any condition that contraindicated use of COC and age ≥35 years in current smokers. Women were recruited over a 15 month period and were followed for a period of 12 months (13 treatment cycles). Study assessments were carried out at enrolment and at 3, 6 and 12 months. The end-of-study final evaluation was completed before 12 months if a woman discontinued study participation early.
The study was conducted in full conformance with the principles of the Declaration of Helsinki, and fully adhered to the Guideline for Good Clinical Practice, International Conference on Harmonization Tripartite Guideline and local laws. Independent ethics committees of the participating centres approved the study protocol; women provided informed consent prior to participation in the study.
Assessments
The primary efficacy outcome was continuation of treatment, defined as the number of treatment cycles completed over 12 months and assessed using a daily diary. Secondary outcomes included treatment satisfaction, menstrual cycle-related symptoms and libido. Treatment satisfaction was assessed with a 7 point scale ranging from ‘very unsatisfactory’ to ‘very satisfactory’ at 3, 6 and 12 months or at the final study visit. Additionally, treating physicians rated treatment satisfaction at 12 months or at the final study visit using the Clinical Global Impression (CGI) 7 point scale that ranged from ‘very unsatisfactory’ to ‘very satisfactory’. Evaluation of menstrual cycle-related symptoms included headache, breast pain/tenderness, swelling (abdominal swelling and oedema), dysmenorrhoea and mood disturbance. Symptoms were assessed using 5 point scales ranging from ‘absent’ to ‘serious’, and the total symptom score was summed across the individual symptoms. The assessment of menstrual cycle-related symptoms at enrolment was retrospective: women were asked to assess symptoms related to their last three menstrual cycles prior to initiating NOMAC/E2. Additionally, treating physicians rated the degree of improvement in overall menstrual cycle-related characteristics and symptoms at 12 months or at the final study visit using the CGI 7 point scale that ranged from ‘much worse’ to ‘much improved’. Level of libido was rated on a 5 point scale that ranged from ‘very poor’ to ‘very satisfactory’ at 3, 6 and 12 months or at the final study visit.
Safety was examined through reports of adverse events (AEs) from study enrolment to the final study visit. Treatment emergent AEs (TEAEs) and treatment-related AEs were assessed. Physicians rated AEs as unrelated (no reasonable possibility) or related (reasonable possibility) to treatment, and AE severity (mild/moderate/severe) and AE seriousness (yes/no). AEs were coded by MedDRA version 16.0 (www.meddra.org) and used preferred terms.
Data analysis
The primary outcome of continuation of NOMAC/E2 treatment was assessed using Kaplan–Meier survival analysis and is reported as the discontinuation-free probability estimate at day 365 with 95% confidence intervals (CIs). Change in the menstrual cycle-related total symptom score was examined using general linear models for repeated measures with 95% CIs. This analysis used a univariate test for within-subject effects using mixed models (Proc Mixed; SAS Institute, Cary, NC, USA) to fit models with a variety of error covariance matrices and evaluate the patterns of covariance matrices (defined as type H covariances) to satisfy the Huynh–Feldt condition, by applying a sphericity test. Adjustment to numerator and denominator degrees of freedom was used to obtain an unbiased estimate of the effects. Treatment satisfaction, CGI physician ratings and libido were examined descriptively using percentages within response categories. AEs were summarised by the percentage of women experiencing any AE and by individual AEs. Analyses included all enrolled women and were completed using SAS version 9.2 or later (SAS Institute, Cary, NC, USA). Numerical data are expressed as mean ± standard deviation (SD). For all statistical analyses, p-values <.05 were considered significant.
Results
A total of 298 women from 17 centres were enrolled ( and ). The mean age was 29.2 ± 7.4 years, 82.2% (245/298) of women were non-smokers, 11.1% (33/298) were overweight (body mass index [BMI] 25.0–30.0 kg/m2) and 1.7% (5/298) were moderately obese (BMI 30.0–35.0 kg/m2). Previous contraception was reported by 80.5% (240/298) of women, including COC (56.7% [169/298]), barrier contraception (35.9% [107/298]) and natural contraceptive methods (10.7% [32/298]). Mean time from NOMAC/E2 prescription to study enrolment was 44.6 ± 35.0 days. Among the 298 enrolled women, no additional information was available for six women. Accordingly, all subsequent analyses were performed on 292 women. Examination of participant disposition showed that 200/292 women (68.5%) continued study participation through 12 months (), and study completion was similar between women with prior COC use (69.5% [116/167] of women) and women with no prior COC use (67.2% [84/125]). Reasons for early discontinuation of study participation included those unrelated to treatment (17/292 [5.8%]), those related to treatment (51/292 [17.5%]) and those lost to follow-up (24/292 [8.2%]). Conservatively, women lost to follow-up were grouped with those with early discontinuation due to treatment.
Figure 1. Participant disposition. Reasons for discontinuation of NOMAC/E2 included treatment-related AEs, poor compliance, dissatisfaction and decision to change contraceptive method. Discontinuation of NOMAC/E2 that was not treatment-related included no longer needed contraception, scheduled surgery and desire for pregnancy.
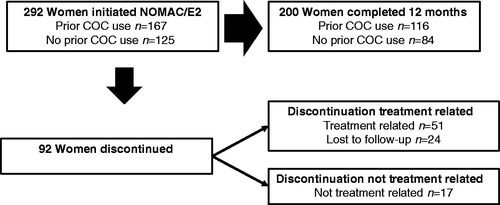
Table 1. Demographic and clinical characteristics of women receiving NOMAC/E2 (N = 298).
Table 2. Menstrual cycle and contraceptive method history of women receiving NOMAC/E2 (N = 298).
Treatment continuation
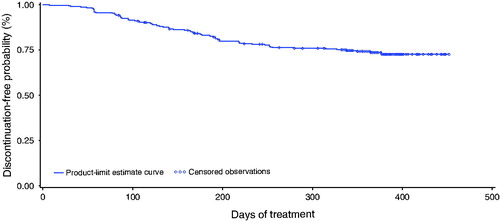
Kaplan–Meier analysis showed that the probability of NOMAC/E2 continuation from enrolment to day 365 was 73.7% (95% CI 68.0%, 78.5%; ). Among women with prior COC use before starting NOMAC/E2, the probability of treatment continuation to day 365 was 74.2% (95% CI 67.8%, 80.6%). Among women with no prior COC use, the probability of treatment continuation to day 365 was 70.7% (95% CI 61.5%, 78.1%).
Figure 2. Probability of treatment continuation for 12 months among women receiving NOMAC/E2. The Kaplan–Meier discontinuation-free probability estimate from enrolment to day 365 was 73.7% (95% CI 68.0%, 78.5%). Censored women (n = 217) included treatment completers (n = 200) and women who discontinued for reasons not related to treatment (n = 17). Discontinuation events (n = 75) included women who discontinued treatment due to treatment-related AEs, poor compliance, dissatisfaction or decision to change contraceptive method (n = 51), and women lost to follow-up (n = 24).
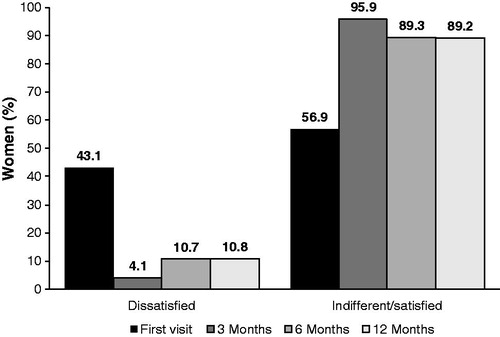
Treatment satisfaction, menstrual cycle-related symptoms and libido
Satisfaction with NOMAC/E2 increased from 56.9% (37/65) of women at the time of initial evaluation performed within 3 months of NOMAC/E2 use to 89.2% (58/65) of women at the final visit performed 12 months after initial evaluation (). Among the 28 women dissatisfied at enrolment, 92.9% (26/28) were satisfied with treatment at the final visit; only 13.5% (5/37) of those satisfied at enrolment were dissatisfied at the final visit. Additionally, physician ratings of each woman’s treatment satisfaction at 12 months showed unsatisfactory in 7.0% (14/200), indifferent in 9.0% (18/200) and satisfactory to very satisfactory in 84.0% (168/200).
Women reported a significant improvement in menstrual cycle-related symptoms (p < .0001) during NOMAC/E2 treatment (). The menstrual cycle-related total symptom rating declined from 6.04 ± 4.32 at enrolment to 3.25 ± 3.05 at 3 months of NOMAC/E2 treatment, and further declined to 2.62 ± 2.74 at 12 months. Women with prior COC use before initiating NOMAC/E2 and women without prior COC use were similar in their report of menstrual cycle-related symptoms at enrolment and decline in symptoms over 12 months with NOMAC/E2 (). Physicians rated cycle characteristics and symptoms at 12 months as worsened in 20% (40/200), unchanged in 14.5% (29/200) and slightly to much improved in 65.5% (131/200) of women.
Table 3. Change from enrolment in menstrual cycle-related symptoms of women receiving NOMAC/E2.
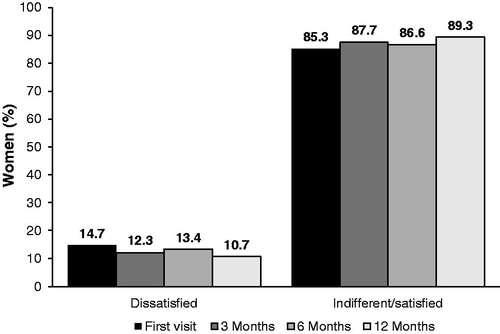
Libido was not affected by NOMAC/E2 treatment. Unsatisfactory libido was reported by 14.7% of women at the enrolment visit and by 10.7% after a follow-up of 12 months ().
Safety
TEAEs were reported by 44.2% (129/292) of women; 38.7% (113/292) of women experienced AEs that were considered possibly treatment-related. The most common treatment-related AEs (reported by 2–13% of women) were metrorrhagia, amenorrhoea, headache, abdominal distension, breast discomfort, mood changes and acne (). Less than 2% of women reported weight gain (n = 4; 1.4%) or decreased libido (n = 2; 0.7%). Two serious AEs were reported, including primary mediastinal large B cell lymphoma, considered unrelated to treatment, and a pregnancy. The pregnancy occurred in a 22-year-old single woman with a BMI 24.2 kg/m2. She reported that her previous use of COC had been interrupted for headache. No information was available on her use of NOMAC/E2 or the pregnancy outcome.
Table 4. Treatment-related AEs reported by ≥2% of women receiving NOMAC/E2 (N = 292).
Treatment discontinuation due to AEs occurred in 31/292 (10.6%) women. The AEs most commonly associated with treatment discontinuation were headache (drug withdrawal headache, n = 1; other headache or migraine, n = 11) and mood changes (n = 7). Treatment discontinuation due to metrorrhagia occurred in 3/292 (1.0%) of women, suggesting that most women with metrorrhagia were experiencing breakthrough spotting or light bleeding.
Discussion
Findings and interpretation
Efficacious and tolerable COC that supports treatment continuation, satisfaction and adherence is essential to prevent COC discontinuation and potential subsequent use of less effective contraceptive methods [Citation11], and further reduce the number of unplanned pregnancies. NOMAC/E2 may be an especially good match for women who prefer COC based on natural estrogen. In the current study, women who selected NOMAC/E2 for contraception during routine clinical practice showed high probability of treatment continuation from study enrolment to 12 months.
Differences and similarities in relation to other studies
Most women reported satisfaction with NOMAC/E2 throughout the study. This was consistent with the significant improvement in menstrual cycle-related symptoms reported by women and their physicians. The significant improvement in menstrual cycle-related symptoms, including headache, breast pain/tenderness, swelling, dysmenorrhoea and mood disturbance, observed at 3 months and with continued improvement to 12 months, is consistent with previous studies examining NOMAC/E2 [Citation6,Citation7]. The high NOMAC/E2 continuation rate is also consistent with the low frequency of AEs resulting in treatment discontinuation. The report of satisfactory libido by most women in the current study is in line with the report of improved sexual function among women who chose to switch to NOMAC/E2 from another COC due to dissatisfaction with sexual desire during their previous treatment [Citation14]. The amenorrhoea experienced by some women is similar to findings from previous NOMAC/E2 studies and is likely related to the long half-life of NOMAC and 24/4 day NOMAC/E2 regimen [Citation4,Citation5].
Concern about side effects is frequently reported by women as their primary reason for COC dissatisfaction and discontinuation, including concern about side effects related to EE and consideration of switching contraception to reduce exposure to hormones [Citation11,Citation12,Citation15–17]. In a real-world clinical practice study of women in Spain, 21.1% of women discontinued COC use for treatment-related reasons, including poor cycle control, side effects, method failure and ‘other’ [Citation18]. In our study of women receiving NOMAC/E2 in routine clinical practice, treatment-related reasons for discontinuation, including AEs or other reasons, was lower at 17.5% of women. Non-treatment-related discontinuation in our study, in 5.8% of women, was similar to a real-world clinical practice study of COC continuation in Spain that reported 6.6% (including women with pregnancy desire and change in sexual habits), whereas our participants lost to follow-up were fewer (8.2% vs 19.3%) [Citation18]. The continuation rate of NOMAC/E2 in our study appears to be comparable to, or higher than, COC continuation rates reported in other population-based cohorts or real-world studies [Citation16–19].
Strengths and weaknesses of the study
Strengths of this study include the real-world examination of a large sample of women receiving NOMAC/E2 for contraception during routine clinical practice. Real-world evaluations of contraceptive outcomes complement the findings of controlled clinical trials because clinical trial participants are often specially selected and may not be representative of the general population of COC users, and clinical trial methods such as free contraception and regular participant follow-up may not accurately reflect COC continuation in real life [Citation19,Citation20]. In our study, women received their prescription for NOMAC/E2 during routine clinical care and study participants independently answered questions about their experience with NOMAC/E2. Because participants were not encouraged to continue NOMAC/E2 or provided with any financial or other support, the effect of study participation on treatment continuation was probably minimal.
Real-world prospective observational studies of clinical practice outcomes also complement findings from retrospective health claims database studies. Database studies provide important information on the use of contraceptive treatment across large populations of women [Citation21]; however, many relevant variables, such as change in menstrual cycle-related symptoms, are not captured in these databases. Such clinical information, necessary to understanding the reasons underlying continuation of COC, as well as potential targets for contraceptive counselling, can be gathered in real-world observational studies.
The current observational study excluded women receiving other COC formulations, which prevented direct comparisons among types of COC. The study findings should be considered preliminary and warrant further comparative investigation of COC continuation and satisfaction among women who select NOMAC/E2 vs EE-based COC.
Conclusion
NOMAC/E2 is the first monophasic 24/4 day COC regimen based on E2, which is structurally identical to the endogenous 17β-E2 that is naturally produced by the ovaries. Previous studies have indicated a preference for COC based on natural estrogen among some women, and improved quality of life among women who switched to COC based on natural estrogen. In our study, the real-world experience of women who were prescribed NOMAC/E2 during routine clinical practice indicated very good treatment continuation and high satisfaction with treatment through 12 months. The non-contraceptive benefits of NOMAC/E2 included significantly improved menstrual cycle-related symptoms of headache, breast pain/tenderness, swelling, dysmenorrhoea and mood disturbance. Our findings provide further evidence that NOMAC/E2 COC meets contraceptive and non-contraceptive needs and supports treatment continuation among women seeking contraception based on natural estrogen.
Acknowledgements
The authors wish to thank Lynanne McGuire, of MedVal Scientific Information Services, for providing medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines’.
Disclosure statement
ACa has served as a speaker for Teva Italia, MSD, Gedeon Richter and Bayer. MV is a consultant for Bayer and Gedeon Richter. GB is an employee of Teva Italia and previously worked for MSD. CBa, MN, ACi, CBe, LC, VDL, EC and AV report no conflicts of interest.
Additional information
Funding
References
- Egarter C, Frey Tirri B, Bitzer J, et al. Women’s perceptions and reasons for choosing the pill, patch, or ring in the CHOICE study: a cross-sectional survey of contraceptive method selection after counseling. BMC Women’s Health. 2013;13.
- Burkman R, Bell C, Serfaty D. The evolution of combined oral contraception: improving the risk-to-benefit ratio. Contraception. 2011;84:19–34.
- Chabbert-Buffet N, Gerris J, Jamin C, et al. Toward a new concept of ‘natural balance’ in oral estroprogestin contraception. Gynecol Endocrinol. 2013;29:891–896.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reprod Health Care. 2011;16:430–443.
- Westhoff C, Kaunitz AM, Korver T, et al. Efficacy, safety, and tolerability of a monophasic oral contraceptive containing nomegestrol acetate and 17β-estradiol: a randomized controlled trial. Obstet Gynecol. 2012;119:989–999.
- Witjes H, Creinin MD, Sundstrom-Poromaa I, et al. Comparative analysis of the effects of nomegestrol acetate/17β-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea. Eur J Contracept Reprod Health Care. 2015;20:296–307.
- Grandi G, Napolitano A, Xholli A, et al. Effect of oral contraceptives containing estradiol and nomegestrol acetate or ethinyl-estradiol and chlormadinone acetate on primary dysmenorrhea. Gynecol Endocrinol. 2015;31:774–778.
- Lete I, de la Viuda E, Perez-Campos E, et al. Effect on quality of life of switching to combined oral contraception based on natural estrogen: an observational, multicentre, prospective phase IV study (ZOCAL Study). Eur J Contracept Reprod Health Care. 2016;21:276–284.
- European Medicines Agency. Zoely 2.5 mg/1.5 mg film-coated tablets. Summary of product characteristics; 2016 [accessed 2016 May 17]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/0012 13/WC500115831.pdf.
- Lete I, Chabbert-Buffet N, Jamin C, et al. Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: a literature review. Eur J Contracept Reprod Health Care. 2015;20:329–343.
- Huber LR, Hogue CJ, Stein AD, et al. Contraceptive use and discontinuation: findings from the contraceptive history, initiation, and choice study. Am J Obstet Gynecol. 2006;194:1290–1295.
- Wigginton B, Harris ML, Loxton D, et al. A qualitative analysis of women’s explanations for changing contraception: the importance of non-contraceptive effects. J Fam Plann Reprod Health Care. 2016;42:256–262.
- Lete I, Barbadillo N, Ugarte L, et al. A cross-sectional study of the choice of oral estrogen contraceptives in women seeking contraceptive counseling: what type of pill do women prefer after being counseled? Gynecol Obstet. 2015;5:100321.
- Caruso S, Cianci S, Cariola M, et al. Improvement of low sexual desire due to antiandrogenic combined oral contraceptives after switching to an oral contraceptive containing 17β-estradiol. J Women’s Health (Larchmt). 2017;26:728–734.
- Hooper DJ. Attitudes, awareness, compliance and preferences among hormonal contraception users: a global, cross-sectional, self-administered, online survey. Clin Drug Investig. 2010;30:749–763.
- Johnson S, Pion C, Jennings V. Current methods and attitudes of women towards contraception in Europe and America. Reprod Health 2013;10.
- Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267–272.
- Lete I, Perez-Campos E, Correa M, et al. Continuation rate of combined hormonal contraception: a prospective multicenter study. J Women’s Health (Larchmt). 2012;21:490–495.
- Moreau C, Bouyer J, Bajos N, et al. Frequency of discontinuation of contraceptive use: results from a French population-based cohort. Hum Reprod. 2009;24:1387–1392.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011;11:CD003553.
- Murphy PA, Brixner D. Hormonal contraceptive discontinuation patterns according to formulation: investigation of associations in an administrative claims database. Contraception. 2008;77:257–263.
Appendix
BOLERO Study Investigators
Dr Luigi Alio, MD, P.O. Civico e Benfratelli di Palermo, Palermo, Italy
Dr Carlo Bastianelli, MD, Dipartimento di Scienze Ostetriche e Ginecologiche e Scienze Urologiche, La Sapienza Università di Roma, Rome, Italy
Prof. Chiara Benedetto, MD, PhD, Dipartimeto di Scienze Chirurgiche, Università degli Studi di Torino, Turin, Italy
Dr Giuseppe Borrelli, MD, Teva Italia Srl, Assago, Milan, Italy
Dr Sandra Bucciantini, MD, Azienda Ospedaliero Universitaria Careggi, Florence, Italy
Prof. Angelo Cagnacci, MD, PhD, Clinica Ginecologica e Ostetrica, Azienda Sanitaria Universitaria Integrata di Udine, Udine, Italy
Dr Luana Calanni, MD, Clinica Ostetrica e Ginecologia, Ospedale Policlinico San Martino, Genoa, Italy
Prof. Salvatore Caruso, MD, Dipartimento Chirurgia Generale e Specialità Medico Chirurgiche, Azienda Ospedaliero Universitaria Policlinico-Vittorio Emanuele, Catania, Italy
Prof. Irene Cetin, MD, Azienda Ospedaliera-Polo Universitario, Milan, Italy
Prof. Antonio Cianci, MD, Dipartimento Chirurgia Generale e Specialità Medico Chirurgiche, Azienda Ospedaliero Universitaria Policlinico-Vittorio Emanuele, Catania, Italy
Prof. Ettore Cicinelli, MD, 2° Unità Operativa di Ginecologia ed Ostetricia, Dipartimento di Medicina e Oncologia (DIMO), Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Bari, Italy
Prof. Vincenzo De Leo, MD, Dipartimento di Medicina Molecolare e dello Sviluppo, Azienda Ospedaliera Universitaria Senese, Siena, Italy
Dr Francesco De Seta, MD, IRCCS Materno Infantile Burlo Garofolo, Trieste, Italy
Prof. Costantino Di Carlo, MD, Azienda Ospedaliera Universitaria Federico II, Naples, Italy
Prof. Lorenza Driul, MD, Azienda Ospedaliera Universitario S. Maria della Misericordia di Udine, Udine, Italy
Dr Manuela Farris, MD, PhD, La Sapienza Università di Roma, Rome, Italy
Prof. Simone Ferrero, MD, Università degli Studi di Genova, Genoa, Italy
Dr Franca Fruzzetti, MD, Azienda Ospedaliero Universitaria Pisana, Pisa, Italy
Dr Antonio Maiorana, MD, P.O. Civico e Benfratelli di Palermo, Palermo, Italy
Prof. Diego Marchesoni, MD, Azienda Ospedaliera Universitario S. Maria della Misericordia di Udine, Udine, Italy
Prof. Carmine Nappi, MD, Azienda Ospedaliera Universitaria Federico II, Naples, Italy
Prof. Rossella Nappi, MD, Fondazione IRCCS, Policlinico San Matteo, Pavia, Italy
Dr Manuela Neri, MD, Clinica Ostetrica e Ginecolocica, Dipartimento di Scienze Chirurgiche, Università degli Studi di Cagliari, Azienda Ospedaliero Universitaria di Cagliari, Policlinico Universitario Duilio Casula, Monserrato, Cagliari, Italy
Dr Francesca Pampaloni, MD, Azienda Ospedaliero Universitaria Careggi, Florence, Italy
Prof. Anna Maria Paoletti, MD, Azienda Ospedaliero Universitaria di Cagliari, Policlinico Universitario Duilio Casula, Monserrato, Cagliari, Italy
Prof. Felice Petraglia, MD, Azienda Ospedaliera Universitaria Senese, Siena, Italy
Prof. Pasquale Scagliola, MD, Azienda Ospedaliera Spedali Civili di Brescia, Brescia, Italy
Prof. Pierluigi Venturini, MD, Università degli Studi di Genova, Genoa, Italy
Prof. Michele Vignali, MD, PhD, Dipartimento di Scienze Biomedicine per la Salute, Università degli Studi di Milano, P.O. Macedonio Melloni, Milan, Italy
Prof. Annibale Volpe, MD, Facoltà di Medicina e Chirurgia Materno-Infantili e dell’Adulto, Università degli Studi di Modena e Reggio Emilia, Modena, Italy