Abstract
Objective
To compare systemic exposure to levonorgestrel (LNG) released from commercially available intrauterine systems (IUSs), a subdermal implant, and oral contraceptives.
Methods
An integrated population pharmacokinetic (popPK) analysis of data from over 3400 individuals in ten clinical studies with six different LNG-releasing contraceptives (four long-acting reversible contraceptives [LARCs: LNG-IUS 8, 12, and 20, initially releasing LNG 14, 17.5, and 20 μg/day, a subdermal implant initially releasing LNG 100 μg/day according to label]; progestin-only pill [POP: LNG 30 μg/day]; and combined oral contraceptive [COC] pill [LNG 100 μg/day and ethinylestradiol 20 μg/day]), was conducted to generate a popPK model. LNG release rates, and total and unbound serum/plasma LNG concentrations with LARCs were estimated over the indicated period of use; maximum (Cmax) and average (Cav) serum LNG concentrations were estimated at steady state for oral contraceptives. Influence of body weight on LNG PK was also investigated.
Results
Serum LNG concentration with LARCs increased with increasing daily LNG release rate, being lowest with LNG-IUS 8, higher with LNG-IUS 12 and LNG-IUS 20, and highest with the subdermal implant (1.7–2.1-times that with LNG-IUS 20). Compared with early serum LNG concentrations with LNG-IUS 20, Cav and Cmax were 1.7- and 4.5-fold higher with POP, and 8.6- and 18-fold higher with COC. Total LNG bioavailability was >97% for the LNG-IUSs and 66–80% with other contraceptives. Serum/plasma LNG concentrations decreased with increasing body weight.
Conclusions
Among the contraceptives examined, COC had the highest and LNG-IUSs the lowest systemic exposure to LNG. Systemic LNG concentration was inversely correlated to body weight.
摘要
目的:比较从市面上有售的宫内系统(IUSs)、皮下埋植剂和口服避孕药中释放出来的全身暴露LNG的情况。
方法:对10项临床研究中超过3400人的数据进行了综合群体药代动力学(popPK)分析, 这些研究包括6种不同的LNG释放避孕药以生成popPK模型(4种长效可逆避孕药[LARCs:LNG-IUS 8、12和20, 最初释放LNG 14、17.5和20ug/天, 根据标签, 最初释放LNG 100ug/天的皮下埋植剂];仅含孕激素的药片[POP:LNG 30ug/天];以及联合口服避孕药[COC]片[LNG 100ug/天和炔雌醇20ug/天])。评估LNG释放率, 以及LARCs的总和及游离血清/血浆LNG浓度, 估计口服避孕药稳态时的最大血清LNG浓度(Cmax)和平均血清LNG浓度(Cav)。研究了体重对LNG PK的影响。
结果:LARCs的血清LNG浓度随LNG日释放率的增加而升高, LNG-IUS 8组最低, LNG-IUS 12和LNG-IUS 20组较高, 皮下埋植剂最高(是LNG-IUS 20组的1.7∼2.1倍)。与采用LNG-IUS 20的早期血清LNG浓度相比, 采用POP的Cav和Cmax分别高1.7倍和4.5倍, 采用COC的Cav和Cmax分别高8.6倍和18倍。LNG-IUS的总生物利用度>97%, 其他避孕药的生物利用度为66-80%。血清/血浆LNG浓度随体重增加而降低。
结论:在所检查的避孕药中, COC的水平最高, 而LNG-IUS的全身LNG暴露水平最低。全身LNG浓度与体重呈负相关。
Introduction
Since the introduction of a combined hormonal contraceptive in 1960 [Citation1], progestins have been used alone and in combination with oestrogens in hormonal contraception [Citation2–4]. Various administration routes with different mechanisms of action have been employed [Citation2]. Levonorgestrel (LNG) is a potent progestin of the 19-nortestosterone class, with gestagenic properties, and is commonly the active ingredient in intrauterine systems (IUSs), subdermal implants, progestin-only pills (POPs), and, together with ethinylestradiol (EE), in combined oral contraceptives (COCs). LNG-releasing IUSs (LNG-IUSs) have a good safety profile and are effective and convenient [Citation5–10]. LNG is released from the IUS directly into the uterine cavity, and acts by thickening cervical mucus and suppressing endometrial maturation [Citation11]. Therefore, a lower daily dosage is sufficient for contraceptive efficacy than with COCs or subdermal implants, which reach their site of action via systemic circulation.
Three LNG-IUSs were included in this analysis, LNG-IUS 8 (Jaydess®/Skyla®, Bayer AG) [Citation12], LNG-IUS 12 (Kyleena®, Bayer AG) [Citation13], and LNG-IUS 20 (Mirena®, Bayer AG) [Citation14]. The newer, lower-dose LNG-IUS 8 and LNG-IUS 12 devices have smaller T-frames than the LNG-IUS 20 [Citation12,Citation13] or devices such as the LNG20 (Liletta®, Allergan, Inc.) [Citation10], and thus use smaller-diameter insertion tubes (LNG-IUS 8 and LNG-IUS 12, 3.8 mm; LNG-IUS 20, 4.4 mm). Smaller sizes may suit women with a narrow cervical canal and/or a small uterine cavity. LNG-IUS 8 and LNG-IUS 12 were evaluated in a 3-year Phase II and a pivotal Phase III study in nulliparous and parous women [Citation5,Citation6]. The Phase III trial was extended, based on residual content analyses at 3 years that suggested LNG-IUS 12 might be effective over 5 years [Citation7], the same period of labelled use as for LNG-IUS 20. As with LNG-IUS 20, Pearl Index data from the Phase III trial showed that the two new low-dose LNG-IUSs were highly efficacious over 3 (LNG-IUS 8) to 5 years (LNG-IUS 12), both having good safety profiles and high continuation and user-satisfaction rates [Citation6,Citation7,Citation15].
In addition to the three LNG-IUSs, a subdermal implant (Jadelle®, Bayer AG) [Citation16], a POP (Microlut®/Norgeston®, Bayer AG) [Citation17], and a COC (Miranova®, Bayer AG) [Citation18], each containing LNG, were included in our analysis. The subdermal implant and POP mainly act by thickening cervical mucus and suppressing endometrial maturation, whereas COCs mainly inhibit ovulation [Citation16]. Ovulation inhibition with subdermal implants and POPs is inconsistent and dose dependent, with ovulation inhibited in 45–85%, and 50% of menstrual cycles with implants and POPs, respectively; POPs also reduce cilial activity in fallopian tubes [Citation19].
This publication presents in vivo release and pharmacokinetic (PK) data from the 5-year Phase III extension study of LNG-IUS 12 [Citation7] and compares them with data for other LNG-IUSs, the subdermal implant, POP, and COC, using an integrated population PK (popPK) analysis to investigate systemic drug exposure associated with each contraceptive. To facilitate this comparison, it is essential to determine serum/plasma concentrations of unbound, pharmacologically active LNG, allowing to exclude factors that can influence PK, such as body weight, serum concentrations of EE, and concentrations of sex hormone-binding globulin (SHBG) [Citation20–23]. This approach provides a uniform method of comparison among different studies and thus yields a comprehensive overview and understanding of LNG PK across different products and routes of administration.
Materials and methods
Data reported for six products were included in the integrated analysis of LNG PK: (1) POP providing LNG 30 µg once daily [Citation17]; (2) COC providing LNG 100 µg/day plus EE 20 µg/day [Citation18]; (3) subdermal implant labelled to release LNG ∼ 100 µg/day 1 month after insertion [Citation16]; (4) LNG-IUS 20, initial LNG release rate of ∼20 μg/day [Citation14]; (5) LNG-IUS 12, initial LNG release rate of ∼17.5 μg/day [Citation13]; and (6) LNG-IUS 8, initial LNG release rate of ∼14 μg/day [Citation12]. Dosing and release-rate information is from the respective product labels. Model development (including data from all administration routes) was based on studies summarised and referenced in Supplementary Table S1.
The popPK analysis integrated data from ten clinical studies and provides a single popPK model of LNG for all six LNG-containing contraceptives. The model was developed based on data obtained with the LNG-IUSs and POP, and thereafter also applied to the COC and the subdermal implant. The model included intravenous data and determined the typical absolute bioavailability of each LNG-containing contraceptive. Details of model development and validation are published elsewhere [Citation24].
In vivo release rates were estimated for the LNG-IUSs and the subdermal implant at the same time points used to estimate individual serum/plasma concentrations. Additionally, average LNG release rates for the LNG-IUSs and the subdermal implant were estimated over 1, 3, and 5 years after placement.
For the three LNG-IUSs and the subdermal implant, individual serum/plasma concentrations of LNG (total and unbound) and SHBG were estimated and compared across the products at representative time points between 24 days and 1825 days (5 × 365; i.e., 5 years) after placement. For POP and COC, individual average (Cav), maximum (Cmax), and minimum (Cmin) serum/plasma concentrations of LNG (total and unbound) and SHBG were estimated on Day 1 and at steady state (Day 21). It should be noted that data included refer to both serum and/or plasma; “serum” and “plasma” are used interchangeably throughout the remainder of the manuscript. Serum concentrations of LNG take around 2–3 weeks to reach steady state after initiation of POP or COC treatment owing to their once-daily administration and their elimination half-life. Thus, comparisons of LNG serum concentration were made between Cav or Cmax at steady state (Day 21) for POP and COC, and concentrations were estimated at Day 25 for the LNG-IUSs and the implant.
A covariate analysis was also conducted to assess the effect of variables such as body weight on the PK of LNG.
Results
In vivo LNG release rates from LNG-IUSs and the implant
Estimated daily in vivo LNG release rates for the three LNG-IUSs and the implant are listed () and compared graphically () over the intended period of use. Average LNG release rates over the first year equated to approximate doses of 8 µg/day for LNG-IUS 8, 12 µg/day for LNG-IUS 12, 20 µg/day for LNG-IUS 20, and 44 µg/day for the subdermal implant (). The shape of the release rate curves for the four products differed (), especially in the first year, during which release rates declined by 56% for LNG-IUS 8, 36% for LNG IUS 12, 13% for LNG-IUS 20, and 28% for the implant. Compared with the first year, the decline in LNG release rates lessened from Year 1 until the end of the indicated period of use for LNG-IUS 8 (7%), LNG-IUS 12 (23%), and the implant (14%); the release rate for LNG-IUS 20 decreased more steadily, declining by 43% from Years 1 to 5 (; ). At the end of the indicated period of use, the daily release rates amounted to 5.5, 7.6, 10.7, and 32.8 µg/day for LNG-IUS 8, 12, and 20, and the subdermal implant, respectively.
Figure 1. Comparison of simulated typical in vivo release rates over the period of use (5 years for subdermal implant, LNG-IUS 20, and LNG-IUS 12, and 3 years for LNG-IUS 8). Solid line: predicted release rate. Limits of shaded area: 5th and 95th percentile of simulations. Simulations are shown for time ≥24 days (few data in the initial phase). IUS: intrauterine system; LNG: levonorgestrel.
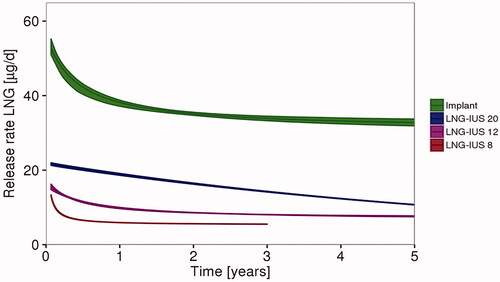
Table 1. Typical model-based estimated in vivo LNG release rates and descriptive statistics of model-based estimated total (and unbound) LNG serum concentrations at representative time points for LNG-IUSs and the subdermal implant, and total (and unbound) Cmax, Cav, and Cmin for POP and COC after Day 1 and Day 21 of oral administration (adapted from [Citation24]).
LNG and SHBG serum concentrations
Estimated serum concentrations of LNG (total and unbound) over time for the LNG-IUSs and subdermal implant were consistent with their respective release rates (). After placement, serum LNG concentrations were lowest for LNG-IUS 8 and increased with LNG-IUS 12 and LNG-IUS 20. The subdermal implant was associated with approximately 2-fold higher LNG serum concentrations over time compared with LNG-IUS 20.
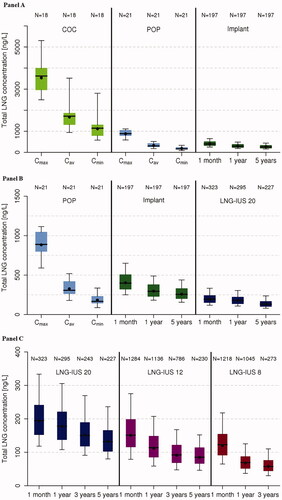
Absolute bioavailability of LNG was estimated to be over 97% for the LNG-IUSs compared with 66–80% for the subdermal implant, POP, and COC (). The daily bioavailable LNG dose with the LNG-IUSs was lower than with the implant, COC or POP both initially and at the end of the intended period of use (). Average LNG exposure (Cav) at steady state with POP was slightly lower than with the implant and approximately 2-fold higher than with LNG-IUS 20 at Day 25. Average exposure to LNG at steady state with COC was 5-fold higher than that with POP, 4-fold higher than with the implant, and nearly 9-fold higher than with LNG-IUS 20 at day 25 ( and ).
Table 2. Estimates of daily bioavailable dose and exposure of LNG among different contraceptive modalitiesTable Footnotea
More pronounced differences were seen when comparing maximum LNG exposure (Cmax) with POP and COC with exposure at Day 25 with the LNG-IUSs and implant (). Maximum LNG exposure with POP was 2-fold higher than with the implant and about 5-fold higher than with LNG-IUS 20; maximum LNG exposure with COC was 4-fold higher than with POP, almost 9-fold higher than with the implant, and 18-fold higher than with LNG-IUS 20. Differences in peak–trough fluctuations between the oral contraceptives, LNG-IUS 20, and the implant are shown in ; LNG serum concentrations of all products are compared in (including descriptive statistics).
Figure 2. Comparison of simulated typical LNG concentrations during the initial phase (A) and towards the end of the fifth year (B) of use (assuming body weight of 65 kg and SHBG baseline concentration of 60 nmol/L). COC: combined oral contraceptive; IUS: intrauterine system; LNG: levonorgestrel; POP: progestin-only pill. COC: 20 µg EE/100 µg LNG; POP: 30 µg LNG; implant release rate: Day 25, 53.0 µg/day; 5 years, 32.8 µg/day; LNG-IUS 20: Day 25, 21.7 µg/day; 5 years, 10.7 µg/day.
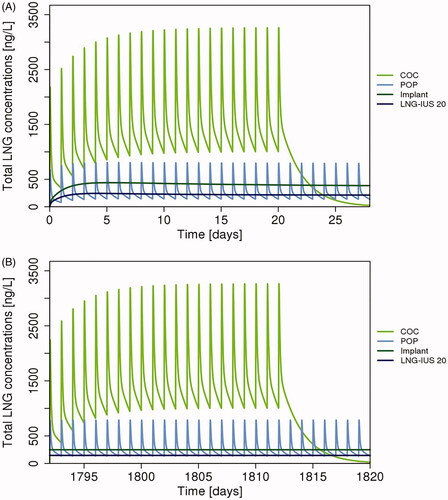
Figure 3. Boxplots of model-based estimated total LNG serum concentrations for: (A) COC and POP (Cmax, Cmin, Cav) at steady state and subdermal implant (1 month, 1 and 5 years); (B) POP (Cmax, Cmin, Cav) at steady state, implant and LNG-IUS 20 (1 month, 1 and 5 years); (C) LNG-IUS 20, LNG-IUS 12, LNG-IUS 8 (1 month, 1, 3, and 5 years). Note: Panel B purposefully includes POP and implant data repeated from Panel A and LNG-IUS 20 data repeated from Panel C in order to provide comparisons on an appropriate scale. COC: combined oral contraceptive; IUS: intrauterine system; LNG: levonorgestrel; POP: progestin-only pill.
For the LNG-IUSs, SHBG concentrations decreased slightly within the first 2 months (LNG-IUS 8, 10%; LNG-IUS 12, 13%; LNG-IUS 20, 17% — typical model-based estimated baseline concentration, 51.5 nmol/L) and subsequently remained relatively constant (Supplementary Table S2). After POP administration, mean SHBG concentrations declined by approximately 20%, from 86.7 nmol/L on Day 1 to 69.0 nmol/L at steady state. For the EE-containing COC, mean SHBG serum concentrations increased by about 60% from 63.2 to 101.0 nmol/L.
Influence of body weight on LNG serum concentrations
The covariate analysis found a significant effect of body weight on the PK of LNG; therefore, total LNG serum concentrations were estimated at different body weights for LNG-IUS 20 after 1-year’s placement, and for POP and COC ().
Figure 4. Simulated total LNG serum concentrations (ng/L) depending on body weight and SHBG baseline concentrations (SBL) for LNG-IUS 20 (1 year after placement), POP and COC (Cav, md). Dark grey shaded area: <min or > max of LNG-IUS 20/POP/COC population. Light grey shaded area: <5th percentile or >95th percentile of LNG-IUS 20/POP/COC population. Solid grey vertical lines: 25th or 75th percentile of LNG-IUS 20/POP/COC population. Dashed grey vertical line: median of LNG-IUS 20/POP/COC population. Cav,md: average serum concentration at steady state; Cy1: serum concentration after 1 year; COC: combined oral contraceptive; IUS: intrauterine system; LNG: levonorgestrel; POP: progestin-only pill; SBL: SHBG baseline concentration; SHBG: sex hormone-binding globulin.
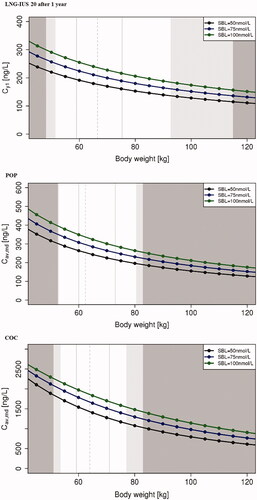
The body weight effect was confirmed for the weight range included in the integrated analysis (48–115 kg for LNG-IUS 20 and 51–83 kg for COC) and was predicted for a broader weight range (45–120 kg). Body weight was identified as a significant covariate of both total clearance and SHBG baseline concentrations with the LNG-IUSs and the implant, and of absolute bioavailability with POP and COC. Clearance increased, and both, SHBG concentrations and absolute bioavailability, decreased with increasing body weight, leading to lower serum LNG concentrations in women with higher body weight.
For LNG-IUS 20, serum LNG concentration was 25% lower in individuals weighing 80 kg (180 ng/L) than in those weighing 55 kg (239 ng/L). POP Cav at steady state was 31% lower in individuals of 80 kg (231 ng/L) than in those of 55 kg (335 ng/L), and COC Cav was 33% lower (1300 ng/L vs 1940 ng/L).
Discussion
Findings and interpretation
Various LNG-containing hormonal contraceptives are available, allowing women to choose the route of administration, duration of use, treatment intervals, mechanism of action, and combination with an oestrogen. These different contraceptive options have distinct differences in systemic exposure to the active progestin (i.e., LNG concentrations in serum/plasma). The present integrated analysis of multiple clinical studies with LNG-containing contraceptives all having good contraceptive efficacy and safety characteristics, indicated that LNG-IUSs are associated with the lowest systemic exposure to LNG among the contraceptive modalities investigated.
In vivo release rates and serum LNG concentrations were estimated over the intended period of use (up to 5 years) for each of the LNG-IUSs and the implant, allowing comparison with daily LNG dosing from oral contraceptives, and with the resulting peak–trough concentrations of serum LNG observed at steady state.
Serum concentrations of LNG associated with each product correspond with the different mechanisms of action each one exploits for contraceptive efficacy. Relatively high systemic concentrations of progestin are needed to cause anovulation, an effect mostly associated with COCs among the products examined here. Subdermal implants and POPs can suppress endometrial maturation and thicken cervical mucus by attaining relatively low systemic concentrations of progestin, and LNG-IUSs can realise the same effects by releasing even less progestin because contraceptive effects are localised to the uterus. Over the 3-year period of use of LNG-IUS 8, systemic concentrations of LNG declined from about 30% to about 22% of the concentrations associated with the subdermal implant over the same period, were <40% of the average steady state LNG concentration with POP, and were always <10% of that with COC.
Accounting for concentrations of SHBG was a key element of our model. In serum, LNG binds both non-specifically to albumin and specifically to SHBG, meaning that only 1–2% of LNG is present unbound, and thus pharmacologically active [Citation21,Citation25]. Therefore, in order to estimate unbound LNG concentrations, total LNG and SHBG concentrations in serum are needed. Concentrations of SHBG in serum are influenced by both LNG and EE, which in turn affect the concentration of total LNG and the fraction of unbound LNG. Concomitant administration of EE (COCs) increases SHBG concentration [Citation26,Citation27], and LNG alone decreases the concentration of SHBG in a dose-dependent manner [Citation20,Citation23]. This dose dependency was seen in our analysis of the three LNG-IUSs, with higher LNG release rates being associated with lower SHBG concentrations. The effect of EE was also evident, being associated with a 60% increase in serum SHBG concentration between baseline and steady state. The LNG dose with COC is approximately 3.3 times that with POP, but the total concentration of LNG at steady state was approximately 5-fold greater with COC than with POP.
Differences and similarities in relation to other studies
Serum LNG concentrations that were estimated for the LNG-releasing devices aligned well with their respective in vivo LNG release rates, and concentrations estimated for LNG-IUS 8 and LNG-IUS 12 were similar to those determined previously in a popPK analysis of Phase III study data (See 14, 15, 28 for LNG-IUS 8 and Supplementary Table S3 for LNG-IUS 12). Similarly, estimated serum LNG concentrations were consistent with previous reports for the other products investigated [Citation16–18,Citation20,Citation29–31], underlining the reliability of the integrated analysis approach. Our analysis also suggests that the LNG release rate determined for the implant at Day 25 in the popPK analysis [Citation14] was lower than that indicated in the product label [Citation16]. This may reflect the small size of the data set for the implant in our analysis compared with the other products, and also the different methods used for bioanalysis and for determining the in vivo release rate.
For the LNG-IUSs and the implant, increased body weight is associated with both decreased serum SHBG and LNG concentrations [Citation32] and increased drug clearence rates, thus reducing serum concentrations of LNG. Our analysis confirmed this, showing an inverse correlation between LNG concentration and body weight. A recent investigation of the pharmacokinetics of COCs in obese women also concluded that increased clearance and distribution led to reduced LNG and EE exposure [Citation33]. These differences in exposure may have little impact on the efficacy of LNG-IUSs, the effects of which are mainly local and, indeed, failure rates in the Phase III trial of LNG-IUS 8 and LNG-IUS 12 were unaffected by body mass index, age or parity [Citation34].
Strengths and weaknesses
The present analysis aimed to provide head-to-head comparisons of systemic LNG exposure for three commonly used LNG-IUSs, a subdermal implant, a POP, and a COC based on one integrated popPK model. The integrated model takes into consideration differences due to administration route (intrauterine, oral, and subdermal), as well as the presence of ethinyl oestradiol in the combined oral contraceptive as well as the effect of factors such as body weight.
As noted in Reinecke et al. there are some limitations that should be considered when interpreting the findings from the PK model. Only a few subjects with body weights close to the lower or upper limit were included, therefore findings for very low or high body weights should be interpreted with caution. Care should also be taken when interpreting absolute bioavailability for the different products as intravenous data were not from the same study and no information on SHBG baseline concentrations was available. However, relevant population characteristics were comparable between the studies. For the implant, results such as the estimated unbound LNG concentrations should be interpreted with particular caution as no SHBG data were available, and data on the LNG concentrations and residual content did not come from the same study.
Relevance of findings
Access to information about in vivo release rates, daily dose for oral contraceptives, and bioavailability across different products can facilitate informed choices among women and clinicians regarding daily systemic drug exposure.
While information on in vivo release rates and serum LNG concentrations can provide useful information when comparing different contraceptives with different routes of administration, it is important to consider other factors such as user adherence and efficacy when discussing method choice. All LNG-based contraceptives are effective if used correctly, but in the real world, COCs and POPs show diminished efficacy with non-compliance [Citation35]. This does not affect LNG-IUSs and hormonal implants, which are independent of user adherence and thus associated with very low Pearl Index scores [Citation12–14]. It is reported that although endometrial changes associated with LNG-IUSs can take several months to develop, thickening of cervical mucus occurs within a few days of placement, so initial failure rates are both low and similar to overall rates [Citation25,Citation36]. As an extra precaution, depending on whether a woman is initiating or switching birth control methods, and the point in her cycle, guidelines typically recommend using a barrier method for up to 7 days after placement [Citation37].
Unanswered questions and future research
Our finding that the LNG release rate determined for the implant at Day 25 in the popPK analysis was lower than that indicated in the product label was unexpected. Further investigation using larger datasets and current bioanalytical methods would be of value to improve the model-based estimations of in vivo LNG release rates for the implant.
Conclusions
Our integrated analysis of six LNG-containing contraceptive products is the first to provide a quantitative head-to-head comparison of systemic exposures for three different LNG-IUSs, a subdermal implant, a POP, and a COC in one popPK model. Effective, reversible contraception is important to women seeking contraceptive advice, but many are concerned about possible long-term side-effects of systemic hormone exposure. Our data indicate that LNG-IUSs are associated with lower systemic exposure to the progestin over their period of use than subdermal implants or daily oral contraceptives, with the latter having the highest exposure. Furthermore, their effectiveness is not susceptible to compliance issues and may be less affected by high body weight than other modalities.
Supplementary Material
Download MS Word (15 KB)Supplementary Material
Download MS Word (16.8 KB)Supplementary Material
Download MS Word (14.5 KB)Acknowledgments
The authors would like to acknowledge Emir Mesic and Henk-Jan Drenth (LAP&P Consultants, BV, Leiden, The Netherlands) for their contributions to the integrated analysis. Editorial support provided by Highfield (Oxford, United Kingdom).
Disclosure statement
Birte Maria Hofmann, Joachim Höchel, Marco Serrani and Martin Merz are employees of Bayer AG, Berlin, Germany.
Isabel Reinecke is an employee of Bayer AB, Solna, Sweden, on behalf of Bayer AG, Berlin, Germany.
Dan Apter's institution received grants from Bayer Pharma AG for the Phase II and Phase III trials of LNG-IUS 8 and LNG-IUS 12. He has also taken part in advisory boards and has been an invited speaker at scientific meetings for Bayer Pharma AG, MSD/Merck, Exeltis, and Gedeon Richter on an ad hoc basis.
Johannes Bitzer has worked as an advisor for and received honoraria from Bayer Health Care, Merck, Teva, Exeltis, Lilly, Boehringer-Ingelheim, Vifor and Gedeon Richter. He has also given invited lectures and received honoraria from Bayer AG, Merck, Johnson and Johnson, Teva, Mylan, Allergan, Abbott, Lilly, Pfizer and Gedeon Richter.
Additional information
Funding
References
- Colton FB. Steroids and “the pill”: early steroid research at Searle. Steroids 1992;57:624–630.
- Royer PA, Jones KP. Progestins for contraception: modern delivery systems and novel formulations. Clin Obstet Gynecol 2014;57:644–658.
- Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord 2011;12:63–75.
- Erkkola R, Landgren BM. Role of progestins in contraception. Acta Obstet Gynecol Scand 2005;84:207–216.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril 2012;97(3):616–622.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol 2013;122:1205–1213.
- Gemzell-Danielsson K, Apter D, Dermout S, et al. Evaluation of a new, low-dose levonorgestrel intrauterine contraceptive system over 5 years of use. Eur J Obstet Gynecol Reprod Biol 2017;210:22–28.
- Eisenberg DL, Schreiber CA, Turok DK, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception 2015;92:10–16.
- Luukkainen T, Allonen H, Haukkamaa M, et al. Five years' experience with levonorgestrel-releasing IUDs. Contraception 1986;33:139–148.
- Costescu DJ. Levonorgestrel-releasing intrauterine systems for long-acting contraception: current perspectives, safety, and patient counseling. Int J Womens Health 2016;8:589–598.
- Lahteenmaki P, Rauramo I, Backman T. The levonorgestrel intrauterine system in contraception. Steroids 2000;65:693–697.
- Bayer HealthCare Pharmaceuticals Inc. Jaydess/Skyla Summary of Product Characteristics; 2019. Available from: https://www.medicines.org.uk/emc/product/5297#gref
- Bayer HealthCare Pharmaceuticals Inc. Kyleena Summary of Product Characteristics; 2019. Available from: https://www.medicines.org.uk/emc/product/769/smpc#gref
- Bayer HealthCare Pharmaceuticals Inc. Mirena Summary of Product Characteristics; 2019. Available from: https://www.medicines.org.uk/emc/medicine/1829#gref
- Apter D, Gemzell-Danielsson K, Hauck B, et al. Pharmacokinetics of two low-dose levonorgestrel-releasing intrauterine systems and effects on ovulation rate and cervical function: pooled analyses of phase II and III studies. Fertil Steril 2014;101:1656–1662.
- Bayer HealthCare Pharmaceuticals Inc. Jadelle Summary of Product Characteristics; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020544s010lbl.pdf
- Bayer plc. Norgeston Summary of Product Characteristics; 2019.
- Bayer HealthCare. Miranova Summary of Product Characteristics; 2015. Available from: https://www.medicines.org.uk/emc/medicine/1834#gref
- McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception 1994;50:S114–S138.
- Fotherby K. Levonorgestrel. Clin Pharmacokinet 1995;28:203–215.
- Kuhnz W, al-Yacoub G, Fuhrmeister A. Pharmacokinetics of levonorgestrel in 12 women who received a single oral dose of 0.15 mg levonorgestrel and, after a washout phase, the same dose during one treatment cycle. Contraception 1992;46:443–454.
- Kuhnz W, al-Yacoub G, Fuhrmeister A. Pharmacokinetics of levonorgestrel and ethinylestradiol in 9 women who received a low-dose oral contraceptive over a treatment period of 3 months and, after a wash-out phase, a single oral administration of the same contraceptive formulation. Contraception 1992;46:455–469.
- Kuhnz W, Schutt B, Woloszczak R. Influence of changes in the concentration of sex hormone-binding globulin in human serum on the protein binding of the contraceptive steroids levonorgestrel, 3-keto-desogestrel and gestodene. J Steroid Biochem Mol Biol 1994;48:573–580.
- Reinecke I, Hofmann B, Mesic E, et al. An integrated population pharmacokinetic analysis to characterize levonorgestrel pharmacokinetics after different administration routes. J Clin Pharmacol 2018;58:1639–1654.
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005;8:3–63.
- Kuhnz W, Blode H, Zimmermann H. Pharmacokinetics of exogenous natural and synthetic estrogens and antiestrogens. Berlin: Springer Verlag; 1999.
- Kalme T, Loukovaara M, Koistinen R, et al. Estradiol increases the production of sex hormone-binding globulin but not insulin-like growth factor binding protein-1 in cultured human hepatoma cells. Fertil Steril 1999;72:325–359.
- Bayer HealthCare AG. NCT00528112 – Cinical Study Report; 2014 April 29 [cited 2019 April 08]. Available from http://trialfinder.bayerscheringpharma.de/html/pdf/91665_Study_Synopsis_CTP.pdf.
- Endrikat J, Blode H, Gerlinger C, et al. A pharmacokinetic study with a low-dose oral contraceptive containing 20 microg ethinylestradiol plus 100 microg levonorgestrel. Eur J Contracept Reprod Health Care 2002;7:79–90.
- Seeber B, Ziehr SC, Gschliebetaer A, et al. Quantitative levonorgestrel plasma level measurements in patients with regular and prolonged use of the levonorgestrel-releasing intrauterine system. Contraception 2012;86:345–349.
- Sivin I, Wan L, Ranta S, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001;64:43–49.
- Koskova I, Petrasek R, Vondra K, et al. Metabolic profile and sex hormone binding globulin (SHBG) in different reproductive phases of Czech women and their relations to weight, body composition and fat distribution. Physiol Res 2009;58:393–402.
- Luo D, Westhoff CL, Edelman AB, et al. Altered pharmacokinetics of combined oral contraceptives in obesity – multistudy assessment. Contraception 2019;99:256–263.
- Gemzell-Danielsson K, Apter D, Hauck B, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low-dose levonorgestrel intrauterine contraceptive systems: subgroup analyses of data from a Phase III trial. PLoS One 2015;10:e0135309.
- Chabbert-Buffet N, Jamin C, Lete I, et al. Missed pills: frequency, reasons, consequences and solutions. Eur J Contracept Reprod Health Care 2017;22:165–169.
- Faculty of Sexual and Reproductive Healthcare. Switching or starting methods of contraception; 2019 August [cited 2019 October 15]. Available from: https://www.fsrh.org/standards-and-guidance/documents/fsrh-ceu-switching-document-feb-2019/.
- Natavio MF, Taylor D, Lewis RA, et al. Temporal changes in cervical mucus after insertion of the levonorgestrel-releasing intrauterine system. Contraception 2013;87:426–431.
