Abstract
Objective
To conduct a secondary analysis of continuation, unwanted effects and cost consequences at 1 year in copper intrauterine device (IUD) users aged under 30 in the European Active Surveillance Study for Intrauterine Devices (EURAS-IUD study) based on IUD type.
Methods
Descriptive and comparative analyses of copper IUD continuation, unwanted effects and estimated cost consequences at 1 year were performed in users aged under 30 based on IUD copper surface area, shape or design, width and arms’ flexibility.
Results
5796 copper IUD users were identified to have been aged under 30 at EURAS-IUD study recruitment and data for 5762 users (99.4%) was analysed. Higher IUD continuation, fewer unwanted effects and lower costs were observed with IUDs of the lowest copper content (<300mm2), horse-shoe frame design, widths 18 mm to <30mm and flexible IUD arms. Discontinuation, unwanted effects and costs were greater with frameless IUDs and framed, ≥30mm width IUDs with 380mm2 of copper and copper bands on their rigid transverse IUD arms.
Conclusions
Significant differences in continuation, reported unwanted effects and estimated costs at 1 year between IUD types were observed in users aged under 30. Although further research is needed, clinicians should consider these findings when counselling and choosing IUD types for younger women.
摘要
目的:根据宫内节育器(intrauterine device, IUD)类型, 对欧洲IUD主动监测研究(EURAS-IUD研究)中30岁以下的带铜IUD使用者1年内的使用持续性、不良反应和成本后果进行二次分析。
方法:根据带铜IUD的铜表面积、形状或设计、宽度和臂的灵活性, 对30岁以下的使用者1年内的使用持续性、不良反应和费用进行描述性和对比分析。
结果:在EURAS-IUD研究中, 发现5796名带铜IUD使用者年龄在30岁以下, 并对5762名使用者(99.4%)的数据进行了分析。使用铜含量最低(<300mm2)、马蹄形设计、宽度18 mm至小于30 mm和臂灵活的IUD, 使用的持续性较长、不良反应较少, 费用也较低。无框IUD, 带框、宽度≥30 mm、有380 mm2铜的IUD, 以及刚性横臂上有铜环的IUD, 停用率、不良影响和成本更高。
结论:30岁以下的IUD使用者在持续使用性、报告的不良反应和1年的费用方面存在显著差异。尽管需要进一步研究, 但临床医生在为年轻女性提供咨询和选择IUD类型时应考虑这些发现。
Introduction
Younger women are choosing to use highly effective contraceptives [Citation1] resulting in lower birth rates [Citation2]. Most births in Europe also now occur in the 30–34 age group [Citation2]. Copper intrauterine devices (IUDs) are among the contraceptive options for these women. Consequently, IUDs with the lowest rates of unwanted effects, discontinuation and cost should ideally be provided. There are many types of IUDs currently available in Europe however no publications identify which of these IUDs are most acceptable to younger women.
The European Active Surveillance Study for Intrauterine Devices (EURAS-IUD study) was a multinational prospective observational study where 61,448 women were provided with a new intrauterine contraceptive [Citation3,Citation4]. More than 30 different copper IUD brands were fitted in 18,370 participants and these brands are still available today. Healthcare professionals (HCPs) from Austria, Finland, Germany, Poland, Sweden and the United Kingdom recruited participants to this study and follow up was for at least one year.
We undertook secondary analyses identifying those IUD users aged 29 or younger at recruitment to the EURAS-IUD study where the primary outcome was continuation at one year and secondary outcomes were the incidence of reported unwanted effects (problems), having to visit a HCP on account of unwanted effects and estimated cost consequences by IUD type.
Materials and methods
Study dataset
Descriptions of the EURAS-IUD study and its full dataset have been previously published [Citation3,Citation4]. For this study, a subset of anonymised one year data on women aged under 30 at the time of copper IUD provision and recruitment to the EURAS-IUD study was analysed. This dataset contained information from both HCPs and IUD users that had been collected from survey questionnaires completed at baseline and one year follow up. Data included country of recruitment, age at recruitment, obstetric history, brand of IUD inserted, reports of any unwanted effects or events (problems including worse bleeding, pain, expulsion, perforation and pregnancy with the IUD), having visited a HCP on account of unwanted effects during IUD use, and if the IUD was still in use at one year follow up (continuation or use status).
The EURAS-IUD study one year follow up questionnaire included the following questions: 'Have you had problems with the IUD?', 'What are they?', 'Did you visit a physician (HCP) because of these problems?', 'Was the IUD removed?', 'Has the IUD fallen out?'… 'Have you been pregnant?', 'Was this pregnancy despite the IUD?'. Women could therefore report more than one problem experienced during IUD use.
‘Worse bleeding’ included reports of spotting; heavier, longer, intermittent, postcoital, and unscheduled bleeding; and any other unacceptable change in menstrual bleeding. ‘Pain’ included any reports that specified: abdominal, genital, back or other pain considered to be related to or arising following the IUD fitting; ‘poking’, ‘pulling’, ‘stinging’, ‘hurting’, ‘cramping’; dysmenorrhoea arising or worsening following the IUD fitting; and dyspareunia. ‘Infection’ included reported ‘infection’, ‘vaginitis’, ‘adnexitis’, ‘endometritis’, ‘inflammation’, pelvic inflammatory disease, urinary tract infection, candidiasis, and bacterial vaginosis. Expulsion included reports of an IUD detected partly or wholly outside of the uterine cavity, warranting removal. Perforation included reports of an IUD detected to have been partially or wholly pierced through the uterus.
Data analysis
Descriptive analyses of the demographics of the total sample and IUD types are reported. All IUDs were grouped according to brand or name stated and categorised into types based on the IUD’s characteristics of copper content, shape or design, width and IUD arms’ flexibility and direction. Where IUD type categorisation was not possible due to missing information, it was described as ‘not specified’ and excluded from the analysis. [] Demographics and IUD types are presented as categorical variables. The primary outcome of IUD continuation or use at one year follow up and secondary outcomes of reported unwanted effects have been presented as binary outcomes based on IUD type. Percentages for all outcomes and their p-values are reported. All p-values have been calculated using the Pearson’s Chi-Square test and Fischer Exact test as appropriate and reported at 5% level of significance.
Box 1. Brands and characteristics of IUDs provided to EURAS-IUD study participants aged under 30.
Cost estimations
Economic modelling to estimate IUD cost consequences at one year based on UK care provision for elements of associated resource use [Citation5–7] and managing unwanted effects [Citation8] was performed. Estimations of costs as a consequence of an IUD type factored in the price of the IUD, the rates of expulsion, discontinuation, pregnancy and perforation, and the incidence of extra visits to a HCP on account of unwanted effects (extra visits excluded rates for expulsion, discontinuation, pregnancy and perforation to avoid double counting or inflating costs).
All IUD provisions were assumed to cost the same, ignoring any cost differences between participating countries and fitting procedures for all IUD types. However, IUD brands differed in price. Hence the average IUD price was calculated per IUD type. The average cost of each IUD type used in the EURAS-IUD study was calculated using the UK British National Formulary [Citation7]. Estimated average IUD prices for the groups of copper content ‘<300mm2’ and ‘380mm2’, ‘unbanded T’ shape or design, ‘≥30mm’ width and IUD arms that ‘flex up’ were slightly higher because these groups included IUD brands like the Nova T380® which cost more (≥£3) than their counterparts. The price of a Gynefix IUD was up to three times the price of some other IUDs that were provided to EURAS-IUD study participants, particularly those IUDs in the group with copper content ‘300 mm2-<380 mm2’. To avoid skewing because of this price difference as well as Gynefix IUDs constituting 1.6% of the IUDs provided and less than 15% of those IUDs in the copper content ‘300 mm2-<380 mm2’ group, the Gynefix IUD price was excluded in the calculation of the average IUD price for the group of IUDs with copper content ‘300 mm2-<380 mm2’.
After their IUD fitting, a user was assumed not to need to visit a HCP unless she wished to get pregnant, needed to remove or replace a time-expired IUD, or experienced a problem (as a consequence of the IUD). Some problems are expected with an IUD, about which the user would have been prior counselled e.g. change in period pattern, heavier and/or more painful periods. These expected problems could resolve spontaneously within the first few months of IUD use or they could only occur to a degree that is tolerated by the user. If problems occurred to a degree that is not tolerated by the IUD user or required intervention(s), then the user would usually be expected to visit a HCP (as a consequence, which will incur additional cost(s) e.g. of a visit to the HCP).
So unwanted effects like bleeding and pain on their own were assumed not to incur additional costs after IUD provision unless the user stated they visited a HCP on account of these problems. Whereas if the IUD had come out (expulsion), the user requested IUD removal for reasons other than to conceive (discontinuation), the user had become pregnant during IUD use (pregnancy), or perforation had been detected, an additional cost after IUD provision was assumed to have been incurred because these unwanted effects usually required extra HCP visit(s) or intervention(s).
Costs included for unwanted effects requiring interventions and extra HCP visits have been based on minimum estimates for healthcare provision in the UK [Citation5,Citation6] and documented care provision to women with complaints during their first year of IUD use who attended a UK sexual health clinic [Citation8]. [] These outcomes were therefore assumed to involve consultations with a HCP (e.g. in a family planning or sexual health clinic) who provided care that included one or more of the following: screening for sexually transmitted infections, pregnancy test, urinalysis, microscopy, transvaginal ultrasound scan, referral e.g. for termination of pregnancy, and provision of an alternative method of contraception [Citation8]. With the assumption that pregnancy with an IUD was unintended, a medical termination of pregnancy was considered the most likely and least expensive option where pregnancy was the outcome. Similarly, an elective laparoscopy procedure as a day case was considered the intervention where perforation was the outcome.
Box 2. Factors and cost estimations as a consequence of IUD provision [Citation5–8]
Monetary amounts in pounds (£) were derived using percentage rates of cost-incurring unwanted effects and extra HCP visits per 100 provisions of each IUD type. These costs for unwanted effects were then combined with the IUD costs to obtain the total costs as a consequence of each IUD type for comparison.
Ethics committee review
Ethical approval was obtained prior to commencement of the EURAS-IUD study. This secondary analysis was exempt from the requirement of further ethical review.
Results
Thirty-three percent of IUD users in the EURAS-IUD study were identified to have been aged under 30 at the time of recruitment (N = 5796) and one year follow up data for 5762 women (99.4%) was included in the analysis (). Majority were 25 to 29 years old (mean age 25.0 years [SD 3.1]), and had previously given birth (66%, n = 3837; mean number of live births 1.6; median [interquartile range, IQR] number of births 1[0–6]). Most IUDs provided were framed (98%, n = 5704) and the Nova T380 was the commonest brand of IUD provided. One-third (32%, n = 1868) of all IUDs were T-shaped IUDs with a copper surface area of 380mm2 and copper bands on their rigid transverse arms (‘gold standard’ IUDs e.g. the CuT380A or TCu380A). Demographic and IUD type information is detailed in . Previous IUD use was reported by 1311 women (22.8%).
Figure 1. EURAS-IUD study participants aged under 30 provided copper intrauterine contraception (IUDs). *Participants could report more than one problem.
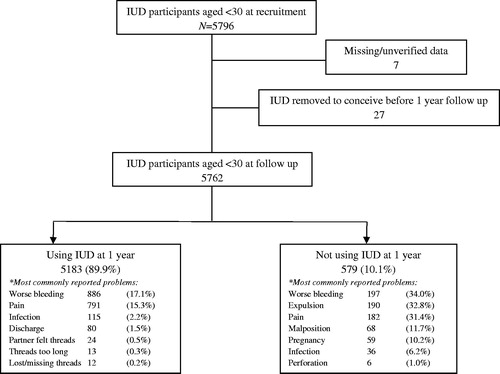
Table 1. Demographics and IUD types of EURAS-IUD participants aged under 30 at recruitment (N = 5796).
Continuation at 1 year was highest in those aged 25–29 years and users of IUDs containing <300 mm2 of copper, IUDs with copper only on the vertical stem or string, and IUDs with flexible arms (). Continuation rates were similar irrespective of previous pregnancy or birth and directly related to age. IUD discontinuation was therefore associated with being of younger age. There was no association between participants’ previous IUD use and continuation at 1 year (p = 0.922).
Table 2. IUD continuation or use status at 1 year follow up* [%, (n)].
More than a third (35.4%, n = 2041) of participants experienced unwanted effects in the first year of their IUD use, including: bleeding 1083 (18.8%), pain 973 (16.9%), expulsion 190 (3.3%), pregnancy 59 (1.0%), and perforation 6 (0.1%). Unwanted effects were lowest for IUDs with <300 mm2 of copper, of horse-shoe design, and width 18 -< 24 mm. Users of IUDs that had 380mm2 of copper, ‘gold standard’ IUDs and frameless IUDs were more likely to report unwanted effects ().
Table 3. Unwanted effects including having to visit a HCP, expulsion and pregnancy at 1 year by IUD type.
Twenty-five percent (n = 1470) had visited a HCP on account of unwanted effects. The incidence of unwanted effects (29%, n = 1503) and having to visit a HCP (17%, n = 891) in those still using their IUD at one year was significantly lower (p = 0.000) than unwanted effects (93%, n = 538) and having to visit a HCP (100%, n = 579) in those who discontinued IUD use ( and ). Further analysis of subgroups is presented in Supplemental online material.
Table 4. Unwanted effects and incidence of visiting a HCP in those participants still using the IUD at 1 year by IUD type.
Table 5. Unwanted effects including having to visit a HCP in those participants not using the IUD at 1 year by IUD type.
For costs estimated as a consequence of the IUD type per 100 provisions, those IUDs of copper content <300mm2, horse-shoe design, widths 18 -< 30mm, and with IUD arms that flex down had the lowest costs while frameless IUDs had the highest costs. ‘Gold standard’ IUDs were associated with the highest cost consequences amongst framed IUD types ().
Table 6. Estimated cost consequences (£) at 1 year per 100 provisions by IUD type.
There were no statistically significant differences between the IUD types regarding incidence of pregnancies in the first year. Eight (13%) of the 59 pregnancies reported were ectopic pregnancies: six in the 25–29 age group and two in the 20–24 age group. Five of the ectopic pregnancies were in ‘gold standard’ IUD users, one in a user of a 380mm2 T-shaped without arm bands IUD, and two using IUDs of copper content 300mm2 -<380mm2, horse-shoe design, width 18 -<24 mm and IUD arms that flex down.
Discussion
Findings and interpretation
Our findings suggest that IUD copper content and size are key factors for unwanted effects, discontinuation and consequent costs at one year in users aged under 30. Greater contraceptive dissatisfaction appeared to be mainly influenced by copper content (either copper distribution on the IUD frame or copper availability in the uterine cavity) and frame compatibility within the uterus (shape/design, width and flexibility). The more localised the copper on an IUD frame and the ability of the frame to conform to the uterine cavity, the more favourable were outcomes at one year. High contraceptive efficacy was maintained with IUDs of copper content <300mm2, suggesting uterine cavity size appeared to also be a factor. The incidence of pregnancy during IUD use was progressively higher with age, having ever been pregnant and ever having a live birth. IUD continuation improving with age could be related to increasing uterine cavity size improving IUD-cavity compatibility; while fewer expulsions associated with IUDs with flexible arms was possibly because their flexibility supported IUD movement within the uterine cavity.
Strengths
This is the first comparison study of its kind to determine which IUD types are more acceptable in younger women. Its findings are specific to younger aged women at a time when there is increasing uptake of intrauterine contraception and less tolerance of unwanted effects. Associations between modern IUD types and unwanted effects have been previously reported [Citation9–11] but not between as many types of IUDs nor in as many younger aged women from routine clinical practice.
Findings from randomised trials particularly those involving blinding tend to be rated highest on the hierarchy of clinical evidence but are not reflective of routine practice, outcomes or costs for clinician decision-making and healthcare provision [Citation12–14]. A number of randomised trials have failed to identify IUD(s) best suited for younger women, therefore an alternative investigative approach is required. This secondary analysis of an existing cohort study provides a low cost approach [Citation15] to support existing data on IUD use in women aged under 30 [Citation16,Citation17].
The EURAS-IUD study’s prospective observational data provides the most recent and largest database of its kind depicting real life experiences with different IUDs. The study obtained responses from both participants and their HCPs. The detailed IUD information enabled categorisations according to IUD copper content, shape or design, width, and arms’ flexibility as well as estimating IUD prices. Outcomes including cost consequences in younger and nulliparous users of different IUDs have never been reported.
Weaknesses
Health care provision and participants’ behaviour, e.g. HCPs’ preferences, IUD types available as well as women’s autonomy and tolerance for unwanted effects, could differ across European countries. These may have influenced the types of IUDs provided, the reporting of unwanted effects, visiting HCPs, and IUD discontinuation. The UK, Germany, Finland and Sweden have similar free provision of IUDs [Citation18]. About half of participants in this study were recruited from the UK and nearly a quarter from Germany. When combined with participants from Finland and Sweden, they made up over 80% of the cohort. Therefore these results may not be applicable to countries where there is a charge for IUD provision or their healthcare costs significantly differ from those of the UK. In this study, there were some IUD categories that had small numbers which may not be representative of those IUD types in larger populations. Groups ‘age 18–19′, ‘never pregnant’ and ‘ever pregnant but no live birth’ also had smaller numbers which may have been insufficient for significant differences between IUD types to be detected within these groups in this study. Other IUDs licenced for use in Europe like the intrauterine ball® and smaller-sized framed IUDs like the Neo-safe 380 Mini® and the Mini TT380 Slimline®, which may be recommended for nulliparous and younger aged women, were not among the IUDs provided to this cohort and hence not included in this study.
Similarities and differences in relation to other studies
Our findings support the existing literature that in younger women intrauterine contraceptives are associated with high continuation and satisfaction rates [Citation19–22] and that unwanted effects increase IUD discontinuation irrespective of parity [Citation23–27]. ‘Gold standard’ and frameless IUDs have higher cost consequences as suggested by more complaints of bleeding, pain and expulsions leading to their higher discontinuation rates [Citation24–26]. IUDs of horse-shoe design and with flexible arms were similarly found to have significantly lower rates of perforation and expulsion [Citation28–30].
Previous studies involving younger women have reported associations between IUD types and outcomes. The 380mm2 copper IUDs had higher rates of discontinuation and complaints of bleeding, pain and expulsions compared to IUDs of lower copper content in studies involving the CuT380A, Cu-safe 300 and Nova T380 [Citation31,Citation32]. Smaller-sized and flexible armed TCu380Ag mini, normal and maxi IUDs had higher continuation rates, fewer expulsions as well as less bleeding and pain when compared with rigid framed TCu380A IUDs in a randomised trial involving 600 parous women (mean parity of 2) aged 20–35 [Citation33]. Continuation rates at 12 months of 84% and 75.8% for the TCu380Ag and TCu380A groups respectively were significantly different, and were highest in TCu380Ag mini IUD users (91.7%) [Citation33]. The rigid framed Gyne T380 Slimline had a significantly higher expulsion rate at one year compared to the flexible armed Nova T380 IUDs in a randomised trial involving 957 mainly parous younger women (mean parity of 2) [Citation34].
Uterine cavity size tends to increase with age, pregnancy and births [Citation35–37]. Significantly more women aged under 30 and of parity ≤2 reported pain with a TCu380Ag IUD (width ≥30mm and rigid frame) than with a Multiload Cu375 IUD (width 18 -< 24mm and flexible arms) in randomised trial involving 1477 women [Citation38]. This finding was similar to other studies involving the CuT380A, Nova T380, Multiload 375 and Cu T-safe 300 [Citation31,Citation39].
‘Gold standard’ IUDs (e.g. CuT380A and TCu380A) have also shown an inverse relationship between age and the incidence of unwanted effects warranting HCP visits, IUD removals and cost consequences. A prospective study of 852 primiparous under 30 year olds had higher pain, bleeding, displacement, expulsion, and IUD removal rates in those 13–19 years compared to those 20–30 years of age, with bleeding the commonest reason for IUD removal [Citation40]. In an earlier study of over 2000 women with the CuT380A IUD, expulsion was the commonest reason for discontinuation at one year and with probabilities of expulsion, pregnancy and discontinuation for bleeding and/or pain higher in those women aged less than 20 years [Citation41]. Significantly higher expulsion rates at one year in women <20 years with the CuT380A IUD were also seen in a similar secondary analysis of data on 2748 women from sites in Africa, Asia and Latin America [Citation42]. IUD evaluations in the US [Citation43–46], of which one was a retrospective review of health insurance claims from 90,489 women [Citation46], have revealed more complaints of dysmenorrhoea and pregnancy as well as a greater likelihood for HCP contact by teenage (<20 years) IUD users compared to those IUD users ≥20 years.
Relevance of findings: implications for clinicians and policymakers
This is the first prospective cohort study to help identify which IUDs best suit women aged under 30 and potential cost savings in IUD provision. It could also improve contraceptive choice, method continuation and overall wellbeing. More research is required to substantiate our findings and this will potentially benefit thousands of younger women in whom rates of contraceptive discontinuation, unintended pregnancies and abortion tend to be higher.
Our study showed expulsion rates as low as 2% with some IUD types and an overall ectopic pregnancy risk of approximately 1 in 1000, reiterating that the IUD is an ideal contraceptive option for younger aged women. It also highlights the need for adequate IUD counselling and HCPs choosing IUD types associated with lower risks of unwanted effects in these women [Citation47]. The recommendation of ‘gold standard’ IUDs being first line needs reconsideration for younger aged and nulliparous women. This could take into account that the ‘gold standard’ IUD’s efficacy appears comparable to IUDs of lower copper content and different designs in these groups of women, and that other risks which may have supported such a recommendation, e.g. infection and perforation, are no more as significant as they were previously.
Open questions and future research
Our study highlights the need for further research into how IUD copper content, design and size affect continuation rates, user experiences and care provision costs as a consequence. Many countries still have only one modern IUD available, which may be possibly hindering research comparing IUD types and improvements to current IUDs.
Conclusion
We observed higher continuation rates, fewer unwanted effects and less associated costs with IUDs containing <300mm2 of copper, of narrow widths and with flexible arms in participants aged under 30 recruited to the EURAS–IUD study. There was no significant difference in failure rates between IUD types irrespective of pregnancy history or parity, and IUD discontinuation was associated with younger age. These findings should support further research into improving IUD outcomes as well as inform clinicians’ counselling and IUD type decision-making with the aim of enhancing continuation, minimising unwanted effects and potentially reducing costs.
Supplemental Material
Download MS Word (91.3 KB)Supplemental Material
Download MS Word (33.8 KB)Supplemental Material
Download MS Word (62 KB)Acknowledgments
We are grateful to: EURAS-IUD study sponsor and researchers Bayer PLC and ZEG Berlin respectively for permissions and providing a subset of the EURAS-IUD study data; Senior Health Economists Ifigeneia Mavranezouli of the University College London/RCOG National Guideline Alliance and Stephen Rice of Newcastle University for their professional advice; and ZEG Berlin’s EURAS-IUD study team and Emeritus Professor John Guillebaud for their valuable comments on the paper.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- United Nations Population Division. Contraceptive use by method 2019: data booklet. New York (NY): Department of Economic and Social Affairs; 2019.
- United Nations Statistics Division. United Nations Demographic Yearbook. Fertility: Table 10 - Live births by age of mother and sex of child, general and age-specific fertility rates: latest available year, 2009–2018. New York (NY): Department of Economic and Social Affairs; 2019.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279..
- Heinemann K, Reed S, Moehner S, et al. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception. 2015;91(4):280–283.
- National Schedule of NHS Costs. 19 National cost collection data. 2018 [Accessed 2020 Aug 28]. Available from: https://www.england.nhs.uk/national-cost-collection/.
- Unit Costs of Health and Social Care. University of Kent, Canterbury: personal social services research Unit (PSSRU); 2019 [Accessed 2020 Apr 21]. Available from: https://www.pssru.ac.uk/pub/uc/uc2019/services.pdf.
- British National Formulary. BMJ Group and the Royal Pharmaceutical Society of Great Britain; 2020 [Accessed 2020 Mar 3]. Available from: https://bnf.nice.org.uk/medicinal-forms/intra-uterine-contraceptive-devices-copper.html.
- Akintomide H, Barnes P, Brima N, et al. Higher discontinuation rate with a standard-sized compared to a small-sized 'gold standard' copper intrauterine device: a case-control review. BMJ Sex Reprod Health. 2019;45(4):263–268.
- O'Brien P. The effects of increasing the copper load on IUD performance: a systematic review. Eur J Contracept Reprod Health Care. 2004;9(Suppl 1):93.
- O'Brien P, Marfleet CC. Frameless versus classical intrauterine device for contraception. Cochrane Database of Syst Rev. 2005;(1):CD003282. DOI:10.1002/14651858.CD003282.pub2
- Kulier R, O'Brien P, Helmerhorst FM, et al. Copper containing, framed intra-uterine devices for contraception. Cochrane Database Syst Rev. 2007;(4):CD005347. DOI:10.1002/14651858.CD005347.pub3
- Mahajan R. Real world data: Additional source for making clinical decisions. Int J Appl Basic Med Res. 2015;5(2):82.
- Cziraky M, Pollock M. Real-world evidence studies. Applied Clinical Trials; 2020 [Accessed 2020 Apr 22]. Available from: http://www.appliedclinicaltrialsonline.com/real-world-evidence-studies?pageID=1.
- Heikinheimo O, Bitzer J, Garcia RL. Real-world research and the role of observational data in the field of gynaecology – a practical review. Eur J Contracept Reprod Health Care. 2017;22(4):250–259.
- NHS Centre for Reviews and Dissemination, University of York. Preventing and reducing the adverse effects of unintended teenage pregnancies. Effective Health Care (Bulletin) 1997;3(1). ISSN: 0965–0288. Available from: https://www.york.ac.uk/media/crd/ehc31.pdf
- Public Health England. Health promotion for sexual and reproductive health and HIV: strategic action plan, 2016 to 2019; 2015. Available from: https://www.gov.uk/government/publications/sexual-and-reproductive-health-and-hiv-strategic-action-plan
- National Institute for Health and Care Excellence. NICE clinical guideline 30: long-acting reversible contraception; 2005 [updated July 2019]. Available from: https://www.nice.org.uk/guidance/cg30.
- Country-by-Country Information. European Consortium for Emergency Contraception (ECEC); 2015 [Accessed 2020 Apr 22]. Available from: https://www.ec-ec.org/emergency-contraception-in-europe/country-by-country-information-2/.
- Friedman JO. Factors associated with contraceptive satisfaction in adolescent women using the IUD. J Pediatr Adolesc Gynecol. 2015;28(1):38–42.
- Sanders JN, Turok DK, Gawron LM, et al. Two-year continuation of intrauterine devices and contraceptive implants in a mixed-payer setting: a retrospective review. Am J Obstet Gynecol. 2017;216(6):590.e1–590.e8.
- Bateson D, Harvey C, Trinh L, et al. User characteristics, experiences and continuation rates of copper intrauterine device use in a cohort of Australian women. Aust N Z J Obstet Gynaecol. 2016;56(6):655–661.
- Dewan R, Bharti N, Mittal A, et al. Early IUD insertion after medically induced abortion. Eur J Contracept Reprod Health Care. 2018;23(3):231–236.
- Jatlaoui TC, Riley HEM, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception. 2017;95(1):17–39.
- Simonatto P, Bahamondes MV, Fernandes A, et al. Comparison of two cohorts of women who expulsed either a copper-intrauterine device or a levonorgestrel-releasing intrauterine system. J Obstet Gynaecol Res. 2016;42(5):554–559.
- Bachofner M, Blickenstorfer K, Hutmacher J, et al. Intrauterine device continuation rates and reasons for discontinuation in a Central European clinic with a high standard of care and ultrasound follow-up: a retrospective cohort study. Eur J Contracept Reprod Health Care. 2018;23(6):407–414.
- Diedrich JT, Desai S, Zhao Q, et al. Association of short-term bleeding and cramping patterns with long-acting reversible contraceptive method satisfaction. Am J Obstet Gynecol. 2015;212(1):50.e1–50.e8.
- Schmidt EO, James A, Curran KM, et al. Adolescent experiences with intrauterine devices: a qualitative study. J Adolesc Health. 2015;57(4):381–386.
- Chi IC. The multiload IUD-a U.S. researcher's evaluation of a European device. Contraception. 1992;46(5):407–425.
- Wilson JC. A New Zealand randomized comparative study of three IUDs (Nova-T, MLCu375, MLAgCu250): 1-, 2- and 3-year results. Adv Contracept. 1992;8(2):153–159.
- Khan SA, Amin Fouzia UZ, et al. A comparative trial of copper T 380 and Cu 375 IUCD. J Ayub Med Coll Abbottabad. 2010;22(3):185–187.
- Nahidi F, Jalalinia S. Comparing the complications of 2 copper intrauterine devices: T380A and Cu-Safe 300. East Mediterr Health J. 2008;14(1):95–102.
- Cox M, Tripp J, Blacksell S. Clinical performance of the Nova T380 intrauterine device in routine use by the UK Family Planning and Reproductive Health Research Network: 5-year report. J Fam Plann Reprod Health Care. 2002;28(2):69–72.
- Kriplani A, Sehgal R, Konar H, et al. A 1-year comparison of TCu380Ag versus TCu380A intrauterine contraceptive devices in India. Int J Gynaecol Obstet. 2019;145(3):268–277.
- Haugan T, Skjeldestad FE, Halvorsen LE, et al. A randomized trial on the clinical performance of Nova T380 and Gyne T380 Slimline copper IUDs. Contraception. 2007;75(3):171–176.
- Kurz KH. Cavimeter uterine measurements and IUD clinical correlation. In: Zatuchni GI, Goldsmith A, Sciarra JJ, editors. Intrauterine contraception: advances and future prospects. Philadelphia: Harper and Row; 1984. p. 142–162.
- Holm K, Mosfeldt Laursen E, Brocks V. Pubertal maturation of the internal genitalia: an ultrasound evaluation of 166 healthy girls. Ultrasound Obstet Gynecol. 1995;6(3):175–181.
- Da Costa AG, Filho FM, Ferreira AC, et al. Uterine volume in adolescents. Ultrasound Med Biol. 2004;30(1):7–10.
- Cole LP, Potts DM, Aranda C, et al. An evaluation of the TCu 380Ag and the Multiload Cu375. Fertil Steril. 1985;43(2):214–217.
- Ragab A, Hamed HO, Alsammani MA, et al. Expulsion of Nova-T380, Multiload 375, and Copper-T380A contraceptive devices inserted during cesarean delivery. Int J Gynaecol Obstet. 2015;130(2):174–178.
- Rasheed SM, Abdelmonem AM. Complications among adolescents using copper intrauterine contraceptive devices. Int J Gynaecol Obstet. 2011;115(3):269–272.
- Escudero F, Gonzales GF, Delgadillo L, et al. Factors associated with discontinuation rates of the copper T380A IUD in a Peruvian public hospital. Adv Contracept. 1999;15(4):303–311.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60(3):155–160.
- Howard B, Grubb E, Lage MJ, et al. Trends in use of and complications from intrauterine contraceptive devices and tubal ligation or occlusion. Reprod Health. 2017;14(1):70.
- Ravi A, Prine L, Waltermaurer E, et al. Intrauterine devices at six months: does patient age matter? Results from an urban family medicine federally qualified health center (FQHC) network. J Am Board Fam Med. 2014;27(6):822–830.
- Alton TM, Brock GN, Yang D, et al. Retrospective review of intrauterine device in adolescent and young women. J Pediatr Adolesc Gynecol. 2012;25(3):195–200.
- Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121(5):951–958.
- Akintomide H. Improving information on intrauterine contraception: providing advice in primary care. Br J Gen Pract. 2019;69(679):98–99.
