Abstract
Background
Spermicides have been identified as a potentially attractive alternative to hormonal contraceptives and/or intrauterine devices. Thus, this study aimed evaluating the efficacy and local tolerance of benzalkonium chloride (BKC) and myristalkonium chloride (MKC) contained in Pharmatex® vaginal formulations and compare them with nonoxynol-9 (N-9), the most common active ingredient in topical vaginal contraceptives.
Methods
Human normozoospermic samples were assessed for motility, viability, acrosome status and penetration ability after exposure to control, N-9 or different BKC and MKC doses for 0 and 10 minutes. Local tolerance on HeLa cells was evaluated by the Trypan-blue and MTT assays.
Results
Exposure to BKC and MKC reduced acrosome integrity while promoting total immobilisation and complete loss of sperm viability (p < .001, n = 15). Both compounds also compromised sperm penetration ability upon exposure (p < .001, n = 15). N-9 induced the same outcomes (p < .001, n = 15); nevertheless, it was more toxic to HeLa cells than BKC and MKC (p < .05, n = 14).
Conclusions
BKC and MKC present strong in vitro spermicidal activity at lower doses than N-9 and were better tolerated after immediate exposure than N-9. Available Pharmatex® galenic formulations were as effective as products based on N-9.
摘要
背景:杀精子剂被鉴定为激素避孕药和/或宫内节育器的潜在有吸引力的替代方案。因此, 本研究旨在评估Pharmatex®阴道制剂中苯扎氯铵(BKC)和肉豆蔻基苄基二甲基氯化铵(MKC)的疗效和局部耐受性, 并将其与壬苯醇醚-9(N-9)进行比较, N-9是局部阴道避孕药中最常见的活性成分。
方法:在暴露于对照、N-9或不同的BKC和MKC剂量0和10分钟后, 评估人精液量正常样品的运动性、活力、顶体状态和穿透能力。通过台盼蓝和MTT测定评估HeLa细胞的局部耐受。
结果:暴露于BKC和MKC降低了精子顶体的完整性, 同时促进了总固定化并完全丧失精子活力(p <0.001, n=15)。两种化合物在暴露时也损伤了精子穿透能力(p <0.001, n=15)。N-9诱导了相同的结果(p <0.001, n=15);然而, N-9对HeLa细胞比BKC和MKC更有毒性(p <0.05, n=14)。
结论:BKC和MKC相比于N-9低剂量的情况下呈现强大的体外杀菌活性, 并且在直接暴露之后耐受性比N-9更好。可用的Pharmatex® 制剂与N-9的产物一样有效。
Introduction
The use of contraceptive methods has considerably increased over the years and nowadays several are available for the different needs of women. With the advent of oral contraception and intrauterine devices, the research on locally applied vaginal chemical contraceptives decreased [Citation1].
Nevertheless, women are starting to prefer non-hormonal methods, avoiding oral contraceptives due to their side-effects and inconvenient daily intake [Citation2]. Additionally, a careful choice must be taken having in mind the specific medical history, her particular risks in case of pregnancy and her own personal preference [Citation3]. To this extent, when well-advised and informed, barrier methods such as spermicides may represent an alternative option for women. Furthermore, given the 44% rate of unwanted pregnancies reported worldwide between 2010 and 2014, the development of woman-controlled contraceptives is of great value, also helping in the prevention of millions of abortions performed annually [Citation4].
Besides being cost-effective and having minimal side effects, topical vaginal spermicides are available over the counter, with no restrictions, and importantly, are woman-controlled [Citation5,Citation6]. The non-ionic surfactant nonoxynol-9 (N-9) is the most common spermicidal ingredient in topical vaginal contraceptive agents [Citation5,Citation6], but adverse consequences to female health were clearly demonstrated [Citation7]. N-9 initially appeared to exhibit antimicrobial and antiviral properties, but evidence has suggested that this compound is less effective and more toxic than what was originally assumed [Citation5,Citation6]. Given that any vaginal contraceptive agent should be both spermicidal and non-toxic to the human cervico-vaginal epithelium, N-9 fell short of this standard by promoting alterations in the natural protective barriers of vaginal microflora and damage to cervical and vaginal mucosa, potentiating the development of sexually transmitted infections (STIs), including the human immunodefiency virus (HIV) [Citation5–7]. Indeed, N-9 has challenged the definition of a non-ionic surfactant, because though displaying a hydrophilic head group, a hydrophobic tail and carry no charge, it was far away from being relatively non-toxic. Cationic rather than non-ionic surfactants were then considered strong candidates for safer vaginal spermicides. These surfactants have a positively charged hydrophilic head that allows both chemical modification and introduction of a desirable moiety according to its specific end, and earlier studies [Citation8,Citation9] have, in fact, suggested cationic compounds as vaginal antiseptics and their possible use without harming the vaginal epithelium.
Benzalkonium chloride (BKC) and myristalkonium chloride (MKC), both cationic surfactants and quaternary ammonium salts [Citation10], were reported to possess antimicrobial and antiviral properties [Citation11–13] and have been used in a series of vaginal commercial forms. Accordingly, the Laboratoire INNOTECH International possesses in its portfolio a wide variety of vaginal contraceptives, ranging from creams and capsules to pessaries and mini-pessaries containing BKC, and tablets containing MKC, under the brand name Pharmatex®. BKC – a mixture of compounds mostly containing 12, 14 or 16 carbon atoms in their carbon chains [Citation14,Citation15] – has been shown to present great spermicidal activity [Citation12–13] while no reports on MKC, a constituent of BKC only comprising carbon chains of 14 carbon atoms [Citation14,Citation15], are available in the literature to our knowledge. The data regarding BKC in terms of toxicity on the cervical-vaginal epithelium is, at this point, somewhat conflicting, ranging from mild to more severe effects, not liable to treatment discontinuance [Citation12,Citation16–20].
In summary, consistent in vitro studies addressing the efficacy and toxicity of both BKC and MKC are lacking. Importantly, these studies should be performed on human cells, as several reports have shown that different resistance levels to spermicides may be species-related [Citation21]. The present in vitro study aimed at mimicking the in vivo situation as much as possible, and by comparison with the untreated control and N-9, (1) address the efficacy of a single dose of the active ingredients BKC and MKC contained in Pharmatex® formulations in human spermatozoa and (2) determine their safety on HeLa cells, a human cell line that mimics the human vaginal/cervical epithelium. N-9 was used in the present study as a positive control.
Material and methods
Reagents
Reagents were supplied by Sigma-Aldrich (St. Louis, MO, USA), unless otherwise indicated. Both BKC and MKC were provided by Laboratoire Innotech International S.A.S. (Paris, France) while N-9 was purchased from Abcam (Cambridge, UK). Pharmatex® galenic preparations (pessaries, mini-pessaries, capsules, cream and tablets), as well as the competitive reference product nonoxinol 120 mg vaginal suppositories from AMCAPHARM Pharmaceutical GmbH were supplied by the sponsor for complementary purposes.
To mimic the in vivo situation as much as possible, BKC, MKC and N-9 concentrations were chosen having in mind (1) their quantity in the commercially available Pharmatex® vaginal products and nonoxynol 120 mg vaginal suppositories and (2) the highest volumes of vaginal fluid from healthy donors that according to the available literature can reach to 8.0 mL/day depending on cycle and arousal [Citation22,Citation23]. The concentrations are further given in .
Table 1. Active principle concentrations to which spermatozoa and HeLa cells were exposed to.
The present study was performed in compliance with the OECD Principles on Good Laboratory Practice and subsequent OECD consensus documents, directive 2004/10/EC of the European Parliament and of the Council of 11 February 2004 and codified version of the Portuguese decrew-law n° 99/2000 May 30.
In vitro efficacy study
15 Normozoospermic samples according to the World Health Organisation (WHO) criteria [Citation24] from 15 men negative for HIV-1, HIV-2, Hepatitis B and Hepatitis C were isolated by density gradient centrifugation (500 g × 10 minutes; 80/40 SupraSperm® gradient medium, Origio, Medicult, Jyllinge, Denmark) and allowed to capacitate in Sperm Preparation Medium (SPM®, Origio, Medicult) for at least 3 h at 37 °C and 5% CO2. Sperm cells were then incubated with a specific phosphate buffered saline (PBS) (Oxoid, UK) -supplemented medium containing 0.5 mM MgCl2, 0.9 mM CaCl2, 5.0 mM D-glucose, 1.0 mM Na-pyruvate, 10.0 mM (v/v) Na-lactate, 25.0 mM NaHCO3 and 1.0% (v/v) Pen/Strep (Gibco, ThermoFisher Scientific, UK) with different concentrations of BKC, MKC and N-9 () for up to 10 minutes at 37 °C and 5% CO2. PBS- supplemented medium alone was also used as negative control.
Motility and viability evaluation
Sperm motility was assessed either by phase-contrast microscopy (200x–400x magnification, Nikon Eclipse E200, Nikon Instruments Inc., Melville, NY, USA) in accordance with WHO guidelines [Citation24] or by the Sander-Cramer test [Citation25]. While in the former, at least 200 spermatozoa per condition, in duplicates, were counted and results presented as percentage of motile cells, in the latter, a dichotomous response is given: positive if 100% of cells become immotile in 20 seconds or negative, if not. This was the only test performed at 20 seconds of exposure and duplicates were also performed.
Sperm viability, on the other hand, was evaluated by the eosin exclusion test as previously described [Citation24,Citation26]. At least 200 sperm cells were analysed per condition, in duplicates, by phase-contrast microscopy (400x magnification; Nikon Eclipse E200). Results are presented as percentage of live, unstained, sperm.
Acrosomal status assessment
Acrosome integrity was evaluated using the acrosomal content marker Pisum sativum agglutinin linked to fluorescein isothiocyanate (PSA-FITC), as stated elsewhere [Citation26,Citation27]. At least 200 spermatozoa were counted per condition, in duplicates, using a fluorescence microscope (630x–1000x magnification; Leica DM4000B, Wetzlar, Germany). Sperm acrosome was considered intact when exhibiting a bright green homogeneous fluorescent staining, while spermatozoa displaying either no fluorescence in the acrosomal region, heterogeneous spots of fluorescence, or a single band at the equatorial segment were considered acrosome-reacted. Results are presented as percentage of intact acrosomes.
Sperm penetration ability evaluation by cervical mucus mimicking
To address the ability of spermatozoa to penetrate cervical mucus while being exposed to BKC, MKC and N-9, the capillary test – originally designed by Kremer in 1965 [Citation28] – was performed, with slight modifications to allow for an evaluation of shorter times of exposure. Briefly, BKC, MKC or N-9 were added to the solution of methylcellulose dissolved in the PBS-supplemented medium described above and 5 cm flattened capillary tubes were further filled by capillary action. Open-ended capillary tubes were placed in eppendorfs containing 1 × 106 spermatozoa for 0 or 10 minutes at 37 °C and 5% CO2 and then observed under phase-contrast microscopy (100x magnification, Nikon Eclipse E200). Sperm migration distance, penetration density and migration reduction were determined [Citation24], and sperm penetration ability was then classified as ‘poor’ if migration distance <1 cm, highest penetration density (number of sperm at 4.5 cm minus at 1 cm) equals zero and migration reduction equals zero, or ‘fair’ if any other combination have occurred. Each condition was carried out in duplicates.
In vitro safety study
Given their suitability in mimicking the human vaginal/cervical epithelium, HeLa cells (American Type Culture Collection, Manassas, USA) [Citation9,Citation25] were incubated in a Dulbecco’s modified Eagle’s medium (DMEM)- containing 1.0% (v/v) Na-pyruvate, 1.0% (v/v) aminoacids and 1.0% (v/v) Pen/Strep with BKC, MKC or N-9 at 37 °C and 5% CO2. Tolerance to the compounds was then addressed by both the Trypan blue exclusion test and the MTT assay. The vehicle DMEM-supplemented medium was used as a negative control.
Trypan blue exclusion test
HeLa cells were washed with PBS and gently mixed with the trypan blue solution (1:1) and observed under an inverted bright field microscope (100x magnification, Leica DMI3000B). At least 200 cells were scored per condition, in duplicates. Results are presented as percentage of viable (unstained) cells in relation to the untreated control, i.e., (% of unstained cells after exposure to the compound/% of unstained cells after exposure to the negative control) × 100.
MTT assay
The cytotoxicity of BKC, MKC and N-9 was also assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [Citation29,Citation30]. Cells were incubated with 0.5 mg/mL MTT for 90 minutes at 37 °C and 5% CO2.and the resultant formazan crystals were dissolved in 0.04 N acidified isopropanol. Solvents were measured at 570 nm and 620 nm. An internal MTT control with no cells was also carried out. Each condition was carried out in duplicates and results are presented as the percentage in relation to the untreated control, i.e., [(delta value with the compound – delta value with MTT internal control)/(delta value with the untreated control – delta value with MTT internal control)] × 100.
Statistical analysis
Statistical analysis was carried out using the SPSS software version 24.0 for Windows. Sample sizes for spermatozoa and HeLa cells experiments were estimated by power analysis using a power value of at least 80% to detect a 10% difference in the proportion of viable cells between groups, a significance below 0.05 (p < .05) and confidence intervals of 95% as criteria. Normal distribution was checked for all variables using the Shapiro Wilk test and comparisons between groups were carried out by the Kruskall-Wallis and the Mann-Whitney tests. Results are expressed as median and interquartile range.
Moreover, for the ‘positive’ or ‘negative’ nominal data used for the Sander-Cramer test and the ‘poor’ or ‘fair’ classification upon sperm penetration ability assessment, the Chi-Square and Fisher tests were used. p ≤ .05 were considered statistically significant.
Results
In vitro efficacy study
Sperm motility and viability
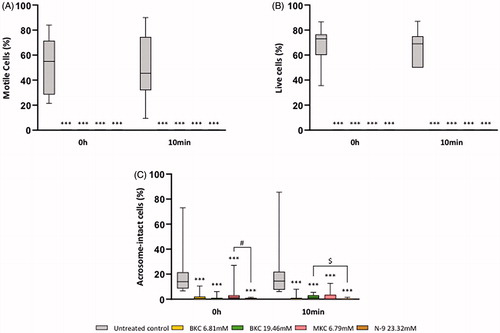
BKC, MKC and N-9 completely immobilised human spermatozoa upon immediate contact and following 10 minutes of exposure, being significantly different from the untreated control at both times (, p < .001, n = 15). When BKC and MKC were compared with N-9, no differences were determined at both timepoints (, p > .05, n = 15). In accordance, the Sander-Cramer test inequivocally showed that all 15 samples treated with the untreated control were negative but positive when treated with N-9, BKC and MKC. Indeed, all active ingredients significantly induced a different response from the untreated control (p < .001, n = 15), whereas no differences were found between the BKC, MKC and N-9 (p > .05, n = 15). In parallel with the loss of motility, sperm viability was also drastically affected (). Independently of the duration of exposure, spermatozoa completely lost their viability upon incubation with both BKC and MKC, something that was also observed with N-9 (, p < .001 in relation to the untreated control, n = 15). Yet, as expected, no differences were noticed between BKC, MKC and N-9 (, p > .05, n = 15). In support of these findings, the exact same outcomes were observed for both parameters when spermatozoa were exposed to the actual galenic formulations to which they would contact with in an in vivo scenario (Supplementary figure 1A–C). Importantly, although the doses in Pharmatex® forms were lower than in nonoxinol 120 mg vaginal suppositories, they retrieved the same outcomes, and no differences were detected when comparing any Pharmatex® formulation with nonoxinol 120 mg vaginal suppositories (Supplementary figure 1A and C, p > .05, n = 15), showing the same effectiveness. In accordance, the Sander-Cramer test showed that regardless the formulation used, all samples were found positive, while the same samples exposed to the untreatead control were negative (Supplementary figure 1B, p < .001, n = 15).
Figure 1. Sperm motility (A), viability (B), and acrosome integrity (C) following exposure to either the untreated control, BKC, MKC or N-9. Results are presented as percentage of (A) motile cells (i.e., progressive + in situ), (B) live, unstained cells and (C) intact acrosomes. *** represents significant differences at p < .001 when compared to the untreated control. # represents a significant difference at p < .01 between N-9 and MKC. $ represents a significant difference at p < .05 between N-9 and 19.46mM BKC.
Sperm acrosome status
Only sperm cells with an intact acrosome are capable of fertilising an oocyte. Yet, BKC, MKC and N-9 were found to decrease the percentage of spermatozoa with intact acrosomes upon immediate exposure and after 10 minutes of treatment when compared with the untreated control, suggesting a premature induction of acrosome reaction (, p < .001, n = 15). Moreover, when BKC and MKC were compared to N-9, no significant differences were noticed, except between MKC and N-9 upon immediate exposure (, p < .01, n = 15), and between the highest BKC dose and N-9 following 10 minutes of treatment (, p < .05, n = 15). Yet, these differences were not observed when comparisons between Pharmatex® formulations and nonoxinol 120 mg vaginal suppositories were performed (Supplementary figure 1D, p > .05, n = 15). Nevertheless, as observed for the active ingredients, all Pharmatex® galenic formulations and AMCAPHARM’s nonoxinol 120 mg vaginal suppositories were found to substantially decrease the percentage of spermatozoa with intact acrosomes when compared with the untreated control following immediate contact and after 10 minutes of exposure (Supplementary figure 1D, p < .001, n = 15).
Sperm penetration ability
To address sperm penetration ability, 3 parameters were analysed: migration distance, penetration density and migration reduction.
Migration distance
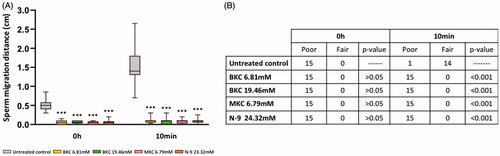
A higher migration distance was noted at time zero in spermatozoa exposed to the untreated control when compared to those exposed to the active ingredients, a difference further enhanced following 10 minutes of exposure (, p < .001, n = 15). Furthermore, no statistical differences between N-9, BKC and MKC were noticed either after immediate contact or 10 minutes of exposure (, p > .05, n = 15).
Figure 2. Sperm penetration capacity assessment following exposure to either the untreated control, BKC, MKC or N-9. (A) Sperm migration distance, (B) number of human sperm samples classified as ‘poor’ or ‘fair’ for penetration ability. ***Represents significant differences at p < .001 when compared to the untreated control.
Penetration density and migration reduction
As expected, no sperm cells were detected at 1.0 cm and 4.5 cm in any experimental condition analysed upon immediate exposure, meaning no sperm penetration density. Thus, the migration reduction (difference between ranks obtained for the number of spermatozoa at 1.0 cm and 4.5 cm) was equal for all conditions following immediate contact (p > .05, n = 15).
However, following 10 minutes of exposure a higher number of sperm cells per field were observed at 1.0 cm upon exposure to the untreated control in relation to MKC, BKC and N-9 (5.01 ± 1.99 vs 0.00 ± 0.00 for; p < .001, n = 15). Consequently, the rank at 1 cm for the untreated control was higher than the rank at 1 cm for BKC, MKC and N-9. Furthermore, as the rank at 4.5 cm was always the same in all the tested conditions (given that 10 minutes is not enough time for sperm to reach the 4.5-cm mark even in the untreated control group), it was not surprising to observe a higher migration reduction in the untreated control. Moreover, as BKC, MKC and N-9 behaved similarly, no differences in sperm penetration density and migration reduction were determined after 10 minutes (p > .05, n = 15).
Overall classification
While upon immediate exposure the same ‘poor’ classification was attributed to all sperm samples tested either with the untreated control or any active ingredient (, p > .05, n = 15), the 10-minute exposure showed significant differences in classification between the untreated control and all the active ingredients, meaning that sperm cells exposed to BKC, MKC and N-9 presented a worse penetration ability than spermatozoa incubated with the untreated control (, p < .001, n = 15). No differences were detected between N-9 and the active ingredients MKC and BKC following 10 minutes of exposure (, p > .05, n = 15).
In vitro safety study
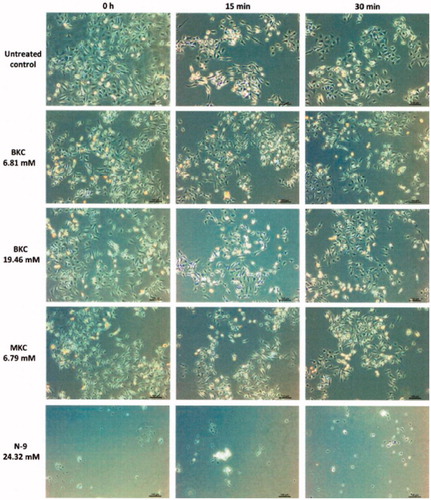
Either using the Trypan blue or the MTT tests, BKC, MKC and N-9 strongly promoted HeLa cell toxicity at all times. In fact, a rough visual analysis under an inverted phase-contrast microscope was sufficient to initially determine clear signs of toxicity such as HeLa cells detachment and floating cell clusters upon N-9 exposure at any time of exposure, and some sort of MKC-induced cell vacuolisation, similar to a dotty aspect, already at time zero (). Only BKC doses were the ones that upon a quick evaluation did not show differences in comparison to the untreated control (), but found to induce toxicity as well, possibly via different and undisclosed mechanisms of action. In reality, as determined by the Trypan Blue test, all HeLa cells lost their viability upon exposure to both BKC and MKC at all concentrations, which was also observed for N-9 (data not shown; p < .001, n = 14). Indeed, the same findings were obtained in experiments with all Pharmatex® and AMCAPHARM’s galenic formulations to which HeLa cells would be in contact with, supporting our results (data not shown; p < .001, n = 14).
Figure 3. Representative image of HeLa cells after exposure to either the untreated control or the active principles BKC, MKC and N-9. Cells were roughly observed under an inverted phase-contrast microscope. Cell detachment was observed upon N-9 exposure as shown by the lower quantity of cells present at the bottom of the multiwell plate and floating cell clusters. Dotty spots indicative of some sort of cell vacuolisation was found upon MKC treatment. These alterations were not present in the untreated control.
Additionally, the same pattern of response was noticed with the MTT test when all active ingredients were compared to the untreated control (, p < .001, n = 14), an outcome also detected when HeLa cells were exposed to all Pharmatex® forms and nonoxinol 120 mg vaginal suppositories (Supplementary figure 2, p < .001, n = 14). However, in sharp contrast to the findings observed with the Trypan blue test, N-9 was found to promote more toxicity than both BKC and MKC upon immediate exposure (, p < .001, n = 14). Statistical differences were also detected between Pharmatex® formulations and nonoxinol 120 mg vaginal suppositories (Supplementary figure 2). Indeed, a more toxic effect was detected with nonoxinol 120 mg upon immediate exposure in comparison with Pharmatex® pessary, mini-pessary and cream (Supplementary figure 2, n = 14).
Figure 4. HeLa cells toxicity determined by the MTT test following exposure to either the untreated control, BKC, MKC or N-9. Results are presented as the percentage in relation to the untreated control, i.e [(delta value with the active ingredient – delta value with MTT internal control)/(delta value with the untreated control – delta value with MTT internal control)] × 100. ***Represent significant differences at p < .001 when compared to the untreated control. & represent significant differences at p < .001 in comparison with N-9.
![Figure 4. HeLa cells toxicity determined by the MTT test following exposure to either the untreated control, BKC, MKC or N-9. Results are presented as the percentage in relation to the untreated control, i.e [(delta value with the active ingredient – delta value with MTT internal control)/(delta value with the untreated control – delta value with MTT internal control)] × 100. ***Represent significant differences at p < .001 when compared to the untreated control. & represent significant differences at p < .001 in comparison with N-9.](/cms/asset/496a22b5-6f7a-4d47-999f-aa52c0513fe9/iejc_a_1900563_f0004_c.jpg)
Discussion
Findings and interpretation
Using a very robust sample size not often observed in this type of in vitro studies, we herein report for the first time that N-9, BKC and MKC at the concentrations used herein display spermicidal activity by interfering with human sperm motility, viability and acrosome status, besides strongly affecting sperm penetration ability in a matrix mimicking midcycle female cervical mucus. Indeed, it has long been reported that N-9, the most often used spermicide in vaginal contraceptives [Citation5–7], is able to disrupt sperm plasma membrane and induce loss of acrosome integrity, completely immobilising spermatozoa and promoting viability loss upon immediate/short contact [Citation31]. This non-ionic surfactant and the cationic surfactants BKC and MKC were found to have spermicidal activities that are not statistically different from each other, possibly acting above their critical micellar concentration (concentration above which surfactant molecules associate with phospholipids, creating ‘‘holes’’ and disrupting the cell membrane), thus producing detergent-unspecific cell damage. BKC and MKC were indeed able to produce a complete loss of motility and viability. Importantly, both BKC and MKC induced these effects at lower concentrations than N-9, which was also observed with all Pharmatex® galenic preparations tested (pessary, mini-pessary, capsule, cream and tablet).
Sperm acrosome integrity was also sharply affected by BKC and MKC, suggesting that even if no effects in sperm motility and viability were found, premature acrosome reaction would certainly prevent pregnancy. Indeed, no sperm cells possess fertilising ability in vivo if their acrosome reacts before being in the vicinity of the oocyte. Although it seems that N-9 may induce more acrosome reaction when compared with BKC and MKC at specific time points, this was not the case when galenic formulations were compared. Indeed, Pharmatex® and AMCAPHARM’s galenic preparations were found similar and a previous study performed by our group has inclusively showed that when compared with a formulation containing a lower quantity of N-9 (75 mg), Pharmatex® mini-pessary at this dose more severely affected acrosome integrity, inducing premature acrosome reaction [Citation32], suggesting that this difference among N-9 and both BKC and MKC may not be so significant. Furthermore, attesting their great spermicidal activity in vitro, the Sander-Cramer test also revealed a complete immobilisation of spermatozoa upon exposure in all sperm samples used.
Moreover, if sperm cells enter the cervical mucus immediately upon ejaculation, instead of moving in the surrounding vaginal and cervical secretions, the vaginal contraceptive agent may not contact with spermatozoa, which may lead to a potential failure of the contraceptive method. We found that both sperm migration distance and sperm penetration ability were severely affected upon exposure. Importantly, while after exposure to the untreated control sperm migrated as expected, in both BKC and MKC groups sperm migration did not reach 0.5 cm in any timepoint addressed, and were not expected to migrate more after 10 minutes as they presented no motility at this point (data not shown). Indeed, this same pattern was obtained with N-9, showing the efficacy of these compounds as contraceptive agents even when dissolved in a viscous cervical mucus-mimicking matrix, which is not surprising since BKC has been reported to penetrate and thicken cervical mucus [Citation12,Citation33], explaining the short sperm migration distance and poor penetration ability. The effects of MKC on in vitro sperm penetration ability, which were not known up to now, seem to be similar to those observed for BKC.
The tolerance of any potential vaginal contraceptive to human cervicovaginal epithelium is another aspect that should be taken into consideration in this type of studies. While being tremendously effective in compromising human sperm function, N-9, BKC and MKC were found to have an impact on HeLa cells, as were Pharmatex® galenic preparations, an outcome attributed to the detergent nature of the compounds. As in sperm cells, the experiments performed with this suitable human adherent epithelial cell line that mimicks the vaginal/cervical epithelium [Citation9,Citation25] were performed in a great sample size, thus allowing no margin for data misinterpretations.
Indeed, many studies have reported that BKC can affect HeLa or other cells in vitro [Citation17,Citation34,Citation35] as well as promoting vaginal damage/disruption in vivo. Using the MTT assay, which is a finer assay than the Trypan blue test and does not solely rely in the human eye, we were able to determine subtle but significant differences between active ingredients, with N-9 being more harmful to HeLa cells upon immediate contact. With regards to N-9, its toxicity is well described and allows no margin for errors, apparently being far worst than BKC and MKC. Despite the fact that AMCAPHARM’s nonoxinol 120 mg vaginal suppository has never been studied before, the high quantity of N-9 used in its formulation anticipated the findings obtained herein. Indeed, lower N-9 concentrations have shown to promote severe consequences for woman general and reproductive health [Citation7], therefore explaining its use as a positive control in the present study.
Differences and similarities in relation to other studies
Both BKC and MKC were reported to have antimicrobial and antiviral properties [Citation11–13], with spermicidal activity being also demonstrated by the former [Citation11,Citation12]. However, despite being used in vaginal formulations, no information on the toxicity of MKC was available and consistent in vitro studies addressing the efficacy and safety of a single exposure to both compounds were still lacking.
Although lacking studies on MKC, many have reported an adverse effect of BKC on HeLa or other cells in vitro [Citation17,Citation34,Citation35], as well as promoting vaginal damage/disruption. Nevertheless, although in some cases some degree of vaginal/cervical irritation and de-epithelialization has occurred in vivo [Citation12], associated or not with inflammation and some kind of discomfort, many women did not find these effects crucial to discontinue BKC treatment [Citation18]. Furthermore, the acceptability of vaginal contraceptives containing BKC was shown to be better than that of N-9 [Citation19,Citation20]. Pregnancy rates were also considered moderate in contraceptives containing BKC [Citation18], but mainly because of the lack of information and/or mistakes in the application of the spermicide rather than due to their poor contraceptive ability. Indeed, the success of local contraceptives not only depends on their complete/incomplete vaginal coverage but also on the information of users, their compliance with the usage rules and their personal motivation [Citation3,Citation14,Citation15].
As observed in our study, N-9 was also found by others to induce cellular toxicity in vitro [Citation36]. Along with this, genital irritation and epithelial disruption that can increase the risk of transmission of STIs has been reported. One such example was the study regarding the frequent use of N-9 by female commercial sex workers, in which a consistent association between genital lesions and HIV transmission was determined [Citation37]. Studies in women using N-9 has often indicated genital irritation detected by colposcopy unrelated to symptoms [Citation38]. In light of this information, and given that many studies also failed to demonstrate any benefit [Citation39], clinical research into N-9 was suspended, leading to a recommendation by the WHO against its use [Citation40].
Strenghts and weaknesses
Local vaginal contraception is perhaps the oldest method of fertility control. With minimal systemic involvement, it was also considered one of the safest methods of contraception. Indeed, the few papers available describing potential BKC systemic effects have shown no/negligible adverse reactions [Citation15,Citation41], and even its inadvertent use at the start of a pregnancy does not induce foetal malformation in humans nor passes to breast milk according to Liebert [Citation15].
However, the lack of interest and innovation in this field coupled with the introduction of oral contraceptive pills and intrauterine devices in the 1960s made the available vaginal contraceptives somewhat outdated, resulting in poor efficacy and acceptability [Citation1]. Nevertheless, local contraceptives can be preferred over other contraceptive methods by women after medical counselling [Citation2,Citation3,Citation6]. Nowadays, FSRH supports the use of hormonal contraception and intrauterine devices to a large extent, but women may opt for vaginal contraceptives inclusively when rarer sexual intercourse occurs and/or when a decline in fertility has been associated. Furthermore, vaginal contraceptives are easy to use, available over the counter and, contrary to the male condom, woman-controlled. To this extent, Pharmatex® products may represent a more suitable alternative than N-9.
Unanswered questions and future research
The potential of Pharmatex® active ingredients and related galenic forms as effective vaginal contraceptives was determined in this in vitro study. Further clinical trials are necessary to address their safety in vivo.
Conclusions
The present in vitro study was performed with the interest of mimicking as far as possible the vaginal environment in vivo. We herein report that in these experimental conditions both BKC and MKC included in Pharmatex® products present strong spermicidal activity, being as effective as N-9 but at lower concentrations. Furthermore, BKC and MKC were found less harmful to the the human vaginal/cervical cell line than N-9 in vitro.
Supplemental Material
Download JPEG Image (125.6 KB)Supplemental Material
Download JPEG Image (214.4 KB)Acknowledgements
The authors would like to thank Marta Baptista, Carla Verissimo, Rosa Fernandes, Sara Escada-Rebelo and Paula Mota for their valuable scientific and technical inputs and John Jones for language editing.
Disclosure statement
The present study was impartially performed. The sponsor did not interfer in study design nor in the collection, analysis and interpretation of data. Briefly, R.S.T. and J.R.S. established the concept and design. M.I.A., R.A.S. and A.F.S. acquired the data. R.S.T. wrote the paper and M.I.R., R.A.S., A.F.S., A.P.S., T.A.S and J.R.S. contributed to the analysis and interpretation of the results, drafting, revising and approving the paper. C.G., V.J., Y.M. and F.R. provided the resources to successfully deliver the study. The contents of this study do not necessarily reflect the view of the sponsor.
Additional information
Funding
References
- Gupta G. Microbicidal spermicide or spermicidal microbicide? Eur J Contracept Reprod Health Care. 2005;10(4):212–218.
- Fruzzetti F, Perini D, Fornaciari L, et al. Discontinuation of modern hormonal contraceptives: an Italian survey. Eur J Contracept Reprod Health Care. 2016;21(6):449–454.
- Serfaty D. Update on the contraceptive contraindications. J Gynecol Obstet Hum Reprod. 2019;48(5):297–307.
- Bearak J, Popinchalk A, Alkema L, et al. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: estimates from a Bayesian hierarchical model. Lancet Glob Health. 2018;6(4):e380–e389.
- Lech MM. Spermicides 2002: an overview. Eur J Contracept Reprod Health Care. 2002;7(3):173–177.
- Batár I. State-of-the-art of non-hormonal methods of contraception: II. Chemical barrier contraceptives. Eur J Contracept Reprod Health Care. 2010;15(2):89–95.
- Iyer V, Poddar SS. Update on nonoxynol-9 as vaginal spermicide. Eur J Contracept Reprod Health Care. 2008;13(4):339–350.
- Vieira OV, Hartmann DO, Cardoso CM, et al. Surfactants as microbicides and contraceptive agents: a systematic in vitro study. PLoS One. 2008;3(8):e2913.
- Inácio ÂS, Mesquita KA, Baptista M, et al. In vitro surfactant structure-toxicity relationships: implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One. 2011;6(5):e19850.
- Belec L, Tevi-Benissan C, Bianchi A, et al. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J Antimicrob Chemother. 2000;46(5):685–693.
- Courtot AM, Nikas G, Gravanis A, et al. Effects of cholic acid and ‘Protectaid’ formulations on human sperm motility and ultrastructure. Hum Reprod. 1994;9(11):1999–2005.
- Mauck CK, Baker JM, Barr SP, et al. A phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9. Postcoital testing and colposcopy. Contraception. 1997;56(2):89–96.
- Harrison JJ, Turner RJ, Joo DA, Stan MA, et al. Copper and quaternary ammonium cations exert synergistic bactericidal and antibiofilm activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52(8):2870–2881.
- Serfaty D. Contraception in breastfeeding women: place for spermicides. J Gynecol Obstet Biol Reprod. 2015;44(1):18–27.
- Serfaty D. Contraception during perimenopause: the spermicides option. J Gynecol Obstet Hum Reprod. 2017;46(3):211–218.
- Aubeny E, Colau JC, Nandeuil A. Local spermicidal contraception: a comparative study of the acceptability and safety of a new pharmaceutical formulation of benzalkonium chloride, the vaginal capsule, with a reference formulation, the pessary. Eur J Contracept Reprod Health Care. 2000;5(1):61–67.
- Fichorova RN, Bajpai M, Chandra N, et al. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod. 2004;71(3):761–769.
- Marinov B. Our experience in the application of local contraceptives. Akush Ginekol. 2004;43(3):48–51.
- Xu JX, Huang ZR, Wu Y, et al. Contraceptive efficacy of bioadhesive benzalkonium chloride gel in comparison with nonoxynol-9 gel. Zhonghua Fu Chan Ke Za Zhi. 2006;41(10):706–709.
- Li W, Huang Z, Wu Y, et al. Effectiveness of an optimized benzalkonium chloride gel as vaginal contraceptive: a randomized controlled trial among Chinese women. Contraception. 2013;87(6):756–765.
- Arambasić BM, Subotin ML, Stanić M. Comparative in vitro study of nonoxynol-9: effects on human, bull and boar spermatozoa. Contraception. 1992;45(3):229–237.
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–95.
- Zaveri T, Hayes JE, Ziegler GR. Release of tenofovir from carrageenan-based vaginal suppositories. Pharmaceutics. 2014;6(3):366–377.
- World Health Organization (WHO). WHO laboratory manual for the Examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010.
- Jain RK, Jain A, Maikhuri JP, et al. In vitro testing of rationally designed spermicides for selectively targeting human sperm in vagina to ensure safe contraception. Hum Reprod. 2009;24(3):590–601.
- Portela JM, Tavares RS, Mota PC, et al. High glucose concentrations per se do not adversely affect human sperm function in vitro. Reproduction. 2015;150(1):77–84.
- Mota PC, Tavares RS, Cordeiro M, et al. Acute effects of TCDD administration: special emphasis on testicular and sperm mitochondrial function. Apjr. 2012;1(4):269–276.
- Kremer J. A simple sperm penetration test. Int J Fertil. 1965;10(3):209–215.
- Jain RK, Maikhuri JP, Kiran Kumar ST, et al. Novel disulphide esters of carbothioic acid as potent, non-detergent spermicides with low toxicity to Lactobacillus and HeLa cells in vitro. Hum Reprod. 2007;22(3):708–716.
- Tavares RS, Portela JMD, Sousa MI, et al. High glucose levels affect spermatogenesis: an in vitro approach. Reprod Fertil Dev. 2017;29(7):1369–1378.
- Schill WB, Wolff HH. Ultrastructure of human spermatozoa in the presence of the spermicide nonoxinol-9 and a vaginal contraceptive containing nonoxinol-9. Andrologia. 1981;13(1):42–49.
- Tavares RS, Silva AF, Escada-Rebelo S, et al. Book of abstracts: The 15th Congress of the European Society of contraception and reproductive health. Eur J Contracept Reprod Health Care. 2018;23(sup1):60–61.
- Erny R, Siborni C. The effect of benzalkonium chloride on ovulatory cervical mucus. Acta Eur Fertil. 1987;18(2):109–111.
- Korting HC, Herzinger T, Hartinger A, et al. Discrimination of the irritancy potential of surfactants in vitro by two cytotoxicity assays using normal human keratinocytes, HaCat cells and 3T3 mouse fibroblasts: correlation with in vitro data from a soap chamber assay. J Dermatol Sci. 1994;7(2):119–129.
- Herold BC, Kirkpatrick R, Marcellino D, et al. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob Agents Chemother. 1999;43(4):745–751.
- Patton DL, Wang SK, Kuo CC. In vitro activity of nonoxynol 9 on HeLa 229 cells and primary monkey cervical epithelial cells infected with Chlamydia trachomatis. Antimicrob Agents Chemother. 1992;36(7):1478–1482.
- Rustomjee R, Abdool Karim Q, Abdool Karim SS, et al. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS. 1999;13(12):1511–1515.
- Roddy RE, Cordero M, Cordero C, et al. A dosing study of nonoxynol-9 and genital irritation. Int J STD Aids. 1993;4(3):165–170.
- Richardson BA, Lavreys L, Martin HL, Jr, et al. Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: a randomized clinical trial. Sex Transm Dis. 2001;28(7):394–400.
- WHO/CONRAD Technical Consultation on Nonoxynol-9. Geneva: WHO; Summary Report 9–10 October 2001.
- Creatsas G, Guerrero E, Guilbert E, et al. A multinational evaluation of the efficacy, safety and acceptability of the Protectaid contraceptive sponge. Eur J Contracept Reprod Health Care. 2001;6(3):172–182.
