Abstract
Purpose
The Kyleena® Satisfaction Study (KYSS) aimed to assess satisfaction and continuation with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 (Kyleena®) in routine clinical practice and to evaluate factors that influence satisfaction.
Materials and methods
This prospective, observational, multicentre, single-arm cohort study, with 1-year follow-up, was conducted in Belgium, Canada, Germany, Mexico, Norway, Sweden, Spain and the United States from 2017 to 2018. During routine counselling, women who independently selected to use LNG-IUS 12 were invited to participate in the study. KYSS assessed LNG-IUS 12 satisfaction, continuation and safety.
Results
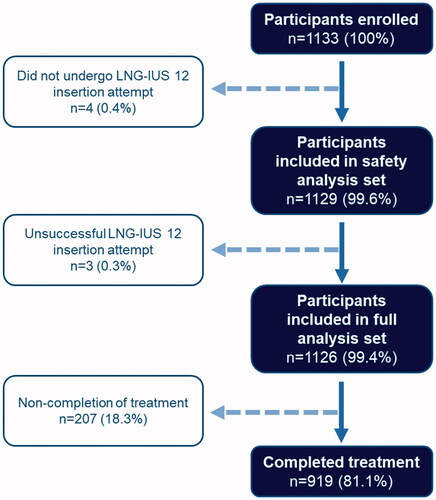
Overall, there were 1126 successful LNG-IUS 12 placements, with insertion attempted in 1129 women. Most participants (833/968, 86.1%, 95% CI 83.7–88.2%, with satisfaction outcome data available) reported satisfaction with LNG-IUS 12 at 12 months (or at the final visit if the device was discontinued prematurely). Satisfaction was not associated with age, parity or motivation for choosing LNG-IUS 12. The majority of women (919/1129, 81.4%) chose to continue after 12 months. Discontinuation was not correlated with age or parity. Overall, 191 women (16.9%) reported a treatment-emergent adverse event.
Conclusions
Results from KYSS provide the first real-world evidence assessing LNG-IUS 12, and demonstrate high satisfaction and continuation rates irrespective of age or parity. Clinical trial registration: NCT03182140
LNG-IUS 12的满意度和续用:来自真实世界kyleena®满意度的研究 摘要
目的:Kyleena®满意度研究(Kyleena® Satisfaction Study, KYSS)旨在评估左炔诺孕酮宫内节育系统(LNG-IUS)12(Kyleena®)在常规临床实践中的满意度和续用情况, 并评估影响满意度的因素。
材料和方法:这项前瞻性、观察性、多中心、单臂队列研究, 随访1年, 于2017年至2018年在比利时、加拿大、德国、墨西哥、挪威、瑞典、西班牙和美国进行。在常规咨询期间, 邀请独立选择使用LNG-IUS 12的妇女参与研究。KYSS评估了LNG-IUS 12的满意度、续用率和安全性。
结果:共有1129例女性尝试放置LNG-IUS 12, 其中1126例成功放置。大多数参与者(833/968, 86.1%, 95%可信区间83.7-88.2%, 满意结果数据可用)报告在12个月时(如果提前停用, 在最后一次就诊时)对LNG-IUS 12满意。满意度与年龄、产次或选择LNG-IUS 12的动机无关。大多数妇女(919/1129, 81.4%)在12个月后选择继续使用LNG-IUS 12。停用与年龄或产次无关。总的来说, 191名妇女(16.9%)报告了一次治疗中的不良事件。
结论:KYSS的结果提供了第一个评估LNG-IUS 12的真实世界证据, 并证明LNG-IUS 12的满意度和续用率很高, 与年龄或产次无关。临床试验注册:NCT03182140。
Introduction
Long-acting reversible contraceptives (LARCs), which comprise hormonal intrauterine systems (IUSs), subdermal implants, and non-hormonal intrauterine devices (IUDs), are highly effective methods of contraception that do not rely on user compliance [Citation1,Citation2]. Despite this, LARCs are used less frequently, especially among younger women, than user-dependent methods such as oral contraceptive pills and barrier methods [Citation3–5]. Furthermore, prevalence of LARC use in women of reproductive age differs between countries, ranging from an estimated 20% in Sweden and 15% in Norway to 7% in Germany and less than 5% in Spain [Citation6,Citation7]. In a number of countries, the acceptance and use of LARCs is growing [Citation6,Citation7].
The lower adoption rate of LARCs in some areas and some populations may be due to misconceptions and perceived barriers among both health care providers and patients regarding the use of LARCs, particularly intrauterine contraception (IUC). This may include the belief that IUC is not suitable for young or nulliparous women, fears regarding adverse events (such as expulsion or uterine perforation), and anxiety about potential pain and difficulty with insertion of the device [Citation8–11].
There is little evidence to support these concerns; LARCs are associated with favourable efficacy and safety profiles regardless of age or parity [Citation12–15], and with high satisfaction and continuation rates in clinical trials [Citation14,Citation16–18] and real-world surveys [Citation19]. In addition, previously published data from the Kyleena® Satisfaction Study (KYSS) interim analysis have shown that most women rate the insertion pain of the IUS as either none or mild and that clinicians consider placement to be easy in the majority of cases [Citation20].
Levonorgestrel (LNG)-releasing IUS 12 (LNG-IUS 12, also known as Kyleena®) has demonstrated contraceptive efficacy for 5 years of use [Citation21]. LNG-IUS 12 was developed with a smaller insertion tube which causes less insertion pain [Citation20,Citation21]. In addition, LNG-IUS 12 has other potential benefits such as lower levels of levonorgestrel and lower rates of amenorrhoea in women who desire this [Citation17,Citation18,Citation21]. KYSS is a multinational, observational study that aims to provide the first real-world evidence of LNG-IUS 12 satisfaction. Continuation, safety profile and satisfaction with the menstrual bleeding profile of LNG-IUS 12 are also reported.
Materials and methods
Study design and participants
KYSS (NCT03182140) is a prospective, observational, multinational, multicentre, single-arm cohort study with a 1-year follow-up, investigating satisfaction with LNG-IUS 12 in a real-word setting. This study was conducted in Belgium, Canada, Germany, Mexico, Norway, Sweden, Spain, and the United States from 2017 to 2018. Approval from the independent ethics committee/institutional review board (see details in Supplementary Table 1) was obtained for all participating centres. The methodology of this study has been described in detail in the previously published interim analysis, which was conducted after the last woman completed her initial visit at first attempt of LNG-IUS 12 insertion [Citation20]. It should be noted that due to a delay in participant recruitment due to protocols for study acceptance, Mexico completed enrolment after the other countries and was thus not included in the interim analysis.
The decision to use an LNG-IUS was made independently by the women after a discussion with their health care provider at participating study centres. These discussions were not prompted or scripted; the health care providers gave their routine counselling, after which the women could decide to use any contraceptive method, including LNG-IUS 12. Women who independently chose to use LNG-IUS 12 were subsequently informed about the study and invited to participate. Women could have used any other contraceptive methods prior to inclusion (including no contraception, i.e., new users) and there were no age restrictions; all women who were eligible for LNG-IUS 12 were able to participate. Exclusion criteria for the study, according to local market authorisation, included contraindications for LNG-IUS, mental incapacity to consent, and participation in other clinical trials with interventions outside routine clinical practice. Written informed consent was obtained from all participants.
Primary and secondary outcomes
The primary endpoint for this study was the overall satisfaction rate with LNG-IUS 12 at the end of the observation/final visit (i.e., after 12 months or at premature discontinuation) per country. Secondary endpoint analyses included satisfaction with LNG-IUS 12 at the end of the observation/final visit stratified by previously used contraceptive method and by reason for switching from previous contraceptive method, and satisfaction with menstrual bleeding profile with LNG-IUS 12 at the end of the observation/final visit. Other endpoints included satisfaction at the end of the observation/final visit stratified by parity and age. Satisfaction ratings were based on the five-item Likert scale: ‘very satisfied’, ‘somewhat satisfied’, ‘neither satisfied nor dissatisfied’, ‘dissatisfied’ and ‘very dissatisfied’ [Citation22].
Safety data including adverse events and reasons for early discontinuation (i.e., before the planned end of the observation period at 12 months) were collected. Data on adverse events were reported spontaneously by the participants or their health care provider. Adverse events were categorised and summarised according to the assessed relationship to LNG-IUS 12 and seriousness of the adverse event. An adverse event was considered treatment emergent if it occurred from the day of the first insertion attempt until the end of the observation period. The incidence of treatment-emergent adverse events is summarised descriptively by Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class and MedDRA Preferred Term.
Statistical analyses
Statistical differences in main population characteristics between countries were analysed by either Kruskal–Wallis test or chi-squared test. The influence of potential prognostic factors on the primary outcome of overall satisfaction with LNG-IUS 12 and the secondary outcome of satisfaction with menstrual bleeding profile were investigated by multiple logistic regression analysis. The inclusion of population characteristics into the multiple logistic regression models was decided upon based on the univariate association of each parameter obtained from chi-squared test/Fisher’s exact test for categorical parameters and one-way analysis of variance for continuous parameters. A P-value of P < .2 was used as a cut-off for inclusion (although age was additionally included as a parameter). Multiple logistic regression analyses were calculated with adjustment for between-country variability by inclusion of country as stratum in multiple logistic regression models. Time-to-event analysis based on the Kaplan–Meier method was used to assess the influence of country and other factors of interest (parity, age, etc.) on premature discontinuation due to expulsion or removal of LNG-IUS 12. Women who discontinued prematurely or who completed the study without a reported expulsion or removal were censored at the date of last follow-up visit. Significance of each factor was obtained by the log-rank test.
All analyses were performed using SAS® software, version 9.4 SAS® [Statistical Analysis Systems, SAS (2013). The SAS System for Windows, Release 9.4. Statistical Analysis Systems Institute, Cary, NC] and generic macros [Citation23].
Results
Baseline demographics and study population
Overall, 1133 women were enrolled in the study (), four of whom did not undergo an LNG-IUS 12 insertion attempt and were therefore not included in the safety analysis set. Of the 1129 participants who underwent an LNG-IUS 12 insertion attempt, insertion was unsuccessful in three women (0.3%). Thus, a total of 1126 women were valid for the full analysis set, over half of whom were nulliparous (584 women, 51.9%). Baseline demographics of the study population are shown in .
Table 1. Baseline demographics (full analysis set).
The selection of countries and country-specific sample sizes were determined according to recruitment feasibility. The population characteristics of mean age, parity, body mass index (BMI) and previous contraceptive method varied between countries (P < .0001 for each aforementioned category). Canada had the lowest mean age at 24.2 years (±7.0 years) and Spain had the highest at 36.2 years (±6.8 years). Similarly, Canada had the highest proportion of nulliparous participants in the full analysis set (87/99, 87.9%) and Spain had the lowest proportion (29/102, 28.4%).
Most participants had been using a form of contraception prior to initiating use of LNG-IUS 12 (809/1126, 71.8%) (). Oral contraceptives were the most common prior method of contraception and were used by 371 participants (32.9%), followed by IUS in 169 participants (15.0%), and barrier methods in 168 participants (14.9%) (Supplementary Table 2).
A variety of factors were associated with a participant’s decision to select LNG-IUS 12 for contraception (Supplementary Table 3). The most common reason was the desire to avoid the need for a daily, weekly, or monthly contraceptive routine, as stated by 386 (34.3%) of the 1126 women included in the full analysis set. High contraceptive efficacy was also an important reason, stated by 302 participants (26.8%). Motivation for choice of LNG-IUS 12 differed significantly between countries (P ≤ .02). The explicit wish to use a low dose of hormones was a more frequent reason for Swedish (39/100, 39.0%) and German (158/506, 31.2%) women, and was least frequent among US women (11/99, 11.1%). Issues related to convenience and comfort were more important in some countries than others; for example, selection of LNG-IUS 12 due to desire for an improved bleeding pattern was more common among Belgian women (26/100, 26.0%) than among US women (12/99, 12.1%).
Satisfaction with LNG-IUS 12
Satisfaction with LNG-IUS 12 at the end of the observation period (at 12 months), or at the final visit if the device was discontinued early, was assessed as the primary study objective. Of the 1126 women in the full analysis set, 968 participants had available satisfaction outcome data. Overall, 833/968 participants [86.1%, 95% confidence interval (CI) 83.7–88.2%] reported satisfaction with the device. The majority of women (603, 62.3%, 95% CI 59.2–65.4%) reported being ‘very satisfied’ with LNG-IUS 12, with a low proportion (24 women, 2.5%) reporting being ‘very dissatisfied’ with LNG-IUS 12 at the end of the observation period/final visit (). However, it should be noted that for 158/1126 (14.0%, 95% CI 1.6–3.7%) women in the full analysis set, no satisfaction outcome data were available, mostly due to preterm removal of the LNG-IUS 12 device or change of contraception method.
Figure 2. Satisfaction of participants with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 overall and by country at end of observation/final visit. Full analysis set (N = 1126). Data missing for 158 participants. Percentages have been rounded to one decimal place and thus may not total 100% exactly.
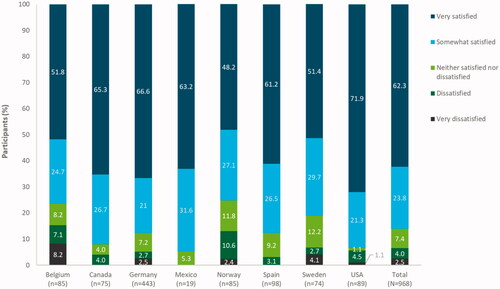
Most partipants in each country included in the survey were satisfied with LNG-IUS 12; however, these rates differed between countries (P < .001) (), ranging from 64/85 women (75.3%, 95% CI 64.7–84.0%) with available satisfaction data in Norway to 18/19 women (94.7%, 95% CI 74.0–99.9%) in Mexico. Norway had the lowest proportion of women who reported being ‘very satisfied’ with LNG-IUS 12 (41/85, 48.2%, 95% CI 37.3–59.3%) and the USA had the highest proportion of ‘very satisfied’ women (64/89, 71.9%, 95% CI 61.4–80.9%).
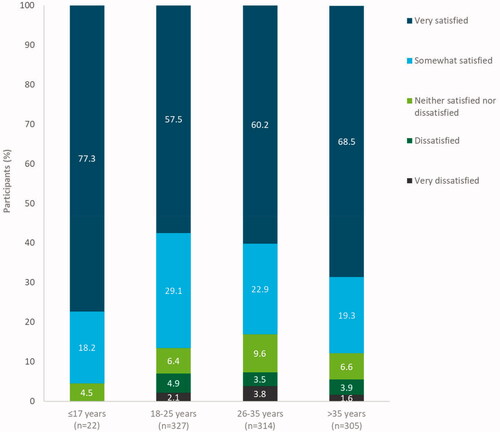
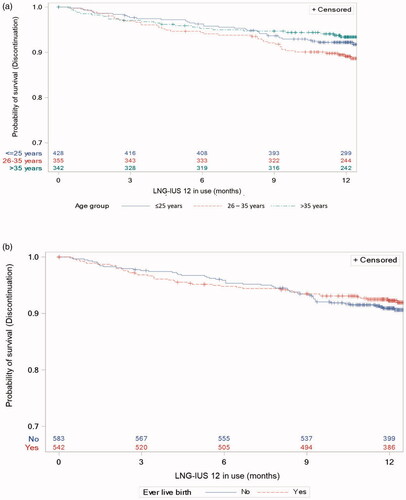
The majority of participants in all age groups were satisfied with LNG-IUS 12 at the end of the observation/final visit (), ranging from 261/314 women (83.1%, 95% CI 78.5–87.1%) aged 26–35 years to 21/22 women (95.5%, 95% CI 77.2–99.9%) of patients aged ≤17 years. The subgroup aged 18–25 years had the lowest proportion of women reporting being ‘very satisfied’ with LNG-IUS 12 and the highest proportion of ‘very satisfied’ patients was found in the subgroup of women aged ≤17 years (). No significant association between age and satisfaction was found (P = .396).
Figure 3. Satisfaction of participants with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 by age at end of observation/final visit (pooled countries). Full analysis set (N = 1126). Data missing for 158 participants. Percentages have been rounded to one decimal place and thus may not total 100% exactly.
Satisfaction rates were similar for both parous and nulliparous women; 406/477 (85.1%, 95% CI 81.6–88.2%) parous women and 427/491 (87.0%, 95% CI 83.7–89.8%) nulliparous women reported being satisfied with LNG-IUS 12. No significant association was observed between parity and satisfaction with LNG-IUS 12 (P = .092).
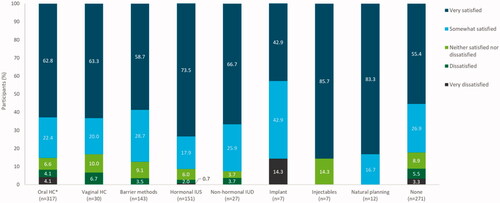
The overall satisfaction rate with LNG-IUS 12 stratified by previous contraceptive method ranged from 223/271 (82.3%, 95% CI 77.2–86.6%) women in the subgroup with no previous contraceptive method to 100% in subgroups including the natural family planning subgroup (12/12 women) (). For all prior methods except the implant, the majority of participants reported being ‘very satisfied’ with their LNG-IUS 12 device. No significant difference in satisfaction rates was observed between participants who had previously used IUS and those who had used other or no methods of contraception prior to initiating LNG-IUS 12 (P = .268).
Figure 4. Satisfaction of participants with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 by prior contraceptive method at end of observation/final visit (pooled countries). HC: hormonal contraception; IUD: intrauterine device; IUS: intrauterine system. *Combined oral contraceptives or progestogen-only pills. Full analysis set (N = 1126). Data missing for 158 participants. Contraceptive method groups ‘transdermal HC’ and ‘back-up contraception’ were not included due to low participant numbers (n = 1 and n = 2, respectively). However, 100% of these participants were ‘very satisfied’. Percentages have been rounded to one decimal place and thus may not total 100%. exactly.
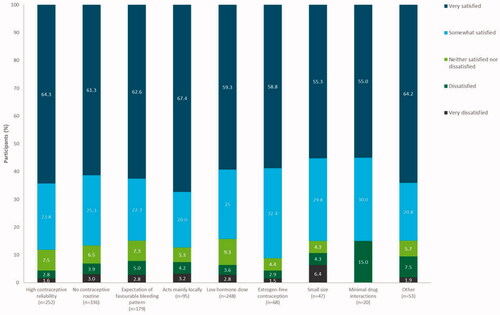
Additionally, when satisfaction was stratified by motivation for switching from previous contraceptive method, most participants reported being satisfied with LNG-IUS 12, regardless of the motivation for choosing LNG-IUS 12 (). Satisfaction rates were similar between the reported motivators, ranging from 84.3% (209/248, 95% CI 79.1–88.6%) in participants who selected LNG-IUS 12 due to the desire for a low hormone dose to 91.2% (62/68, 95% CI 81.8–96.7%) among women who wished to have estrogen-free contraception.
Figure 5. Satisfaction of participants with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 by motivation for selecting LNG-IUS 12 at end of observation/final visit (pooled countries). Participants could give more than one reason for selecting LNG-IUS 12. Full analysis set (N = 1126). Percentages have been rounded to one decimal place and thus may not total 100% exactly.
Continuation and adverse events with LNG-IUS 12
Of the 1129 participants in the safety analysis set, 919 women (81.4%) chose to continue with the device at the end of the observation period (). Of the 210 women who did not continue in the study until the planned end of the observation period at 12 months, half of these (105 women, 9.3% of the safety analysis set study population) were lost to follow-up.
Table 2. Discontinuation with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 overall and by country at end of observation/final visit.
Premature discontinuation rates due to expulsion or removal of LNG-IUS 12 differed significantly between countries (P = .0074) (), ranging from 5/102 women (4.9%) in Spain to 19/100 women (19.0%) in Sweden (). No correlation was found between age or parity and discontinuation (). We found that previous use of an IUS was correlated with greater continuation rates of LNG-IUS 12 (P = .03) and that discontinuation was more common in women who were dissatisfied with their previous method compared to women who were satisfied with their previous method (P = .02).
Figure 6. Kaplan–Meier estimates for premature discontinuation due to expulsion or removal of LNG-IUS 12, by country, with number of participants at risk. BEL: Belgium; CAN: Canada; DEU: Germany; ESP: Spain; LNG-IUS: levonorgestrel-releasing intrauterine system; Mex: Mexico; NOR: Norway; SWE: Sweden; USA: United States of America.
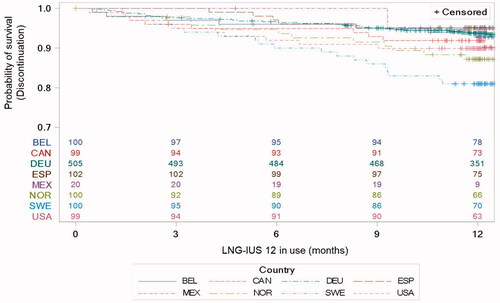
Figure 7. Kaplan–Meier estimates for premature discontinuation, (a) by age and (b) by parity, with number of participants at risk. LNG-IUS: levonorgestrel-releasing intrauterine system.
In total, 100 women (8.9%) either had the device removed or expelled the device before 12 months of use. In the interim KYSS analysis, we reported that 24 women (2.2% of the 1101 participants included in the interim study population) had the device removed within 3 months of use [Citation20]. Based on this and the discontinuation curves depicted in and , there does not appear to be any specific timepoint associated with greater discontinuation of LNG-IUS 12.
Overall, 191 (16.9%) women reported treatment-emergent adverse events and 138 (12.2%) of these were considered related to LNG-IUS 12 (). No uterine perforations were reported. Six women (0.5%) reported device expulsion (). Two intrauterine pregnancies (0.2% of participants) and one ectopic pregnancy (0.1% of participants) were reported with LNG-IUS 12 in situ. The two intrauterine pregnancies resulted in spontaneous abortion. Overall, 74 women (6.6%) reported bleeding profile disorders such as heavy menstrual bleeding and irregular bleeding (data not shown). Dysmenorrhoea was reported by 13 women (1.2%), ovarian cysts by 13 women (1.2%) and pelvic pain by 11 women (1.0%) (data not shown). Other common adverse events included gastrointestinal disorders (reported by 37 women, 3.3%), infections/infestations (reported by 26 women, 2.3%) and psychiatric disorders (reported by 20 women, 1.8%).
Table 3. Summary of treatment-emergent adverse events with levonorgestrel-releasing intrauterine system (LNG-IUS) 12 at end of observation/final visit.
Adverse events were the main reason for removal of the LNG-IUS 12, leading to discontinuation in 69/1129 women (6.1%) (). Of the treatment-emergent adverse events leading to discontinuation, the most common were psychiatric disorders (including depression) (0.9%), followed by acne (0.7%), pelvic pain (0.6%), dysmenorrhoea (0.5%), metrorrhagy (0.4%), and menorrhagy (0.3%) (Supplementary Table 4).
Satisfaction with menstrual bleeding profile
In the first follow-up at 4–12 weeks after LNG-IUS 12 placement, 499/821 (60.8%, 95% CI 57.3–64.1%) women with satisfaction outcome data available reported being satisfied with their menstrual bleeding profile. At the end of the observation period at 12 months, or final visit if the participant discontinued early, 632/887 (71.3%, 95% CI 68.1–74.2%) women with available outcome data reported satisfaction with their menstrual bleeding profile. Satisfaction with previous contraceptive method, concomitant disease and moderate pain during LNG-IUS 12 insertion were correlated with satisfaction with bleeding profile (P = .003, P = .045 and P = .073, respectively). The choice to use LNG-IUS 12 due to the desire for no contraceptive routine also correlated with bleeding profile satisfaction (P = .034).
Discussion
Findings and interpretation
The results from KYSS demonstrate high satisfaction and continuation rates with LNG-IUS 12 across all countries included in this study. These rates compare favourably with both prior clinical trials of LNG-IUS 12 and rates reported in trials using other methods of reversible contraception [Citation14, Citation16–18,Citation37]. However, significant differences in these rates between countries in KYSS were observed. Belgium, Norway and Sweden had a lower proportion of satisfied women and a higher early removal rate than other countries.
It should be noted that of the 14% of discontinuations reported in this study, 50% were due to loss to follow-up and 50% for premature removal or expulsion. Thus, while the overall discontinuations for some countries may appear to be higher, the number of women choosing to have the device removed was low. Additionally, satisfaction data were not available for some participants, and therefore, satisfaction rates for these women were unknown. Although premature discontinuation and satisfaction did vary between countries, these results provide further evidence for the high continuation rate with LNG-IUS 12, which is another indicator of user satisfaction.
Population variances between the countries may account, at least in part, for the observed differences in satisfaction and continuation rates. The age and parity of women differed between countries, as well as the main reasons for choosing an LNG-IUS. In addition, although most women in the study had used a prior method of contraception, the most common method of contraception varied between countries. There are several potential reasons for these population variances. For example, some health care providers may have misplaced concerns, particularly surrounding the use of IUSs in nulliparous women, which may have led to a selection bias towards parous populations, who also tend to be older, in some countries [Citation24,Citation25]. In addition, government policies and costs may affect the study population included [Citation25].
The variance in satisfaction and continuation rates between countries could also be due to the overall prevalence of use between countries. IUS use is common in some of the countries studied (such as the Nordic countries) and uncommon in others (such as Spain) [Citation6,Citation7]. Women from a low-use country who select an IUS for contraception may be more motivated to manage side-effects and to continue with that method compared with women in countries where IUS use is widespread. Sweden, in particular, had lower continuation rates in this study but also has a higher prevalence of IUS use in the general population [Citation6].
In addition, differences in counselling between the participating countries and centres may also have led to the observed differences in satisfaction and continuation between countries. As the health care providers in this study were asked to simply provide women with their routine counselling, this was not standardised and therefore women in some countries and some centres may have received more thorough counselling than others. It is known that counselling can affect contraceptive acceptance and continuation rates [Citation26–28]. Thus, this may also be a factor behind the country differences.
Interestingly, this study revealed that parity did not affect satisfaction or continuation rates. Similarly, age was not associated with satisfaction or continuation. This is important because it is a misconception among some health care providers and the public that IUSs are unsuitable for young, nulliparous women [Citation8–11]. LNG-IUS 12 has previously been shown to be safe and efficacious in nulliparous women of all ages [Citation14,Citation17,Citation18]. Furthermore, previously published data from the KYSS interim analysis have also demonstrated that insertion of LNG-IUS 12 is easy and results in none or mild pain even in young, nulliparous women where it is often (erroneously) assumed that insertion will be painful and difficult [Citation20]. The data presented here provide further evidence that LNG-IUS 12 is suitable for women irrespective of parity or age, by demonstrating high satisfaction and continuation across these subgroups.
Participants in this study had used a wide variety of prior contraceptive methods before switching to LNG-IUS 12. Regardless of the previous method used, satisfaction with LNG-IUS 12 was high; furthermore, previous IUS use was not associated with higher satisfaction rates than previous use of no or other contraceptive methods. Some differences in satisfaction were observed, but firm conclusions cannot be drawn from direct comparisons between the prior contraception subgroups due to the fact that some subgroups (such as the implant, transdermal hormonal contraception, or back-up contraception subgroups) contained an extremely small number of participants.
Satisfaction with LNG-IUS 12 was also high regardless of motivation for switching from the prior method of contraception. It is interesting to note that even those who chose to use LNG-IUS 12 due to a desire for an improved bleeding pattern had high satisfaction rates. This is despite the potential initial bleeding pattern changes associated with LNG-IUS, where bleeding may be more frequent and/or prolonged for the first 3 months of use [Citation29]. Unfavourable bleeding pattern changes are a common cause of dissatisfaction and discontinuation of LARCs [Citation30–34], and concerns about these changes can prevent some women from considering using LARCs [Citation25,Citation35,Citation36].
Although it is possible that irregular bleeding and spotting were underreported, the number of women reporting bleeding issues was low overall, and the number discontinuing due to this was even lower. The rate of device removal (for any reason) at 4–12 weeks as reported in the KYSS interim analysis was 2.2% compared with 8.3% at the end of 12-month observation period [Citation20], demonstrating that discontinuation was not excessively high in the first 3 months of use. Women’s satisfaction with their menstrual bleeding profile was lower at 4–12 weeks of use than at the end of the observation period; however, at both follow-up time-points, most women were satisfied. Thus, for women with concerns about bleeding profile changes caused by usage of LNG-IUS, these findings should be reassuring.
This study builds on the already established favourable safety and efficacy profile of LNG-IUS 12 [Citation14,Citation17,Citation18]. Adverse events, particularly serious adverse events or those leading to discontinuation, were uncommon and there were very few cases of contraceptive failure and expulsion, and no cases of uterine perforation.
Strengths and weaknesses
As previously described, one weakness of this study is the number of participants for whom no satisfaction data were available, as these participants who were lost to follow-up may impact the overall satisfaction rates. Other limitations of the study include the relatively small sample sizes for some subgroups (e.g., in the analyses on satisfaction stratified by prior methods of contraception and motivation for choosing LNG-IUS 12). The sample size for Mexico was also much smaller than that of other countries included in the study. Another limitation is that this study was not designed to investigate the interaction of age, race, ethnicity, education level or socioeconomic status. In addition, the mean BMI of the study population was 24.4 and the majority of participants were white. Therefore, these results may not be generalisable to all other populations.
Similarities and differences in relation to other studies
Prior clinical trials have demonstrated high satisfaction and continuation rates associated with LNG-IUS 12 use [Citation14,Citation17,Citation18]. The results from KYSS build on this further and provide the first evidence in a real-world setting for satisfaction with LNG-IUS 12. Continuation was similar to that seen in other clinical trials of LNG-IUS 12 [Citation14,Citation17,Citation18]. Satisfaction and continuation rates in this study compare favourably with those reported in trials using other methods of contraception [Citation14,Citation16–18,Citation37]. The rate of adverse events, in particular expulsion, also compared favourably to rates reported in prior trials [Citation14,Citation17,Citation18]. Real-world evidence bridges the gap between clinical trials and routine clinical practice, as the data from observational studies reflect the diversity of patients and clinical scenarios [Citation38–40]. Thus, it is anticipated that the findings from KYSS will be applicable to an even larger, more inclusive, population of patients, providers, and health care systems and settings.
Open questions and future research
This study provides insight into the influence that factors such as previous method of contraception, age and parity may have on satisfaction with IUSs, as well as adding to the understanding of women’s motivation for choosing LARCs such as IUSs. Understanding the effects of these variables on satisfaction rates for a given contraceptive method may help health care providers to identify which method(s) may best suit their patient and assist in the counselling and method selection process. Ultimately, these data and their potential impact on counselling may contribute to an increased uptake of IUSs [Citation26–28].
Conclusions
Overall, this study demonstrates that LNG-IUS 12 is well-tolerated and has high satisfaction and continuation rates irrespective of age, parity or motivation for initiating LNG-IUS 12 usage. The evidence presented here provides further support for the suitability of LNG-IUS 12 regardless of age or parity.
Supplementary Tables 1-4
Download MS Word (58.4 KB)Acknowledgements
The authors thank all the investigators for their participation and continued commitment to the study. The authors acknowledge funding from Bayer AG, Berlin, Germany, and acknowledge Highfield, Oxford, UK, for providing medical writing assistance with funding from Bayer AG.
Disclosure statement
Dale Stovall has received honorarium from Bayer for educational lectures. Thomas Römer has received honoraria for lectures and advisory board participation from Bayer, Exeltis, Gedeon Richter, Hexal and Theramex. Gilbert Donders has been a member of a medical advisory board for Bayer Belgium since 2010, and his research centre has received fees for lectures and research from Bayer, Gedeon Richter, MSD, Medinova and Mycovia. Brian Hauck is on medical advisory boards for Allergan, Bayer, Merck, Pfizer and Searchlight. Helena Kopp Kallner has received honoraria for lectures from Actavis, Bayer, Exeltis, Gedeon Richter, Merck, Mithra, Natural Cycles, Nordic Pharma and Teva, and teaches courses sponsored by Bayer, Merck and MSD; she has also provided expert opinion for Bayer, Evolan, Exeltis, Gedeon Richter, Merck, Natural Cycles, Teva and TV4, and is an investigator in trials sponsored by Bayer, Gedeon Richter, Mithra and MSD. Julie Salomon has received personal fees from private practice. Michal Zvolanek and Ann-Kathrin Frenz are employees of Bayer AG, Berlin/Wuppertal, Germany. Anja Bauerfeind and Tanja Böhnke are full-time employees of ZEG Berlin/Kantar, Berlin, Germany. Keith Aqua, Terje Sørdal, and Eric Saucedo de la Llata have no conflicts of interest to declare.
Additional information
Funding
References
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366(21):1998–2007.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol. 2015;126(5):917–927.
- Joshi R, Khadilkar S, Patel M. Global trends in use of long-acting reversible and permanent methods of contraception: seeking a balance. Int J Gynaecol Obstet. 2015;131(Suppl 1):S60–S63.
- Agostini A, Godard C, Laurendeau C, et al. Two year continuation rates of contraceptive methods in France: a cohort study from the french national health insurance database. Eur J Contracept Reprod Health Care. 2018;23(6):421–426.
- Lindh I, Skjeldestad FE, Gemzell-Danielsson K, et al. Contraceptive use in the nordic countries. Acta Obstet Gynecol Scand. 2017;96(1):19–28.
- United Nations. Contraceptive use by method 2019. Data booklet. 2019 [cited 2020 Nov 3]. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf.
- Secura GM, Allsworth JE, Madden T, et al. The contraceptive CHOICE project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115.e1–115.e7.
- Hladky KJ, Allsworth JE, Madden T, et al. Women's knowledge about intrauterine contraception. Obstet Gynecol. 2011;117(1):48–54.
- Potter J, Rubin SE, Sherman P. Fear of intrauterine contraception among adolescents in New York city. Contraception. 2014;89(5):446–450.
- Brima N, Akintomide H, Iguyovwe V, et al. A comparison of the expected and actual pain experienced by women during insertion of an intrauterine contraceptive device. Open Access J Contracept. 2015;6:21–26.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123(3):585–692.
- Eisenberg DL, Schreiber CA, Turok DK, et al.; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- Gemzell-Danielsson K, Apter D, Hauck B, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low-dose levonorgestrel intrauterine contraceptive systems: subgroup analyses of data from a phase III trial. PLoS One. 2015;10(9):e0135309.
- Lohr PA, Lyus R, Prager S. Use of intrauterine devices in nulliparous women. Contraception. 2017;95(6):529–537.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117(5):1105–1113.
- Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and mirena. Fertil Steril. 2012;97(3):616–622.
- Nelson AL, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122(6):1205–1213.
- Merki-Feld GS, Caetano C, Porz TC, et al. Are there unmet needs in contraceptive counselling and choice? Findings of the european TANCO study. Eur J Contracept Reprod Health Care. 2018;23(3):183–193.
- Beckert V, Aqua K, Bechtel C, et al. Insertion experience of women and health care professionals in the kyleena® satisfaction study. Eur J Contracept Reprod Health Care. 2020;25(3):182–189.
- Gemzell-Danielsson K, Apter D, Dermout S, et al. Evaluation of a new, low-dose levonorgestrel intrauterine contraceptive system over 5 years of use. Eur J Obstet Gynecol Reprod Biol. 2017;210:22–28.
- Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;22:5–55.
- Liu Y, Nickleach DC, Zhang C, et al. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000Res. 2018;7:1955.
- Black KI, Lotke P, Lira J, et al. Global survey of healthcare practitioners' beliefs and practices around intrauterine contraceptive method use in nulliparous women. Contraception. 2013;88(5):650–656.
- Buhling KJ, Hauck B, Dermout S, et al. Understanding the barriers and myths limiting the use of intrauterine contraception in nulliparous women: results of a survey of european/canadian healthcare providers. Eur J Obstet Gynecol Reprod Biol. 2014;183:146–154.
- Davie JE, Walling MR, Mansour DJ, et al. Impact of patient counseling on acceptance of the levonorgestrel implant contraceptive in the United Kingdom. Clin Ther. 1996;18(1):150–159.
- Modesto W, Bahamondes MV, Bahamondes L. A randomized clinical trial of the effect of intensive versus non-intensive counselling on discontinuation rates due to bleeding disturbances of three long-acting reversible contraceptives. Hum Reprod. 2014;29(7):1393–1399.
- Ali MM, Cleland J, Shah IH. Causes and consequences of contraceptive discontinuation: evidence from 60 demographic and health surveys. 2012. [cited 2020 Jul 10]. Available from: https://www.who.int/reproductivehealth/publications/family_planning/9789241504058/en/.
- Goldthwaite LM, Creinin MD. Comparing bleeding patterns for the levonorgestrel 52 mg, 19.5 mg, and 13.5 mg intrauterine systems. Contraception. 2019;100(2):128–131.
- Dickerson LM, Diaz VA, Jordon J, et al. Satisfaction, early removal, and side effects associated with long-acting reversible contraception. Fam Med. 2013;45(10):701–707.
- Weisberg E, Bateson D, McGeechan K, et al. A three-year comparative study of continuation rates, bleeding patterns and satisfaction in australian women using a subdermal contraceptive implant or progestogen releasing-intrauterine system. Eur J Contracept Reprod Health Care. 2014;19(1):5–14.
- Bahamondes L, Brache V, Meirik O, et al. A 3-year multicentre randomized controlled trial of etonogestrel- and levonorgestrel-releasing contraceptive implants, with non-randomized matched copper-intrauterine device controls. Hum Reprod. 2015;30(11):2527–2538.
- Diedrich JT, Desai S, Zhao Q, et al. Association of short-term bleeding and cramping patterns with long-acting reversible contraceptive method satisfaction. Am J Obstet Gynecol. 2015;212(1):50.e1–50.e8.
- Bateson D, Harvey C, Trinh L, et al. User characteristics, experiences and continuation rates of copper intrauterine device use in a cohort of australian women. Aust N Z J Obstet Gynaecol. 2016;56(6):655–661.
- Goldstuck ND. Reducing barriers to the use of the intrauterine contraceptive device as a long acting reversible contraceptive. Afr J Reprod Health. 2014;18(4):15–25.
- Hauck B, Costescu D. Barriers and misperceptions limiting widespread use of intrauterine contraception among canadian women. J Obstet Gynaecol Can. 2015;37(7):606–616.
- Rosenstock JR, Peipert JF, Madden T, et al. Continuation of reversible contraception in teenagers and young women. Obstet Gynecol. 2012;120:1298–1305.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - What Is It and What Can It Tell Us? N Engl J Med. 2016;375(23):2293–2297.
- Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on Real-World evidence in health care decision making. Value Health. 2017;20(8):1003–1008.
- Heikinheimo O, Bitzer J, García Rodríguez L. Real-world research and the role of observational data in the field of gynaecology - a practical review. Eur J Contracept Reprod Health Care. 2017;22(4):250–259.