Abstract
Objective
To assess and compare the risk of unintended pregnancy in NOMAC-E2 users with levonorgestrel-containing COC (COCLNG) users in clinical practice.
Study design
In this observational study, new usersFootnote1 of NOMAC-E2 and COCLNG were recruited in Europe, Australia, and Latin America and followed for up to 2 years. Unintended pregnancy was expressed by the Pearl Index (contraceptive failures per 100 women-years [WY]), crude hazard ratios (HRcrude) and adjusted hazard ratios (HRadj).
Results
Overall, 44,559 and 46,754 users were recruited to the NOMAC-E2 and COCLNG user cohorts, respectively. There were 64 unintended pregnancies in NOMAC-E2 users (0.15 per 100 WY; 95% CI, 0.11–0.19) and 200 in COCLNG users (0.41 per 100 WY; 95% CI, 0.35–0.47). The unintended pregnancy risk was statistically significantly lower in the NOMAC-E2 cohort (p<.0001) compared to the COCLNG user cohort. The HRadj of NOMAC-E2 vs COCLNG was 0.45 (95% CI, 0.34–0.60; adjusted for age, body mass index, gravidity, COC user status, education level).
Conclusions
NOMAC-E2 demonstrated superior contraceptive effectiveness compared to COCLNG, likely due to the comparatively short hormone-free interval and possibly reinforced by the long half-life of NOMAC.
与含左炔诺孕酮的复方口服避孕药相比, 诺美孕酮和17β-雌二醇(NOMAC-E2)的使用者意外怀孕:PRO-E2研究的最终结果 摘要
目的: 评估和比较在临床中服用NOMAC-E2妇女与服用含有左炔诺孕酮的避孕药物 (COCLNG)妇女的意外怀孕的风险。
方法: 在这项观察性研究在欧洲、澳大利亚和拉丁美洲招募使用NOMAC-E2和COCLNG的新患者并随访长达2年。Pearl指数(每100名妇女每年年避孕失败[WY])、粗风险比率(HRcrude)和调整后的风险比率(HRadj)用来描述意外怀孕。
结果: 总体来说, 研究分别招募了44559名使用NOMAC-E2妇女和 46,754 名使用COCLNG 队列妇女。 NOMAC-E2 妇女中有64例意外怀孕(0.15/100 WY;95% CI, 0.11–0.19), COCLNG妇女中有200例意外怀孕(0.41/100 WY;95% CI, 0.35–0.47)。 与 服用COCLNG 妇女队列相比, NOMAC-E2 队列的意外怀孕风险在统计学上显著降低 (p<.0001)。 NOMAC-E2 与 COCLNG 的 调整后风险比为 0.45(95% CI, 0.34-0.60;根据年龄、体重指数、妊娠、COC 使用者状态、教育水平进行调整)。
结论: 与 COCLNG 相比, NOMAC-E2 表现出更好的避孕效果, 这可能是由于相对较短的无激素间隔时间, 加上NOMAC 的较长的半衰期。
1. Introduction
Combined oral contraceptives (COCs) act by inhibiting ovulation and are a reliable form of reversible birth control [Citation1]. With perfect use (efficacy or theoretical effectiveness), COCs are associated with a contraceptive failure rate of around 0.3% during the first year of use [Citation1,Citation2]. The higher failure rates observed in ‘real-life’ users are largely attributed to non-compliant use (deviating from instructions on the package inserts, e.g., missed pills [Citation3]) [Citation4] and show marked geographical variation [Citation5]. The European Active Surveillance Study on Oral Contraceptives (EURAS-OC) showed a low ‘real-use’ contraceptive failure rate of 0.75% during the first year of use [Citation6–8], while data from the United States (U.S.) arm of the International Active Surveillance of Women Taking Oral Contraceptives (INAS-OC) showed that the lowest pregnancy rate at one year was 2.1%. In the latter study, of all reported unintended pregnancies, 86% were attributed to non-compliance [Citation9].
A variety of long-established and newer fixed combination oestrogen-progestin formulations are currently marketed worldwide. Since COCs first became available in the 1960s, there have been continuous innovations in relation to drug components, hormone doses, and dosing regimens. Traditionally, COCs have been licenced in a regimen of 21 days of active treatment followed by a 7-day hormone-free interval (in which withdrawal bleeding usually occurs). Newer COCs with a reduced hormone-free interval have been developed with the aim of reducing side effects associated with hormone withdrawal (e.g., withdrawal bleeding or menstrual migraines) [Citation10]. Several studies investigating COCs with a 24/4 day-regimen (24 days active treatment and 4-day hormone-free interval) showed reduced ovarian activity and improved efficacy by extending the duration of active pill intake and shortening the hormone-free interval compared to the traditional 21/7 regimen [Citation9,Citation11].
The ovulation inhibition activity of modern COCs is primarily achieved through the action of progestin and is reinforced by the oestrogen component. The progestins on the market have different characteristics and therefore can inhibit ovulation at different doses [Citation12]. Furthermore, the progestin concentration level required to elicit a biological response is influenced by factors such as route of administration, metabolism, bioavailability, and half-life [Citation13].
Nomegestrol acetate-17β-oestradiol (NOMAC-E2) is a monophasic COC containing a fixed dose of the progestin nomegestrol acetate (2.5 mg) and the synthetic analogue of the endogenous oestrogen 17β-oestradiol (1.5 mg), which is taken for 24 days followed by 4 days of placebo. NOMAC is absorbed rapidly after oral intake (reaching a peak serum concentration within 4 hours) and has a relatively long half-life (approximately 46 hours) [Citation14] in comparison to levonorgestrel (LNG) (approximately 22 hours [Citation13]). NOMAC has a strong affinity for the progesterone receptor and has strong anti-gonadotropic activity and progesterone receptor-mediated anti-oestrogenic activity, moderate anti-androgenic activity, and is devoid of oestrogenic, androgenic, glucocorticoid or mineralocorticoid activity [Citation15]. At dosages of 1.5 mg/day or more, NOMAC effectively suppresses gonadotropic activity and ovulation in women of reproductive age [Citation16].
The Prospective Controlled Cohort Study on the Safety of a Monophasic Oral Contraceptive containing Nomegestrol Acetate (2.5 mg) and 17β-oestradiol (1.5 mg) (PRO-E2) study was designed to fulfil a post-marketing requirement to the European Medicines Agency and focussed on the risk of venous thromboembolism of NOMAC-E2 compared to the regulatory gold standard levonorgestrel-containing COCs (COCLNG) as the primary outcome (results reported separately). A pre-specified secondary outcome included the risk of unintended pregnancy in users of NOMAC-E2 compared to users of COCLNG during real-world use in a population that was representative of the actual users of the preparations (results reported here).
2. Materials and methods
The PRO-E2 study was a large, multinational, controlled, prospective, active surveillance study that followed new users (starters and restarters) of NOMAC-E2 and COCLNG. Starters were first-ever users of any COC. Restarters were restarting COC use after a minimum break of 2 months. Ethical approval was obtained as required by local law and an independent Safety Monitoring and Advisory Council (SMAC) monitored the study.
2.1. Study population
Participants were recruited by health care professionals (HCPs) during routine clinical practice in Australia, Austria, Colombia, France, Germany, Hungary, Italy, MexicoFootnote2, Poland, Russia, Spain and Sweden between August 2014 and September 2019. The overall recruitment target was 101,000 study participants (50,500 NOMAC-E2 users and 50,500 COCLNG users). All women, newly prescribed an eligible COCFootnote3 could participate if they had not used a COC in the past 2 months, signed an informed consent form and completed a baseline questionnaire in the local language.
2.2. Baseline survey and follow-up
Participants completed a baseline questionnaire to capture demographic data, reason(s) for their COC prescription (contraceptive reasons only, contraceptive and non-contraceptive reasons, non-contraception reasons only), personal and family medical history, concomitant medication, exposure to hormonal contraceptives and lifestyle factors. They were followed up directly at 6, 12 and 24 months via mail or e-mail and completed follow-up questionnaires regarding contraceptive use, pregnancy and the occurrence of other outcomes of interest.
Activities to minimise loss of information were implemented within each country as appropriate. These activities included reminding study participants via telephone, email or post and contacting their friends or relatives (based on details provided by the women at enrolment) to try to re-establish contact with the study participants. Additionally, registered letters were sent, national address registries were searched, and face-to-face interviews were conducted.
Self-reported outcomes of interest were reviewed by medical advisers. If necessary, study participants and/or the treating physicians were contacted for further information.
2.3. Evaluation
Unintended pregnancy was measured by the Pearl Index, which calculates the number of contraceptive failures per 100 women-years (WY) of exposure. Pregnancy events were assigned to the exposure at the time of conception (which may have differed from the COC prescribed at study entry). Only first confirmedFootnote4 pregnancies during study participation were included in this analysis. Confirmed pregnancies were included regardless of the reasons for contraceptive use (e.g., contraceptive or non-contraceptive reasons) reported by study participants at baselineFootnote5.
Inferential statistics were based on Cox regression analysis and crude hazard ratios (HRcrude) and adjusted hazard ratios (HRadj) were calculated. An a priori expert model defined in collaboration with the SMAC included the following pre-defined prognostic factors: age, body mass index (BMI = kg/m2), gravidity, COC user status (starter, restarter), and education level at study entry. All statistical analyses were conducted using SAS 9.4 [Citation17].
3. Results
A total of 101,498 women (49,598 NOMAC-E2 and 51,900 COCLNG users) were enrolled in the study. Of these 10,185 reported starting with an ineligible COC or no COC at all. From the remaining 91,313 women, 44,559 were allocated to the NOMAC-E2 and 46,754 to the COCLNG user cohorts.
3.1. Loss to follow-up and dropout
Overall, 16,528 women (16.3% of the study population) were lost to follow-up: 8,137 NOMAC-E2 users (16.4%) and 8,391 COCLNG users (16.2%). A total of 15,941 women (15.7% of the study population) dropped out of the study after indicating they no longer wished to participate. The dropout rate did not differ substantially between user cohorts: 7,457 NOMAC-E2 users (15.0%) and 8,484 COCLNG users (16.3%).
3.2. Baseline characteristics of user cohorts
The characteristics of the 91,313 women who were eligible for the here described analyses are displayed regarding WY of hormonal contraceptive exposure and selected baseline characteristics by user cohort in .
Table 1. Selected baseline characteristics by user cohort.
There were no substantial differences between the cohorts in relation to user status. Starters comprised 62.6% of the NOMAC-E2 cohort and 64.4% of the COCLNG cohort. Restarters comprised 37.4% and 35.6% of the NOMAC-E2 and COCLNG cohorts, respectively.
NOMAC-E2 users had a slightly higher mean age (31.0 ± 8.63 years) than COCLNG users (29.3 ± 8.53 years). A greater proportion of NOMAC-E2 users were aged 35 years or more compared with COCLNG users (32.9% and 26.5%, respectively). Mean weight (NOMAC-E2: 63.3 kg; COCLNG: 63.1 kg) and mean BMI (NOMAC-E2: 23.2; COCLNG: 23.3) were similar in both cohorts. A slightly higher proportion of NOMAC-E2 users reported ever having been pregnant (NOMAC-E2: 57.0%; COCLNG: 54.9%), while nearly identical proportions of NOMAC-E2 users and COCLNG users reported (at study entry) having had at least one live birth (90.7% and 90.9%, respectively).
At study entry, 54.9% of NOMAC-E2 users and 57.1% of COCLNG users reported obtaining a COC prescription for contraceptive reasons only. Contraceptive and non-contraceptive reasons were reported by 33.9% and 33.1% of NOMAC-E2 and COCLNG users, respectively. Meanwhile, 9.5% of NOMAC-E2 users and 8.6% of COCLNG users reported seeking a COC prescription only for non-contraceptive reasons. The most frequent non-contraceptive reasons (as reported by the study participants) included cycle regulation, painful menstrual bleeding and heavy and/or prolonged menstrual bleeding.
NOMAC-E2 users reported having achieved a higher level of education, with 43.4% having had more than a university entrance level education compared to 36.1% of COCLNG users.
3.3. Unintended pregnancies
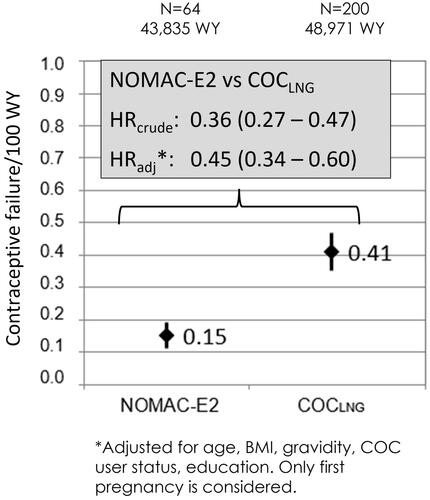
Overall, there were 64 confirmed unintended pregnancies in NOMAC-E2 users (0.15 per 100 WY; 95% CI, 0.11–0.19) and 200 in COCLNG users (0.41 per 100 WY; 95% CI, 0.35–0.47) (). The risk of unintended pregnancy was statistically significantly lower in the NOMAC-E2 cohort compared to the COCLNG cohort (p<.0001). The HRcrude for unintended pregnancy comparing NOMAC-E2 to COCLNG was 0.36 (95% CI, 0.27–0.47) and the HRadj was 0.45 (95% CI, 0.34–0.60).
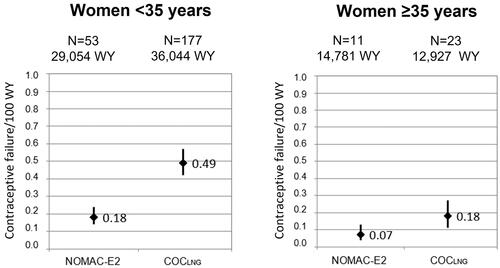
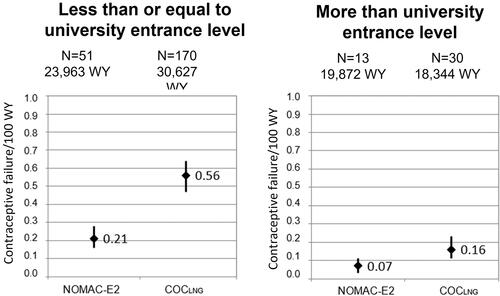
To further investigate whether the higher mean age of NOMAC-E2 users impacted the lower contraceptive failure rate in that cohort (potentially due to reduced fertility), an analysis was performed stratifying by two age categories (women under the age of 35 years and women aged 35 years and older), (). There were 53 unintended pregnancies among women under the age of 35 years in NOMAC-E2 users (0.18 per 100 WY; 95% CI, 0.14–0.24) and 177 in COCLNG users (0.49 per 100 WY; 95% CI, 0.42–0.57). Among women aged 35 years and older, there were 11 unintended pregnancies in NOMAC-E2 users (0.07 per 100 WY; 95% CI, 0.04–0.13) and 23 in COCLNG users (0.18 per 100 WY; 95% CI, 0.11–0.27). In comparison to COCLNG, NOMAC-E2 use appeared particularly effective at preventing unintended pregnancy among women in the lower age strata. In women aged 35 years and older, a similar trend could be observed but no statistically significant difference could be detected. This might at least partially depend on the lower number of unintended pregnancies in this age group. Because the educational level of a study participant could affect her understanding of and compliance with correct COC use, a stratified analysis by educational level was conducted. The numbers of unintended pregnancies in women who reported at study entry that they had less than or equal to a university entrance level education and rates per 100 WY were as follows: 51 in NOMAC-E2 users (0.21 per 100 WY; 95% CI, 0.16–0.28) and 170 in COCLNG users (0.56 per 100 WY; 95% CI, 0.47–0.64) (). Among women who reported more than a university entrance level education at study entry, there were 13 unintended pregnancies in NOMAC-E2 users (0.07 per 100 WY; 95% CI, 0.03–0.11) and 30 in COCLNG users (0.16 per 100 WY; 95% CI, 0.11–0.23).
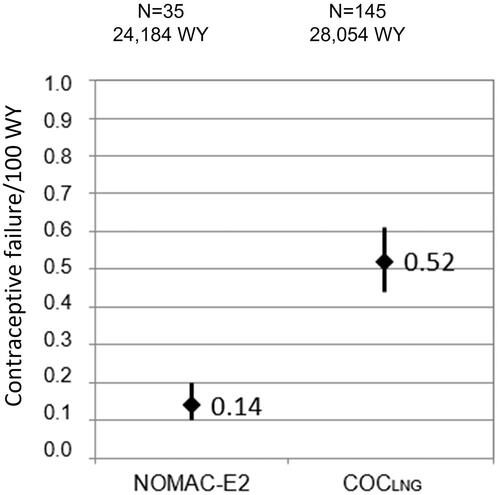
In this real-world study, women who used a COC for non-contraceptive reasons (e.g., cycle control) might not have been at risk of unintended pregnancy because they may not have been sexually active. Therefore, a sensitivity analysis was performed in which the dataset was limited to women who reported at study entry that their reason for COC use was solely to prevent pregnancy. In this analysis, there were 35 unintended pregnancies in NOMAC-E2 users (0.14 per 100 WY; 95% CI, 0.10–0.20) and 145 in COCLNG users (0.52 per 100 WY; 95% CI, 0.44–0.61) (see ).
4. Discussion
The results of the PRO-E2 study have shown that the risk of unintended pregnancy was significantly lower in NOMAC-E2 users compared to COCLNG users. The baseline characteristics between the user cohorts did not differ substantially except in relation to mean age and educational level. The higher mean age of NOMAC-E2 users could impact their rate of unintended pregnancy given that fertility decreases with age [Citation18]. However, an age-stratified analysis showed that the contraceptive effectiveness of NOMAC-E2 was particularly pronounced among younger women (aged under 35 years). Therefore, the lower rate of contraceptive failure in NOMAC-E2 users did not appear to be associated with their higher mean age.
COC use which deviates from the recommended guidelines contributes to the risk of unintended pregnancy. The importance of education and counselling for proper contraceptive use has been highlighted in several studies [Citation19, Citation20] and various social and demographic factors have been shown to be predictive of contraceptive compliance [Citation21–23]. Women with a lower educational level may have more difficulty adhering to a regular intake schedule and complying with instructions on the package insert (e.g., in case of missed pills, vomiting, etc.), as was recently found in a clinical trial [Citation21]. The NOMAC-E2 cohort reported a higher level of education; if their higher educational level contributed to increased compliance, this may have influenced the superior effectiveness of NOMAC-E2 in preventing pregnancy. However, a stratified analysis by educational level showed that NOMAC-E2 was particularly effective at preventing pregnancy among less educated women. Therefore, the lower rate of unintended pregnancy among NOMAC-E2 users was not associated with their higher educational level (and presumably more compliant use).
The superior effectiveness of NOMAC-E2 compared with COCLNG could perhaps be attributed to its longer half-life [Citation13] and an intake regimen with a shorter hormone-free interval (4 days compared to 7 days for most LNG COCs). These characteristics were shown to make NOMAC-E2 more ‘forgiving’ in the sense that contraceptive effectiveness is maintained even when pills are missed [Citation16]. In support of these findings, results from the INAS-OC study showed the improved contraceptive effectiveness of a 24-day regimen of drospirenone when compared to a 21-day dosing schedule [Citation9] and the INAS-SCORE study [Citation24] showed a lower contraceptive failure rate of a multiphasic dienogest/E2-valerate preparation with a short 2-day hormone-free interval compared to LNG COCs with a 7-day hormone-free break.
Based on the real-world approach of PRO-E2 study, women sometimes used COCs for non-contraceptive reasons (e.g., cycle control) and were perhaps not at risk of pregnancy because they were not sexually active. A sensitivity analysis restricting the dataset to women who reported that their COC prescription was for contraceptive reasons only showed similar results to those of the overall cohort analysis. However, it is important to note that reasons for COC use were asked only at study entry and these reasons may have changed during the course of follow-up (e.g., a patient who started COC use only for contraceptive reasons may have separated from her partner but continued using the COC for its other benefits).
Apart from the usual limitations that apply to observational research [Citation25], a further limitation of the PRO-E2 study was a higher loss to follow-up (LTFU) rate than in previous similar studies. This results in part from the implementation of the General Data Protection Regulation (GDPR) in May 2018 and emergence of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic towards the end of the study adversely impacting upon the ability of the investigators to communicate with the study participants. Importantly, however, LTFU rates were non-differential between the NOMAC-E2 and COCLNG cohorts. Another limitation was that a direct comparison with a COCLNG, which has the same 24/4 day regimen as NOMAC-E2, was not possible, as such a preparation is not currently on the market [Citation10]. Based on the primary study objective (to assess the risk of venous thromboembolism), the regulatory gold standard COCLNG was chosen as comparator. As contraceptive effectiveness was a secondary objective of the study, the chosen comparator is not the most effective contraceptive available on the market.
The study benefitted from several strengths. Only new users were enrolled; selection bias introduced by the inclusion of prevalent users was eliminated. Relying upon patient reports ensured that almost all outcomes of interest were captured and these self-reports were subsequently confirmedFootnote6 via the women or their physicians. The study design also enabled the capture of important potential confounders such as age, BMI, gravidity and education level. Furthermore, precise information on COC use (specific COCs, stopping and switching patterns) was captured and women contributed WY to several (sub-)cohorts depending upon their real-life use. The PRO-E2 study was designed to capture real-world COC use. Study participants were recruited by a broad range of HCPs (e.g., gynaecologists, general practitioners, midwives) and all eligible new users could participate (e.g., there were no medical inclusion or exclusion criteria). Therefore, the generalisability of the results to the general population is high.
In conclusion, the PRO-E2 study found a higher contraceptive effectiveness in NOMAC-E2 users compared to COCLNG users, consistent with the short hormone-free interval of NOMAC-E2 and comparatively long half-life of NOMAC. NOMAC-E2 was particularly effective at preventing pregnancy in younger women and those with a lower educational level.
Acknowledgements
The authors acknowledge the valuable scientific contribution of the SMAC. Their critical review of the data and challenging discussions substantially enhanced the overall quality of the study and the interpretation of the results. SMAC members included David Grimes, Michael Lewis, Robert Reid, Samuel ShapiroFootnote7, Carolyn Westhoff, Ulrich Winkler and Stephanie Teal. Advisors to the SMAC included Jochen Albrecht and Fritz von WeizsäckerFootnote8. The authors also thank the colleagues in the local field organisations who conducted the day-to-day field work. Their remarkable dedication and flexibility despite the challenging circumstances caused by the pandemic ensured the success of the study. The authors also thank the ZEG Berlin staff members (statisticians, data managers, event validation staff, medical advisers) whose contribution to the study was invaluable.
Disclosure statement
Carol Koro and Julia DiBello are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and hold stock/stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Merck Sharp & Dohme Corp. was exclusively licenced by Theramex to sell Zoely in certain territories (other than the U.S. and Canada) until January 2020, and held Marketing Authorisations for the product in the non-EU markets of such territory. In January 2020, these rights were transferred back to Theramex. The rights to sell the product in the U.S. and Canada pursuant to a licence from Teva remained with Merck until spin-off of Organon on June 2, 2021.
Additional information
Funding
Notes
1 First-ever users of an eligible COC or restarting with an eligible COC (same COC as before or a new COC) after a break of at least 2 months.
2 Due to non-compliance with study procedures, data contributed by 625 study participants in Mexico were excluded from these analyses as advised by the SMAC. The local field organisation deviated from the required storage requirements and destroyed 545 of the informed consent forms. After recontacting the women, proof of consent was available for 235 women.
3 1) NOMAC-E2, 2) COCLNG monophasic preparation containing 20-30mcg of ethinylestradiol, 3) COCLNG multiphasic preparation containing up to 40mcg of ethinylestradiol.
4 If there was ambiguity concerning the date of conception or COC use, the woman and/or her HCP were contacted for clarification. The consent form included permission to contact any treating physician to follow up on specific outcomes.
5 Study participants reported their reason(s) for COC use only at study entry; their reason(s) for use may have changed during follow-up (e.g., a woman who began using a COC for contraceptive only reasons may have experienced a relationship change and continued using the COC for non-contraceptive reasons).
6 If there was ambiguity concerning the date of conception or COC use, the woman and/or her HCP were contacted for clarification.
7 Died 19 April 2016.
8 Died 19 November 2019.
References
- Barnett C, Hagemann C, Dinger J, et al. Fertility and combined oral contraceptives - unintended pregnancies and planned pregnancies following oral contraceptive use – results from the INAS-SCORE study. Eur J Contracept Reprod Health Care. 2017;22(1):17–23.
- Mestad RE, Kenerson J, Peipert JF. Reversible contraception update: the importance of Long-Acting reversible contraception. Postgrad Med. 2009;121(4):18–25.
- QuickStats: percentage of women who missed taking oral contraceptive pills* among women aged 15-44 years who used oral contraceptive pills and had sexual intercourse, overall and by age and number of pills Missed – National survey of family growth, United States, 2013-2015†. MMWR Morb Mortal Wkly Rep. 2017;66:965.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011;5:CD004861.
- Trussell J, Portman D. The creeping pearl: Why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception. 2013;88(5):604–610.
- Dinger JC, Heinemann, Lothar AJ, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the european active surveillance study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75(5):344–354.
- Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94(4):328–339.
- Dinger JC, Cronin M, Möhner S, et al. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009;201(3):263.e1–263.e9.
- Dinger J, Minh TD, Buttmann N, et al. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011;117(1):33–40.
- Read CM. New regimens with combined oral contraceptive pills-moving away from traditional 21/7 cycles. Eur J Contracept Reprod Health Care. 2010;15(Suppl 2):S32–S41.
- Klipping C, Duijkers I, Trummer D, et al. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception. 2008;78(1):16–25.
- Bastianelli C, Farris M, Rosato E, et al. Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation. Expert Rev Clin Pharmacol. 2018;11(11):1085–1098.
- Bick AJ, Louw-Du Toit R, Skosana SB, et al. Pharmacokinetics, metabolism and serum concentrations of progestins used in contraception. Pharmacol Ther. 2021;222:107789
- Gerrits MGF, Schnabel PG, Post TM, et al. Pharmacokinetic profile of nomegestrol acetate and 17β-estradiol after multiple and single dosing in healthy women. Contraception. 2013;87(2):193–200.
- Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61(1-2):171–180.
- Ruan X, Seeger H, Mueck AO. The pharmacology of nomegestrol acetate. Maturitas. 2012;71(4):345–353.
- SAS Institute Inc. The SAS system for windows. Cary (NC): SAS.
- Female age-related fertility decline. Committee opinion no. 589. Fertility and Sterility. 2014;101:633–634.
- Culwell KR, Adams Hillard PJ. Patient education and contraceptive compliance. GLOWM. 2009. DOI:https://doi.org/10.3843/GLOWM.10378
- Dempsey A, Choi A. Strategies to improve compliance among oral contraceptive pill users: a review of the literature. OAJC. 2014;5:17.
- Westhoff CL, Torgal AT, Mayeda ER, et al. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012;85(5):465–469.
- Moreau C, Bouyer J, Gilbert F, et al. Social, demographic and situational characteristics associated with inconsistent use of oral contraceptives: evidence from France. Perspect Sex Reprod Health. 2006;38(4):190–196.
- Martínez-Astorquiza-Ortiz de Zarate T, Díaz-Martín T, Martínez-Astorquiza-Corral T, MIA Study InvestigatorsEvaluation of factors associated with noncompliance in users of combined hormonal contraceptive methods: a cross-sectional study: results from the MIA study. BMC Womens Health. 2013;13:38
- Barnett C, Dinger J, Minh TD, et al. Unintended pregnancy rates differ according to combined oral contraceptive - results from the INAS-SCORE study. Eur J Contracept Reprod Health Care. 2019;24(4):247–250.
- Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol. 1991;133(7):635–648.