Abstract
Purpose
To describe the effects of estetrol (E4) 15 mg/drospirenone (DRSP) 3 mg on physical and emotional premenstrual and menstrual symptoms.
Materials and Methods
We used Menstrual Distress Questionnaire (MDQ) data from a phase-3 trial (NCT02817828) in Europe and Russia with participants (18 − 50 years) using E4/DRSP for up to 13 cycles. We assessed mean changes in MDQ-t-scores from baseline to end of treatment in premenstrual (4 days before most recent flow) and menstrual (most recent flow) scores for 4 MDQ domains in starters and switchers (use of hormonal contraception in prior 3 months) and performed a shift analysis on individual symptoms within each domain.
Results
Of 1,553 treated participants, 1,398(90.0%), including 531(38%) starters, completed both MDQs. Starters reported improvements for premenstrual Pain (−1.4), Water Retention (−3.3) and Negative Affect (−2.5); and for menstrual Pain (−3.5), Water Retention (−3.4), and Negative Affect (−2.7) (all p < 0.01). For switchers, no changes were significant except an increase in premenstrual (+1.0, p = 0.02) and menstrual (+1.5, p = 0.003) Water Retention. We observed a change in symptom intensity in >40% of participants for Cramps, Backache and Fatigue (domain Pain), Painful or Tender Breast and Swelling (domain Water Retention) and Mood Swings and Irritability (domain Negative Affect).
Conclusion
E4/DRSP starters experienced significant improvements in the domains Pain, Water Retention and Negative Affect particularly benefiting those with more severe baseline symptoms. Switchers showed minimal changes.
SHORT CONDENSATION
A phase 3 study in Europe and Russia showed that Estetrol/Drospirenone, a new combined oral contraceptive, significantly improved the MDQ scores for domains Pain, Water Retention and Negative Affect in women starting COC use, while switchers showed minimal changes.
Introduction
Menstruation-related symptoms have an important impact on user preferences for contraceptives [Citation1]. When considering method choices, users incorporate information on contraceptive reliability, efficacy, and ease of use along with effects that can impact quality of life such as irregular bleeding, uterine cramping, breast tenderness, mood alterations, anxiety, water retention, concentration and headache [Citation1–3].
Estetrol (E4) is a natural oestrogen marketed first for clinical use in a combined oral contraceptive containing E4 15 mg and drospirenone (DRSP) 3 mg. E4 is produced by the human foetal liver during pregnancy and synthesised from a plant source for clinical use. E4 displays selective tissue activity distinct from other natural and synthetic oestrogens [Citation4]; as such, its clinical effects may be different compared to other oestrogen-containing contraceptives.
In Phase 2 clinical trials, E4/DRSP user acceptability, well-being and treatment satisfaction were compared with E4/LNG over 6 cycles using a self-reported Subject Satisfaction and Health-Related Questionnaire [Citation5]. E4 15 mg/DRSP 3 mg, compared to E4 15 mg/LNG 150 mcg, was associated with higher user acceptability (study completion 72/79 (91.1%) vs 60/80 (80.0%), p = 0.048) and satisfaction at cycle 6 (57/78 (73.1%) vs 38/75 (50.6%), p < 0.001). Well-being was significantly better with E4/DRSP compared to E4/LNG (odds ratio 2.00 [95% confidence intervals 1.13- 3.53]). These findings suggest a benefit of E4/DSRP over E4/LNG.
In two Phase 3 clinical trials, E4/DRSP showed high contraceptive efficacy, a predictable bleeding pattern, and a favourable safety and tolerability profile [Citation6,Citation7]. The studies included a longitudinal evaluation of bothersome menstrual symptoms using the Menstrual Distress Questionnaire (MDQ). The MDQ is a self-reported standard validated instrument for measuring cyclical perimenstrual symptoms and comprised of 46 items clustered in 8 domains: ‘Pain’, ‘Water Retention’, ‘Negative Affect’, ‘Impaired Concentration’, ‘Autonomic Reactions’, ‘Behaviour Change’, ‘Arousal’, and ‘Control’ (Supplementary Table 1) [Citation8]. In this planned secondary analysis, we present the MDQ results for the first 4 domains on physical and emotional premenstrual and menstrual symptoms from the E4/DRSP Phase 3 trial conducted in Europe and Russia.
Materials and methods
Trial design
The E4/DRSP Phase 3 trial in Europe and Russia was a multicentre, open-label, single arm study conducted from June 2016 to April 2018; the details of the study entry criteria and study visit schedule have previously been described (NCT02817828) [Citation6]. Briefly, the trial included healthy, heterosexually active, pre-menopausal participants aged 18–50 years with a body mass index (BMI) ≤35 kg/m2 and a history of regular menstrual cycles (21–35 days) when not using hormones. We included participants who had used oral, transdermal, vaginal, implantable, and intrauterine hormones within the 3 months before enrolment (switchers) and those who had not (starters). Participants received E4 15 mg DRSP 3 mg in a blister pack containing 24 active and 4 inactive tablets to be taken once daily for up to 13 cycles. Enrolled participants had scheduled follow-up evaluations at Cycles 2, 4, 7, and 10 and within 3 weeks of completing Cycle 13. Participants completed a baseline MDQ on or before the first day of E4/DRSP intake and at end of treatment (EoT) from Day 7–14 of Cycle 13 or at an early termination visit. MDQs in local language were used with validated translations except for the Polish translation. Participants rated symptoms occurring during the premenstrual (4 days before most recent flow), menstrual (most recent flow) and intermenstrual (the remainder of the cycle) phases. Items were scored on a 5-point scale ranging from 0 (no experience of symptoms) to 4 (symptoms are present, severe) for the 46 items/symptoms in the eight MDQ domains (Supplementary Table 1).
Analysis
For this analysis, we included all participants who received at least one dose of E4/DRSP and completed both a baseline and EoT MDQ. For each of the two menstrual phases, we excluded a domain score for an individual participant if the score for more than 2 domain items was missing [Citation8]. When a score for one domain item was missing, we calculated the mean score of the remaining items and added it to the total raw score [Citation9]. We calculated the domain raw scores (sum of item scores within each domain) (Supplementary Table 2) were rounded to the nearest whole number and converted to t-scores using the conversion tables from the MDQ-C manual [Citation9]. This conversion allows a comparison of MDQ results between cycles, across cycle phases, and between participants [Citation9].
We focused this evaluation on the 4 domains related to the menstrual symptoms previously demonstrated to be the most bothersome [Citation10]: the physical domains of pain (6 items) and water retention (4 items) and the emotional domains of negative affect (8 items) and impaired concentration (8 items). The domains ‘Behaviour Change,’ ‘Autonomic Reactions’, ‘Arousal’, and ‘Control’ contain questions unrelated to the most bothersome menstrual symptoms. Because bothersome symptoms are typically not prevalent during the intermenstrual phase [Citation8,Citation9], we only evaluated outcomes in the premenstrual and menstrual phases.
We calculated MDQ domain t-scores at baseline and EoT for all participants and stratified by starters and switchers. The distribution of the change from baseline t-scores approximately followed a normal distribution. We compared mean EoT domain t-scores versus baseline using a two-sided paired t-test with α set at 0.05. We evaluated the proportion of observations that were outliers (more than 2 standard deviations larger than the mean) and evaluated outcomes with and without the outliers. We also evaluated outcomes with and without data obtained before Cycle 9 to assess the effect of early discontinuation.
We performed a shift analysis on the individual symptoms within each domain by calculating the proportion of participants shifting between intensity categories (severe, strong, moderate, mild or none) at baseline and EoT. We selected the items with at least 10% of participants with severe or strong complaints at baseline and a change in intensity in at least 40% of participants to create shift analysis figures. For the shift analyses, we defined significant improvement as a more than 10% difference between improvement rate and deterioration rate.
We used SAS® software (version 9.4) for Windows® to perform statistical analyses and considered a p < 0.05 as significant.
Results
Of the 1,553 participants who started study treatment, 1,398 (90.0%) completed both a baseline and EoT MDQ and are included in this analysis. Participants completed most EoT assessments in Cycle 9 − 13 (n = 1,254 [89.7%]), with 91 (6.5%) in Cycle 5 − 8, and 53 (3.8%) in Cycle 1 − 4 (Supplemental Figure 1). Demographics and characteristics for the analysis population are presented in .
Table 1. Demographic characteristics of participants who completed the MDQ at baseline and at end of treatment in the Europe/russia phase 3 trial with E4/DRSP (N = 1398).
The proportion of outliers was less than 5% for all domains and inclusion of their MDQ scores did not modify the overall results. Including the 10.3% of EoT MDQ scores obtained before Cycle 9 did not significantly modify the overall results (data not shown); therefore, we included the MDQ scores of all participants.
MDQ t-scores
T-scores for the 4 main MDQ domains for all participants are displayed in . Mean t-scores decreased significantly from baseline to EoT for menstrual ‘Pain’ (-1.4), and premenstrual and menstrual ‘Negative Affect’ (-0.7 and −1.1, respectively). Raw scores for all domains and t-scores for the other 4 MDQ domains are provided in Supplemental Tables 2 and 3, respectively.
Table 2. Mean baseline, EoT and change from baseline for menstrual and premenstrual MDQ t-scores in the Europe/russia phase 3 trial with E4/DRSP.
T-scores for starters and switchers are presented in and . Overall, in the premenstrual and menstrual phases, numerically mean t-scores at baseline were higher for starters than for switchers for all domains. The mean t-scores for starters (n = 531) decreased significantly from baseline to EoT for premenstrual and menstrual ‘Pain’ (-1.4 and −3.5; respectively), ‘Water Retention’ (-3.3 and −3.4, respectively), and ‘Negative Affect’ (-2.5 and −2.7, respectively). For switchers (n = 867), t-scores increased significantly for premenstrual and menstrual ‘Water Retention’ (+1.1 and +1.5, respectively).
Figure 1. Mean baseline and EoT premenstrual [A] and menstrual [B] MDQ t-scores for starters and switchers in the Europe/Russia phase 3 trial with E4/DRSP.
![Figure 1. Mean baseline and EoT premenstrual [A] and menstrual [B] MDQ t-scores for starters and switchers in the Europe/Russia phase 3 trial with E4/DRSP.](/cms/asset/962865aa-cdb8-474f-a782-fae9667a28e6/iejc_a_2359117_f0001_c.jpg)
Table 3. Mean baseline, EoT and change from baseline for premenstrual and menstrual MDQ t-scores in the Europe/russia phase 3 trial with E4/DRSP - starters and switchers.
MDQ - shift analysis
Seven of the total 26 symptoms had at least 10% of participants with severe or strong symptoms at baseline and showed changes in intensity of more than 40% of participants in either menstrual or premenstrual symptoms among switchers or starters. These symptoms were Cramps, Backache and Fatigue in the domain ‘Pain’, Painful or tender breasts and Swelling in the domain ‘Water retention’, and Mood swings and Irritability in the domain ‘Negative affect’ (). We observed changes primarily in starters, who reported notable improvements (more than 10% difference between participants improving versus worsening) for premenstrual Painful or tender breast, Swelling, Mood swings and Irritability and for menstrual Cramps, Backache, Painful or tender breasts, Swelling, Mood swings and Irritability (). The shifts were more pronounced (skipping two or more intensity levels) for those reporting improvement compared to those reporting worsening ( and Supplemental Figures 2–5). These patterns were observed in both premenstrual and menstrual symptoms, as well as for starters and switchers, although improvement was less prominent for switchers compared to starters.
Figure 2. Shift analysis for premenstrual and menstrual symptom scores for Painful or tender Breasts in the physical domain ‘Water Retention’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
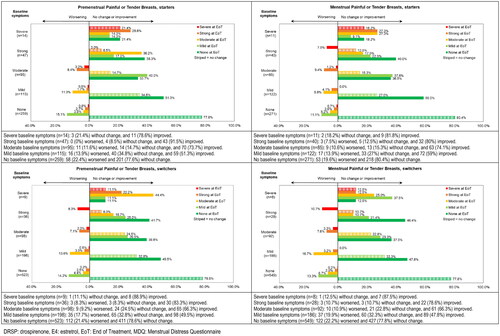
Figure 3. Shift analysis for premenstrual and menstrual symptom scores for Mood Swings in the emotional domain ‘Negative Affect’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
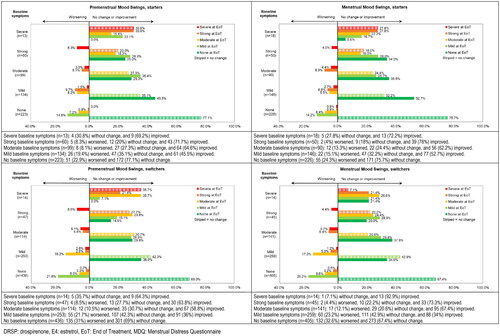
Figure 4. Shift analysis for premenstrual and menstrual symptom scores for Irritability in the emotional domain ‘Negative Affect’ for starters and switchers who completed the MDQ at baseline and at end of treatment in the Europe/Russia phase 3 trial with E4/DRSP.
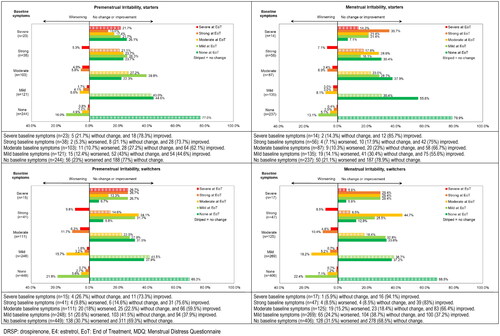
Table 4. Overview of shift analysis with % of participants remaining stable, improving and worsening and % of participants with severe or strong symptoms at baseline for premenstrual and menstrual symptoms in the Europe/Russia phase 3 trial with E4/DRSP - starters and switchers.
In the group of switchers, the percentage of participants with strong or severe symptoms at baseline was lower compared to starters, except for Headache (). Notably menstrual Headache showed more improvement in switchers compared to starters.
More detailed information on the actual shifts per baseline intensity score for the 3 symptoms with changes in over 50% of participants are provided in (Painful or tender breasts, Mood swings and Irritability) and in supplemental Figures 2–5 for symptoms with changes in 40–50% of participants (Cramps, Backpain, Fatigue and Swelling).
Discussion
Findings and interpretation
In this analysis, we used MDQ data from almost 1,400 healthy participants in Europe and Russia who used E4/DRSP for up to 13 months and assessed changes in premenstrual and menstrual scores for the physical and emotional MDQ domains of ‘Pain’, ‘Negative Affect’, ‘Impaired Concentration’ and ‘Water Retention’. Several studies have demonstrated that these domains are the most severely affected by menstruation and most directly impact health-related quality of life [Citation3,Citation11–14]. Overall, mean baseline t-scores were in the normal range and, except for the scores for the domain ‘Impaired Concentration’, mean scores decreased at EoT. For hormonal contraception starters, the decreases in scores for the domains ‘Negative Affect’, ‘Water Retention’, and ‘Pain’ were significant whereas the switchers did not experience improvement. Starters had numerically higher baseline MDQ scores than switchers, potentially reflecting a pre-study beneficial effect on MDQ domains from prior hormonal contraceptive use. Switchers in our study reported minimal to no MDQ score changes, a finding different from starters, suggesting the beneficial effect from their prior hormonal contraceptive was maintained with E4/DRSP.
In the t-score analysis, starters reported improvements for premenstrual and menstrual symptoms across all four domains, with the largest changes noted for menstrual ‘Pain’ and the smallest for menstrual ‘Impaired Concentration’.
Because we enrolled healthy women with low MDQ baseline scores, large changes in mean scores would not be expected. For that reason, we also performed shift analyses to describe changes from baseline for each symptom, providing more details on E4/DRSP impact for a specific sign or symptoms. The shift analysis primarily noted intensity shifts for ‘Cramps’, ‘Backache’ and ‘Fatigue’ in the domain ‘Pain’, for ‘Painful or Tender Breast’ and ‘Swelling (Breast/Abdomen) in the domain ‘Water Retention’, and for ‘Mood Swings’ and ‘Irritability’ in the domain ‘Negative Affect’. Consistent with the outcome of the t-score analysis, limited shifts were observed for symptoms in the domain of ‘Impaired Concentration’.
Switchers reported minimal changes overall, although we did find a limited but statistically significant worsening of premenstrual and menstrual Water Retention. The changes in t-scores were small in comparison to those associated with the observed improvements in the trial. Such a limited worsening of premenstrual and menstrual water retention symptoms has also been described with MDQ evaluations in EE/DRSP users [Citation13].
We did not observe clear changes in the domain Impaired Concentration. These findings align with the results of factor structure analyses conducted by Boyle [Citation15], which indicated that Impaired Concentration is not an independent domain of the MDQ in a sample of young healthy women (mean age 21.1 years), 35% of whom were using an oral contraceptive. Boyle [Citation15] suggested that Impaired Concentration might have emerged as a distinct factor of the MDQ if the analysis had been based on data from users with more severe symptoms.
Results in the context of what is known
Because hormonal contraceptive use can impact menstrual symptoms both positively and negatively [Citation16–18], assessing the effect of a new COC is relevant. Negative effects on well-being and mental health have been linked to the oestrogen to progestogen ratio of contraceptives, age, and predisposing factors such as ongoing mental disorders, psychiatric symptoms, dysmenorrhoea, and premenstrual mood symptoms prior to OC use [Citation19–22]. In addition, menstrual pain symptoms are common among women of reproductive age [Citation23–25] and water retention that causes premenstrual breast tenderness can negatively impact women’s quality of life as well [Citation26–28].
COCs containing ethinylestradiol (EE) and levonorgestrel (LNG) are commonly prescribed. In 2016, Zethraeus and associates [Citation29] reported the results of a double-blind randomised trial in 340 women who received EE 30 μg/LNG 150 μg or placebo for 3 months. Assessments using the Psychological General Well-Being Index and the Beck Depression Inventory showed that EE/LNG use decreased general well-being compared with placebo.
While EE/DRSP is considered frequently as a treatment for premenstrual syndrome (PMS), the data is generally of low-quality [Citation30]. PMS may improve in the first three cycles of use but proven benefit thereafter as well as benefit in patients with less severe symptoms is limited [Citation30]. However, EE/DRSP is a proven treatment for premenstrual dysphoric disorder [Citation30–34]. Overall, EE/DRSP appears more favourable in terms of mood symptoms than combination formulations with other progestins such as LNG [Citation35,Citation36]. A randomised, single-blind, seven cycle study using 21/7-day regimens of EE 30 µg/DRSP 3 mg and EE 30 µg/LNG 150 µg found that MDQ t-scores for the domains Water Retention and Impaired Concentration did not change and were comparable between both COCs [Citation37]. EE/DRSP, however, was significantly better in alleviating negative affect symptoms during the menstrual phase (median t-score decrease −3; p < 0.05). In addition, more subjects in the EE/DRSP group reported significant improvement in physical well-being (60% vs 46%; p < 0.05).
Studies with E2/NOMAC suggest this COC has beneficial effects on menstrual symptoms and well-being/quality of life [Citation11,Citation38]. A pooled analysis from two clinical trials [Citation11] showed that women who used E2/NOMAC (24/4 regimen, n = 2631) for 13 cycles reported decreases in MDQ t-scores for the domains Pain, Water Retention, Negative affect, Impaired Concentration, and Behaviour Change while changes with the comparator EE/DRSP (21/7 regimen, n = 891) were less pronounced. As in our study, starters had higher (i.e., worse) baseline scores than switchers, resulting in numerically greater decreases in t-scores for starters than for switchers (no inferential statistics calculated). Similar, but smaller benefits were observed for E2/NOMAC in the premenstrual phase. The premenstrual MDQ t-scores reported by women using E2/NOMAC were significantly different versus baseline in comparison to those reported by women using EE/DRSP (21/7 regimen), which could be related to a more hormone stable regimen with E2/NOMAC.
Clinical implications
In Phase 2 clinical trials, E4/DRSP caused negligible effects on endocrine, metabolic, and haemostasis parameters [Citation39,Citation40], and showed higher levels of user acceptability, well-being, and satisfaction than E4/LNG combinations [Citation5].
Menstrual symptoms are a common reason for women to discontinue COC use which can increase the risk of unplanned pregnancies if other less reliable or no methods are used [Citation20,Citation41,Citation42]. The positive effects on premenstrual and menstrual symptoms especially for starters as reported here are therefore important and make E4/DRSP a welcome addition to the contraceptive options available to women. Careful structured counselling when starting hormonal forms of birth control should be encouraged, taking into account the wishes of the user, efficacy, safety (e.g., VTE risk), and quality of life impact [Citation43,Citation44].
Research implications
Our analysis of the MDQ data shows a beneficial effect of E4/DRSP on physical and emotional premenstrual and menstrual symptoms, especially in hormonal contraceptive starters. Comparative clinical trials are required to evaluate these effects in relation to other COCs. Additionally, since we assessed the effects of E4/DRSP in a population with few participants having severe or strong symptoms at baseline, studies evaluating E4/DRSP in patients specifically with significant symptoms are needed to understand the impact in those populations.
Strengths and limitations
We performed our analysis in a large sample of users without pre-existing major menstrual complaints thus allowing to assess negative and positive changes which have proven to be important for adherence and satisfaction. We used the validated Moos MDQ questionnaire, which is generally used to track improvement in menstrual symptoms when on contraception and was also used in studies with other COCs. In addition to the standard MDQ t-scores analysis, we also analysed the shifts in individual symptoms within each domain. This approach enabled us to identify the symptoms that are responsible for the changes within the domains. Source data verification was performed as part of study quality control. The main goal of our Phase 3 trial was to assess the contraceptive efficacy and safety of E4/DRSP and the MDQ t-score analysis was a planned secondary objective, while the presentation of the shift analysis was performed post hoc. Consequently, the trial was not specifically designed for MDQ analysis, and no criteria were used to enrol participants with menstrual-related complaints. The trial did not include comparators and predisposing factors were not assessed. Also, information on the specific content of oral contraceptives used before entering the trial was not obtained. A more regular assessment (e.g., at baseline, 3, 6, 9 and 12 months) in women with higher MDQ baseline scores could have added more insight in the timing of the response to E4/DRSP on the burden of menstruation symptoms.
The study did not include the assessment of the individual patient’s rating of the observed changes in the symptoms and, therefore, cannot directly relate statistical to clinical significance, but it provides indirect evidence on patients’ positive or negative experiences related to menstrual symptoms. Shift analyses revealed more informative insights into specific symptom changes, highlighting significant improvements particularly in individuals with more severe symptoms at baseline.
Conclusions
The analysis of MDQ responses of 1,398 E4/DRSP phase 3 trial participants in Europe and Russia showed that starters experienced improvements in the domains Pain, Negative Affect, and Water Retention. Switchers showed minimal changes in the domain Water Retention.
Ethical approval
The study design was based on the Declaration of Helsinki, ICH E6 (R2) Good Clinical Practice guidelines, US Food and Drug Administration and European Medicines Agency (EMA) guidelines. The trial centre Independent Ethics Committees approved the trial at the different study sites. The full list of Ethics committees and approval dates was previously published Gemzell-Danielsson et al. (2022) [Citation6].
Supplemental Material
Download MS Word (710.4 KB)Acknowledgements
The authors would like to acknowledge the contributions of the principal investigators and staff at the 69 centres in Europe and Russia and thank the members of the Estelle Scientific Advisory Boards for their valuable advice. Patricia de Groot, PhD and Silvia Paz, MD, and Mireille Gerrits PharmD (Terminal 4 Communications, Hilversum, the Netherlands) provided Medical Writing support. Fabrice Nollevaux, Maud Hennion, and Clément Laloux (Pharmalex, Belgium) provided statistical support. PRA Health Sciences was the Contract Research Organization for the execution of the study.
Disclosure statement
JB Invited lecturer and advisor receiving honoraria from Bayer AG, MSD, Exeltis, Gedeon Richter, Actavis, Theramex, Labatec, Abbott, Mithra, Libbs.
CB serves on the Advisory Boards of Bayer AG, Bayer Canada, Astellas, BioSyent and has received honoraria from Merck Canada, Pfizer, Lupin Pharma, Astellas and research grants from Incyte, Mylan and Exeltis.
JZ has no conflict of interest to declare.
SW serves on an advisory board for Bayer and MSD.
TP serves on an Advisory Board for Exeltis, Merck and has received honoraria from Astra Zeneca, Exeltis, Ferring, Merck and MSD. Her research is funded by the Finnish Academy, Sigrid Jusélius Foundation, the Finnish Medical Foundation and Roche.
LS serves as a consultant for Bayer Pharmaceuticals (Russia) and for Gedeon Richter (Russia)
IA has served as an ad hoc speaker for Bayer Pharma AG (Russia), TEVA (Russia), Astellas (Russia), Roche Diagnostics Rus LLC (Russia), Avexima, Bionorica (Russia), CSC Pharma, and Aspen Health LLC.
KGD has served as an ad hoc speaker and/or member of advisory boards for Exelgyn, Campus Pharma,HRA Pharma, Exeltis, Bayer AG, Organon (MSD), MedinCell, Gedeon Richter, Natural Cycles, Cirqle and Myovant.
MJ is employee of Mithra Estetra SRL, an affiliate company of Mithra Pharmaceuticals, Liège, Belgium.
MDC has received speaking honorarium from Gedeon Richter, Mayne, OLIC, and Organon, served on an Advisory Board for Gedeon Richter and Mayne, has stock options with Femasys, and has consulted for Curai, Estetra SRL, Medicines360, and Organon. The Department of Obstetrics and Gynaecology, University of California, Davis, receives contraceptive research funding for MDC from Chemo Research SL, Evofem, Femasys, Medicines360, Merck, Sebela, and Sumitomo Pharma.
JMF is a member of the board at Mithra and received financial support for the supervision of this study.
Additional information
Funding
References
- Nappi RE, Fiala C, Chabbert-Buffet N, et al. Women’s preferences for menstrual bleeding frequency: results of the inconvenience due to women’s monthly bleeding (ISY) survey. Eur J Contracept Reprod Health Care. 2016;21(3):242–250.
- Wyatt KD, Anderson RT, Creedon D, et al. Women’s values in contraceptive choice: a systematic review of relevant attributes included in decision aids. BMC Womens Health. 2014;14(1):28.
- Shimamoto K, Hirano M, Wada-Hiraike O, et al. Examining the association between menstrual symptoms and health-related quality of life among working women in Japan using the EQ-5D. BMC Womens Health. 2021;21(1):325.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224(1):85–95. doi: 10.1530/JOE-14-0549.
- Apter D, Zimmerman Y, Beekman L, et al. Estetrol combined with drospirenone: an oral contraceptive with high acceptability, user satisfaction, well-being and favourable body weight control. Eur J Contracept Reprod Health Care. 2017;22(4):260–267.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-Drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern and safety in Europe and russia. BJOG. 2022;129(1):63–71. doi: 10.1111/1471-0528.16840.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: north American phase 3 efficacy and safety results. Contraception. 2021;104(3):222–228. doi: 10.1016/j.contraception.2021.05.002.
- Moos RH. The development of a menstrual distress questionnaire. Psychosom Med. 1968;30(6):853–867. doi: 10.1097/00006842-196811000-00006.
- Moos RH. Menstrual distress questionnaire - Manual. Menlo Park (CA): Mind Garden Inc.; 2010.
- Munro AK, Hunter EC, Hossain SZ, et al. A systematic review of the menstrual experiences of university students and the impacts on their education: a global perspective. PLoS One. 2021;16(9):e0257333. doi: 10.1371/journal.pone.0257333.
- Witjes H, Creinin MD, Sundstrom-Poromaa I, et al. Comparative analysis of the effects of nomegestrol acetate/17 beta-estradiol and drospirenone/ethinylestradiol on premenstrual and menstrual symptoms and dysmenorrhea. Eur J Contracept Reprod Health Care. 2015;20(4):296–307.
- Barrington DJ, Robinson HJ, Wilson E, et al. Experiences of menstruation in high income countries: a systematic review, qualitative evidence synthesis and comparison to low- and Middle-income countries. PLoS One. 2021;16(7):e0255001. doi: 10.1371/journal.pone.0255001.
- Balik G, Hocaoglu C, Kagitci M, et al. Comparison of the effects of PMDD and pre-menstrual syndrome on mood disorders and quality of life: a cross-sectional study. J Obstet Gynaecol. 2015;35(6):616–620.
- Weisberg E, McGeehan K, Fraser IS. Effect of perceptions of menstrual blood loss and menstrual pain on women’s quality of life. Eur J Contracept Reprod Health Care. 2016;21(6):431–435. doi: 10.1080/13625187.2016.1225034.
- Boyle G. Factor structure of the menstrual distress questionnaire (MDQ): exploratory and LISREL analyses. Pers Individ Dif. 1992;13(1):1–15.
- Garforth B, Degnbol H, Terris ET, et al. Elevated plasma oxytocin levels and higher satisfaction with life in young oral contraceptive users. Sci Rep. 2020;10(1):8208. doi: 10.1038/s41598-020-64528-w.
- Leon-Larios F, Vazquez-Valeo CG, Sanchez-Sanchez A, et al. Health-related quality of life in undergraduate women using any contraceptive. Health Qual Life Outcomes. 2019;17(1):90. doi: 10.1186/s12955-019-1157-2.
- Bitzer J. Hormonal contraception and depression: another pill scandal? Eur J Contracept Reprod Health Care. 2017;22(1):1–2. doi: 10.1080/13625187.2016.1269163.
- Bengtsdotter H, Lundin C, Gemzell Danielsson K, et al. Ongoing or previous mental disorders predispose to adverse mood reporting during combined oral contraceptive use. Eur J Contracept Reprod Health Care. 2018;23(1):45–51. doi: 10.1080/13625187.2017.1422239.
- Sanders SA, Graham CA, Bass JL, et al. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception. 2001;64(1):51–58. doi: 10.1016/s0010-7824(01)00218-9.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21(5):347–355.
- Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord. 2002;70(3):229–240. doi: 10.1016/s0165-0327(01)00356-1.
- Grandi G, Ferrari S, Xholli A, et al. Prevalence of menstrual pain in young women: what is dysmenorrhea? J Pain Res. 2012;5:169–174.
- McKenna KA, Fogleman CD. Dysmenorrhea. Am Fam Physician. 2021;104(2):164–170.
- Santer M, Wyke S, Warner P. What aspects of periods are most bothersome for women reporting heavy menstrual bleeding? Community survey and qualitative study. BMC Womens Health. 2007;7:8. doi: 10.1186/1472-6874-7-8.
- Kanat BH, Atmaca M, Girgin M, et al. Effects of mastalgia in young women on quality of life, depression, and anxiety levels. Indian J Surg. 2016;78(2):96–99. doi: 10.1007/s12262-015-1325-5.
- Goyal A. Breast pain. Am Fam Physician. 2016;93(10):872–873.
- Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
- Zethraeus N, Dreber A, Ranehill E, et al. Combined oral contraceptives and sexual function in women-a Double-Blind, randomized, placebo-Controlled trial. J Clin Endocrinol Metab. 2016;101(11):4046–4053. doi: 10.1210/jc.2016-2032.
- Ma S, Song SJ. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2023;6(6):CD006586. doi: 10.1002/14651858.CD006586.pub5.
- Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2009;(2):CD006586. doi: 10.1002/14651858.CD006586.pub3.
- Yonkers KA, Brown C, Pearlstein TB, et al. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106(3):492–501. doi: 10.1097/01.AOG.0000175834.77215.2e.
- Marr J, Niknian M, Shulman LP, et al. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. 2011;84(1):81–86. doi: 10.1016/j.contraception.2010.10.010.
- Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10(6):561–569.
- Bitzer J, Paoletti AM. Added benefits and user satisfaction with a low-dose oral contraceptive containing drospirenone: results of three multicentre trials. Clin Drug Investig. 2009;29(2):73–78. doi: 10.2165/0044011-200929020-00001.
- Mansour D. Experiences with yasmin: the acceptability of a novel oral contraceptive and its effect on well-being. Eur J Contracept Reprod Health Care. 2002;7(Suppl 3):35–41. discussion 42-3.
- Kelly S, Davies E, Fearns S, et al. Effects of oral contraceptives containing ethinylestradiol with either drospirenone or levonorgestrel on various parameters associated with well-being in healthy women: a randomized, single-blind, parallel-group, multicentre study. Clin Drug Investig. 2010;30(5):325–336. doi: 10.2165/11535450-000000000-00000.
- Lete I, de la Viuda E, Perez-Campos E, et al. Effect on quality of life of switching to combined oral contraception based on natural estrogen: an observational, multicentre, prospective phase IV study (ZOCAL study). Eur J Contracept Reprod Health Care. 2016;21(4):276–284.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103(4):213–221. doi: 10.1016/j.contraception.2021.01.001.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102(6):396–402. doi: 10.1016/j.contraception.2020.08.015.
- Lundin C, Danielsson KG, Bixo M, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143. doi: 10.1016/j.psyneuen.2016.11.033.
- Hall KS, White KO, Rickert VI, et al. Influence of depressed mood and psychological stress symptoms on perceived oral contraceptive side effects and discontinuation in young minority women. Contraception. 2012;86(5):518–525. doi: 10.1016/j.contraception.2012.04.010.
- Ali M, Tran NT. Defining counselling in contraceptive information and services: outcomes from an expert think tank. BMJ Sex Reprod Health. 2022;48(2):79–81. doi: 10.1136/bmjsrh-2021-201132.
- Dehlendorf C, Krajewski C, Borrero S. Contraceptive counseling: best practices to ensure quality communication and enable effective contraceptive use. Clin Obstet Gynecol. 2014;57(4):659–673. doi: 10.1097/GRF.0000000000000059.
