Abstract
Background
Contraceptive methods are well-established in their ability to prevent pregnancy and increase individual agency in childbearing. Evidence suggests that contraceptives can also be used to treat adverse conditions associated with menstruation, including abnormal and prolonged uterine bleeding, heavy menstrual bleeding, painful menstruation, endometriosis, uterine fibroids, and premenstrual dysphoric disorders.
This review investigates the effects of contraceptive techniques such as contraceptive pills, and long-acting reversible contraceptives (e.g. intrauterine devices, implants) on menstrual morbidity.
Methods
Over ten databases with no geographical boundaries were searched from inception until October 2023. Study designs were one of the following types to be included: parallel or cluster randomised controlled trials, controlled clinical trials, controlled before and after studies, interrupted time series studies, cohort or longitudinal analyses, regression discontinuity designs, and case-control studies. Ten team members screened the papers in pairs with a Kappa score of more than 7, and Covidence was used. Conflicts were resolved by discussion, and the full papers were divided among the reviewers to extract the data from eligible studies.
Results
Hormonal contraceptives are considered a well-tolerated, non-invasive, and clinically effective treatment for abnormal and prolonged uterine bleeding, heavy menstrual bleeding, painful menstruation, endometriosis, uterine fibroids, and premenstrual dysphoric disorders. Our studies investigating quality of life or well-being in women with heavy menstrual bleeding, endometriosis, or uterine fibroids have found improvements in all dimensions assessed.
Conclusions
Hormonal contraceptives significantly reduce pain, symptom severity, and abnormal bleeding patterns associated with women who suffer from heavy menstrual bleeding, endometriosis, and uterine fibroids.
SHORT CONDENSATION
Hormonal contraceptives significantly reduce pain, symptom severity, and abnormal bleeding patterns associated with women who suffer from heavy menstrual bleeding, endometriosis, and uterine fibroids. Findings can inform clinical practice and policy decisions to ensure that women have access to safe and effective contraceptive options that promote both reproductive and non-reproductive health.
Background
The use of contraception is rising globally, with the number of users having increased from 663 million to 851 million over the last two decades. By the year 2030, it is projected that an additional 70 million women will be using contraceptives [Citation1]. Of the 1.9 billion women of reproductive age (15-49 years), 1.1 billion report the need for contraceptives, which includes access to sexual and reproductive healthcare services and education, yet only 842 million women report meeting those needs [Citation2]. This is often attributed to misconceptions or negative perceptions surrounding contraception. In developing regions, an estimated 257 million women who desire contraceptives refrain from utilising contraceptive techniques for a range of reasons, such as limited access to information or services, as well as inadequate support from their partners or communities. In many countries, data on contraceptives are only available for women of reproductive age who are married or in a union, further highlighting these barriers to access.
The cost burden associated with hormonal contraceptives undeniably poses a significant barrier to access and consistent utilisation, potentially impacting menstrual health outcomes adversely. Financial constraints may lead to irregular use, increasing the risk of unintended pregnancies and health complications. However, amidst these challenges, global initiatives stemming from the International Conference on Population and Development (ICPD) underscore a collective commitment to address these issues. Efforts to ensure free provision of contraceptives within national reproductive health policies signify progress towards equitable access. By addressing economic barriers and promoting reproductive health rights, these initiatives strive to create a future where individuals can make informed choices about their reproductive health, regardless of financial constraints.
Methods of contraception come in three forms: short-acting, long-acting, and one-time barriers. Short-acting contraceptives include the pill (151 million users), injectables (74 million users, patches, and vaginal rings (less than 15 million users). Long-acting contraceptives include intrauterine devices (159 million users), implants (23 million users), and female sterilisation (219 million users). One-time barrier contraceptives include sponges, diaphragms, cervical caps, spermicide, female condoms, and male condoms; the prevalence of use is low for all one-time barrier methods except for male condoms (189 million users).
There is growing evidence of the benefits of contraceptives beyond pregnancy prevention. Within the United States, it is estimated that 1.5 million women take contraceptive pills for reasons other than pregnancy prevention, which has essential indications for women worldwide [Citation3]. Evidence suggests that hormonal contraception may be effective in treating menstrual-related symptoms and disorders such as menorrhagia, dysmenorrhoea, and menstrual morbidity.
Hormonal contraceptive methods can impact a person’s menstrual cycle, but the mechanism by which they do so varies. Combined oral contraceptive (COC) pills, for example, can provide control of the menstrual cycle by thinning the endometrium, thereby reducing menstrual blood loss. Because of this mechanism of action, COCs are often used to treat menorrhagia, a condition that results in excessive blood loss and can significantly impair a person’s quality of life. On the other hand, the levonorgestrel intrauterine system (LNG-IUS) releases levonorgestrel so that a local foreign body reaction characterised by an increase in inflammatory cells is instigated, creating an unfavourable environment for implantation (Polis et al. 2018) as well as mucosal changes of the cervix. After insertion of the intrauterine device (IUD), many women experience a thinning of the endometrium, which lessens the amount of lining to be shed during the menstrual cycle, resulting in diminished menstrual bleeding. Individuals who menstruate often utilise contraceptives to suppress menstrual-related symptoms. In this way, contraceptives offer women control over the timing and number of pregnancies, but also in the management of medical and comfort concerns surrounding menstruation. Progestin-only contraceptive injectables and implants are highly effective, longer-acting contraceptive methods that most women can use in most circumstances.
Individuals who menstruate, health care providers, and program managers must be well-informed about the benefits and risks of each contraception method so that patients can receive high-quality health care. Legislators must also understand the health benefits of contraception to create policies that provide access to contraceptive resources. This study aims to identify and evaluate evidence that focuses on the use of contraceptives and their impact on menstrual morbidity outcomes among women of reproductive age. This review examines the association between the use of hormonal contraceptives and dysmenorrhoea, endometriosis, anaemia, menstrual irregularities, uterine fibroids, and amenorrhoea.
Methods
This systematic review is registered on Prospero (CRD42022332647) and relies on quantitative evidence regarding the use of contraceptives and their impact on non-reproductive outcomes among women of reproductive age. As for the population, we included women of reproductive age (14-49 years of age) presenting to primary healthcare clinics. If the studies were interventional, we included all contraceptive methods that the World Health Organisation (WHO) defines as effective and acceptable. Moreover, we included all therapeutic contraceptives that have been introduced earlier in therapeutic guidelines, such as mifepristone or medroxyprogesterone acetate pills. These are inclusive of 1) short-acting hormonal contraception (e.g. contraceptive pills, patches, and vaginal rings), 2) long-term contraception (e.g. hormonal intrauterine devices, implants, and injections), 3) one-time barrier contraception (e.g. condoms, sponges, diaphragms, cervical caps, and spermicide), 4) permanent contraception (e.g. tubal ligation and vasectomy), and 5) emergency contraception (e.g. morning after pill or IUD). If the studies were observational, contraceptives of all types (as stated above) were considered the main exposure. Any study which mixed contraception with other medications or modalities was excluded. The comparison was considered non-users or placebos. The outcomes of interest included menstrual-related morbidity (e.g. dysmenorrhoea, endometriosis, anaemia, menstrual irregularities, and amenorrhoea). We included studies with the following study designs: parallel or cluster randomised controlled trials, controlled clinical trials, controlled before and after studies, interrupted time series studies, cohort or longitudinal analyses, regression discontinuity designs, and case-control studies. The result of this systematic review is focused on hormonal contraceptives.
Data sources
To counteract the potential for publication bias, a comprehensive search for both published and unpublished studies was conducted from the date of inception until February 2022, with no restrictions on language or geographical location. We ran an updated search till October 2023, and no new related articles that matched our inclusion criteria were found under the title and abstract screening. A variety of databases were consulted, including CINAHL (1981-), OVID Medline (1946-), Embase (1947-), PsycINFO (1800s-), Maternity & Infant Care (1857-), LILACS (1982-), Clinicaltrials.gov (2000-), Web of science (1900-), Scopus (2004-), CENTRAL Database (1996-), and 13 local databases (further details of which can be found in Appendix I).
The search strategy was initiated with a Medline search utilising Medical Subject Headings (MeSH) terms and keywords, as Appendix II outlined independently or in combination. The search strategy was then adapted for the other databases consulted, including CINAHL, OVID Medline, Embase, PsycINFO, POLLINE, Web of Science, CENTRAL Database, Science Citation Index Expanded (SCIEXPANDED), and WHOLIS. In addition, reference lists of full-text papers relevant to the review were searched.
To augment the database search, OpenGrey (www.opengrey.eu), Google, and Google Scholar were utilised to search for relevant grey literature, and the websites of relevant societies and institutions devoted to contraception were consulted (see Appendix I).
We did not implement any language barrier. Studies that were systematic reviews, scoping reviews, narrative reviews, or meta-analyses were excluded from the review, along with a thesis, conference proceedings, commentaries, editorials, news, or protocol-only manuscripts. The search method was inclusive of all contraceptive methods, although the focus was hormonal contraceptives.
The retrieved articles were processed using Mendeley to remove duplicates and exported to Covidence software (Veritas Health Innovation, Melbourne, Australia) for title and abstract screening using eligibility criteria. The full texts of the included studies were then screened for quality appraisal and data extraction. To ensure that the review adequately addressed the main objectives of the study, the team analysed the inclusion criteria and summarised the characteristics of the included studies. The reasons for exclusion at the full-text screening stage were documented in the PRISMA flow diagram and reported in the findings section. Any discrepancies in the evaluation of studies were identified by Covidence and resolved through discussion.
The review team consisted of ten reviewers (SJ, OM, JM, JM, CC, MS, JA, CH, BJ, KC, AO) who screened the papers in pairs and achieved a Kappa score of more than 7. The screening process was managed by SJ, and conflicts were resolved through discussion. The Kappa score was obtained by reviewing ten abstracts at the abstract screening stage and ten full papers at the full-text screening stage. The abstracts were screened independently by two reviewers, each using Covidence. Full papers were divided among the reviewers for data extraction. One reviewer extracted the data, while another checked the extraction. The second reviewer was also responsible for creating forest plots in Revman. The same reviewers were responsible for completing the table of included studies, but there was not sufficient time to request clarifications or additional data from the researchers. Data collection was performed using data extraction forms stored securely on Google Drive and shared among all researchers. Summary tables were then created manually, and data were entered into Revman for analysis.
The studies were categorised into three primary types: randomised controlled trials (RCTs), cohort studies, and case-control studies. A forest plot was generated when two or more studies were available for comparison and outcome. When only a single study was available for a given outcome, a concise summary of the study was produced (see Appendix III) [Citation4–20].
Analysis
We analysed individual women as the unit of analysis, focusing on methods used in original trial reports like intent-to-treat or per-protocol. It compared hormonal contraceptives versus no contraceptive or oral contraceptives versus no use, with subgroup analysis for different contraceptive types. Meta-analysis was conducted when studies compared identical methods, dosages, and regimens, using effect measures like odds ratios, risk ratios, mean differences, or standardised mean differences with 95% confidence intervals. These methods were chosen to ensure valid interpretation, facilitate data pooling, and communicate findings effectively. Despite intending to create forest plots at multiple time points, the follow-up period was shorter than clinically meaningful durations, and only the first and last reference periods were reported for outcomes. Attributes of included studies were recorded in a table, including author, publication year, country, aims, population, contraceptive type, dosage, outcome measures, and effect measures.
Subgroup analysis and sensitivity analysis
In the present study, subgroup analysis was performed to examine the impact of various types of contraception, dosage, and modes of administration on the outcomes, where feasible. Furthermore, sensitivity analysis was carried out to evaluate the robustness of the results by testing their dependence on the study quality. This was accomplished by systematically excluding each study from the analysis. Additionally, a sensitivity analysis was performed to assess the effect of loss to follow-up rates on the results by excluding studies with follow-up loss rates greater than 20%.
Assessment of heterogeneity
In the meta-analysis, we included only those comparisons and outcomes for which we had two or more data points. To assess heterogeneity, we evaluated the differences in study design, target population, and primary outcome measures among the included studies. Fixed and random-effect models were employed to evaluate the homogeneity of trials combined in the meta-analysis. The extent of heterogeneity was measured by Cochrane’s Q, which was calculated as a weighted sum of the squared differences between individual study effects and the pooled effect across studies. The alpha level was set at 0.10, recognising that the chi-square test for heterogeneity is a low-power test. The magnitude of heterogeneity was then assessed using the I2 score, and any score above 50% was investigated for the clinical and methodological diversity of the studies. In combining the data, we excluded studies that used different contraceptive methods, different doses of the same method, or different criteria for defining morbidity.
Due to limited time and resources, we could not contact authors or consult trial protocols for additional information on missing outcomes. Less than 20% of the data were missing. However, we consulted ClinicalTrials.gov to identify the trial protocols and determine the missing outcomes, and we compiled a list of authors for potential future correspondence to obtain the missing information.
Patient and public involvement
No patients were involved in this research. It is a systematic review, so the patients’ priorities, experiences, and preferences did not inform the development of research questions and outcome measures. Patients were not involved in the design of the research. Further, this is not clinical research or randomised clinical trials.
Results
The Prisma chart in demonstrates the number of studies included in the search from different sources and the number of studies screened and included in the review.
Figure 1. PRISMA flow diagram.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
A search was done for outcomes related to menstrual morbidity. A total of 44 studies were included in the analysis.
The total number of included studies was 44, 35 of which were randomised clinical trials (RCTs), one quasi-experimental, and eight observational studies (3 prospective, three retrospective, and two population-based). shows some of the characteristics of RCTs, including country of origin, year of publication, number of facilities, type of health facility, level of health facility, sample size, study design, population, type of contraception studied, the outcome of interest extracted, and quality of study based on study design. Similar data (with exposure instead of intervention) was extracted for observational studies (). Most studies were from 2000 onward, while a handful were published before 2000 (n = 3).
Table 1. Characteristics of RCTs.
Table 2. Characteristics of observational studies.
Women of reproductive health age between 14 and 49 were included. Some studies, however, noted the population as healthy women, while others noted women with particular conditions. For example, 14 studies focused on women with primary or secondary dysmenorrhoea, while 19 focused on endometriosis, eight on uterine fibroids, and five on abnormal or heavy bleeding.
Studies focused on either one type of contraception (e.g. oral contraception, ring/patch, implant, injection, IUD, condoms, sterilisation), a combination of contraceptives, or all hormonal contraceptives.
Outcomes of interest were menstrual health (e.g. endometriosis reoccurrence, uterine fibroids size reduction, heavy menstrual bleeding, and pain). The irregular menstruation outcome was presented in various forms. For instance, we extracted data on menstrual bleeding in the form of the level of haemoglobin (g/l) and pictorial blood loss assessment score. Amenorrhoea by 12 weeks was also collected, as well as recovery of baseline menstrual pattern, which relates to complications before using hormonal contraception. Although no language restrictions were imposed during the search, all included studies were in English ().
Table 3. Global distribution of studies.
Quality Assessment
All included studies were assessed and ranked for quality. Details on this assessment are provided in Appendix IV. (see also ).
Table 4. Modified dawn and black assessment for observational studies.
Randomised clinical trials
Menstrual problems
Meta-analyses were possible for a wide array of menstrual problems, including recovery of baseline menstrual pattern (or, as authors noted, complete recovery), amenorrhoea, menstrual blood loss measured by haemoglobin (g/l), or menstrual bleeding using other tools, namely, ‘pictorial blood loss assessment score’. The severity of menstrual signs was reported as ‘daily reporting of severity of menstrual symptoms’ by several authors.
Recovery of baseline menstrual pattern
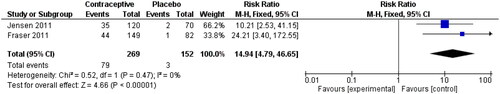
Jensen 2011 [Citation21] studied the effect of Oestradiol Valerate and Dienogest on heavy menstrual bleeding for 28 days in an RCT. Responders were defined as participants with no abnormal bleeding symptoms and achievement of all relevant criteria during the 90-day efficacy interval, and they were compared with non-oral contraceptive pill (OCP) users. The same definition was used by Fraser 2011 [Citation22]. The following analysis is shown in . The risk ratio was reported at 14.94 (95% CI 4.79, 46.65), although the wide confidence interval should be acknowledged when interpreting this result.
Dysfunctional uterine bleeding
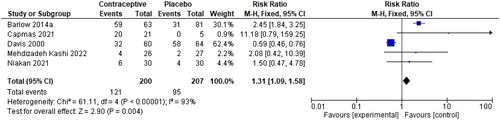
Five RCTs presented data on dysfunctional uterine bleeding or irregular menstrual bleeding, two of which included specific populations of women who had fibroids [Citation23–27]. Compiling the data was possible due to the common symptoms experienced by these women. The effect size reported in is 1.31 (95% CI 1.09, 1.58) with the expected high heterogeneity (I2 = 93%). When Davis and colleagues’ study was removed, the heterogeneity was reduced to 0%, and the effect measures remained on the same side of the line of no effect (2.52, 95% CI 1.88, 3.37).
Figure 3. Irregular menstrual bleeding (events), for the type of hormone, refer to the table of included studies.
Two RCTs [Citation21, Citation22] also reported on the outcome of irregular menstrual bleeding as the change in total days during which patients experienced spotting and bleeding, compared to baseline data for users of contraceptives compared to a placebo group ().
Amenorrhoea
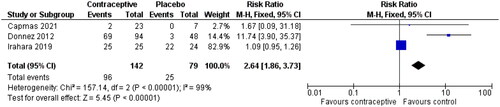
Three studies have been reported to study the impact of OCPs on amenorrhoea [Citation24, Citation28, Citation29]. The time frame for these studies was approximately the same (three months or less). The effect size in reported 2.64 (95% CI 1.86, 3.73) in favour of the placebo group. High heterogeneity is of great concern in this analysis (I2 = 99%).
Menstrual blood loss
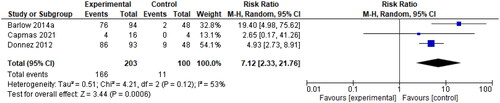
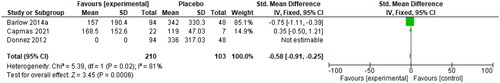
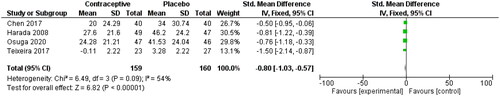
Menstrual blood loss was measured by the Pictorial Blood Loss Assessment Chart (PBAC). A 2021 study by Ko et al. determined that a PBAC cut-off score of 76 has a sensitivity of 93.2% and specificity of 83.0% for the prediction of self-perceived heavy menstrual bleeding [Citation30]. A PBAC score <75 was used as a primary endpoint in two studies comparing the use of 10 mg UPA to a placebo, and a cut-off score of <60 was used in one study with the same intervention. The risk ratio of 7.12 (95% CI 2.33, 21.76) is shown below in . These authors also provided data on PBAC scores at the end of treatment, with an SMD of −0.58 (95% CI −0.91, −0.25), as seen in . Donnez et al. 2012 reported that 70% of patients in the 10-mg group were amenorrheic within the first ten days of the study, which explains the mean PBAC score of 0 for this intervention group [Citation28].
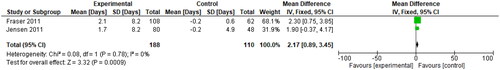
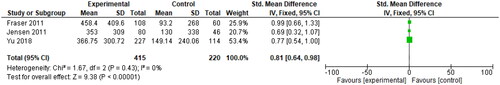
Three RCTs (whose OCP users were on all combined hormonal contraceptive pills) tracked the participants’ mean blood loss (mL) from baseline to the end of treatment through the collection of sanitary items used [Citation21, Citation22, Citation31]. The SMD was reported to be 0.81 (95% CI 0.64, 0.98), as seen in below.
Figure 8. Change in mean blood loss (mL), for type of hormone, refer to the table of included studies.
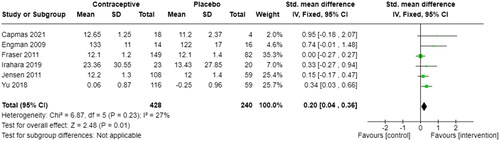
Six studies also measured Menstrual blood loss in haemoglobin level (g/dL) [Citation21, Citation22, Citation24, Citation29, Citation31, Citation32]. In , SMD was reported to be 0.20 (95% CI 0.04, 0.36) with a low heterogeneity of 27%, referring to a low variability.
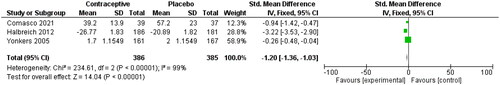
Menstrual symptom severity
Three studies used the ‘daily reporting of severity of menstrual symptoms’ scale to study the severity of menstrual symptoms [Citation33–35]. The SMD for these studies in shows that compared to OCP non-users, OCP users had a −1.20 lower SMD (95% CI −1.36, −1.03). A lower severity score means less/less severe menstrual symptoms.
Figure 10. Severity of menstrual symptoms, for the type of hormone, refer to the table of included studies.
Menstrual symptoms using general tools
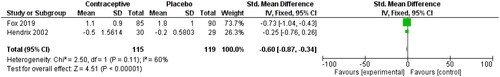
Fox 2019 reported menstrual pain as a continuous variable using the ‘Dysmenorrhoea Daily Diary’, which captured vaginal bleeding, cramping pain score, rescue pain medication use, and the impact of pelvic pain on daily life [Citation36]. Hendrix used a similar tool called the ‘Mood Disorder Questionnaire’ [Citation37]. This questionnaire consists of 47 questions regarding symptoms experienced during menses. Each answer is scored from 0 to 4, with 0 being no experience of symptoms and four being severe. The questions cover eight subscales for distress, including pain, water retention, autonomic reactions, negative effects, impaired concentration, behaviour change, arousal, and control. The effect size in shows a reduction in SMD among OCP users compared to non-users of −0.60 (95% CI −0.87, −0.34) with a moderate heterogeneity level of 60%, which may relate to the self-reported measure.
Endometriosis
Pain associated with endometriosis
OCPs are often used for symptom management as well as family planning in individuals with endometriosis. Several RCTs were dedicated to this concept, and there were abundant comparisons between OCP users and non-users. Pain due to endometriosis, dysmenorrhoea, and dyspareunia were found to be among the most reported outcomes.
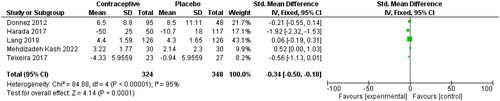
Five studies reported pain associated with endometriosis as a continuous variable. Lower pain was reported for OCP users compared to non-users (SMD: −0.34, 95% CI −0.50, −0.18) in . Heterogeneity in this analysis was high (I2=95%), possibly due to various pain measurement tools [Citation26, Citation28, Citation38–40].
Figure 12. Endometriosis associated with pain (mean scores), for the type of hormone, refer to the table of included studies.
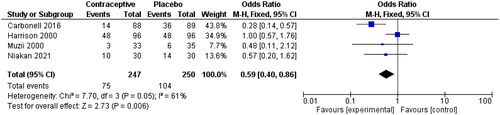
Four studies also reported pain as a dichotomous variable [Citation4, Citation27, Citation41, Citation42]. Like other meta-analyses on pain as a continuous variable, this analysis showed a lower pain incidence among OCP users than non-users (0.59, 95% CI 0.40, 0.86). The I2 was still relatively high (I2=61%), which may be associated with different doses and duration of OCP between studies ().
Dysmenorrhoea associated with endometriosis
Four RCTs were included in as they all reported dysmenorrhoea associated with endometriosis as a continuous variable. The risk ratio was −0.80 for SMD analysis (95% CI −1.03, −0.57) with an I2 value of 54%, indicating a higher level of heterogeneity.
Endometriosis recurrence
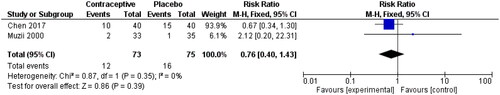
Two studies also reported endometriosis recurrence, as seen in [Citation42, Citation43]. The effect size was 24% lower among OCP users, and the 95% CI crossed the line of no effect (0.40, 1.43).
Leiomyoma size
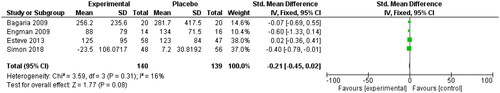
Using OCPs reduced the size of leiomyoma (SMD: −0.21, 95%CI −0.45, 0.02). Four studies examined this relationship [Citation32, Citation44–46]. Simon reported median and interquartile range, suggesting a non-normal distribution of data ().
Uterine fibroids
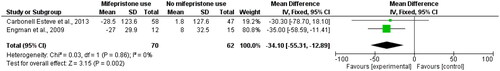
Two RCT studies reported a decrease in the size of myoma volume (ml) following the use of mifepristone (one daily capsule of 5 mg mifepristone or a mifepristone placebo over 3 months for Crabonell Esteve et al. (2013) study and 50 mg mifepristone or placebo every other day during 3 months for the Engman et al. (2009) study compared to non-users. The mean difference was −34.10 with a wide confidence interval (95% CI −55.31, −12.89) and zero heterogeneity in [Citation32, Citation47].
Cohort studies
Endometriosis
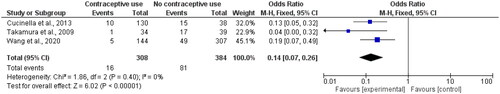
We found three cohort studies that reported prospective endometriosis recurrence among contraceptive versus non-users. Wang and colleagues (2020) investigated the LNG-IUS, and Cucinella et al. (2013) and Takamura et al. (2009) looked at the effect of oral contraceptives. As seen in , the total number of endometriosis recurrence events among contraceptive users was 16 (out of 308) compared to 81 (out of 384) in the control group, leading to an OR of 0.14 (95% CI 0.07, 0.26) with zero heterogeneity [Citation48–50].
Side effects of contraceptives
A significant number of side effects were reported, including, but not limited to, uterine haemorrhage, ovarian haemorrhage, menometrorrhagia, uterine bleeding, headache, abdominal pain, pyrexia, breast pain, hypercholesterolaemia, hypothyroidism, constipation, hypertriglyceridaemia, etc. Some reported side effects are the primary outcomes of interest that other trials aim to study. Although the variety of possible side effects is imposing, it has implications for future studies to elucidate their significance (see Appendix V).
Discussion
Summary of main results
The review examined the relationship between menstrual issues and various types of pain, including abdominal, pelvic, and lower back pain, associated with dysphasia. Initially, 44 studies were identified, primarily consisting of RCTs and cohort studies. For menstrual problems, RCTs focused on outcomes such as recovery of baseline menstrual patterns, dysfunctional uterine bleeding, amenorrhoea, menstrual blood loss, and symptom severity. Contraceptive users generally experienced significant improvements in these outcomes compared to non-users, although high heterogeneity was observed across some analyses. In endometriosis management, RCTs explored pain, dysmenorrhoea, recurrence rates, leiomyoma size, and uterine fibroids. Contraceptive users tended to experience reduced pain, lower recurrence rates, and smaller leiomyoma size. Cohort studies also indicated a lower risk of endometriosis recurrence among contraceptive users. However, variability in study methodologies and interventions highlights the need for further research to optimise treatment outcomes in these areas.
Agreements and disagreements with other studies
In general, our findings are similar to prior studies that have examined the association between contraceptives and non-reproductive health outcomes. Studies investigating quality of life or well-being in women with heavy menstrual bleeding, endometriosis, or uterine fibroids found improvements in all dimensions assessed, which is similar to the findings of Bürger. Our findings on dysmenorrhoea reduction have also been confirmed in a previous study conducted by Iwata and colleagues [Citation51]. We also have strong evidence that contraceptives reduce heavy menstrual bleeding, like the results found by Lethaby [Citation52]. For endometriosis patients, the effects of contraception on the alleviation of symptoms such as pelvic pain and dysmenorrhoea have been shown both in this paper as well as in others. A review conducted by Grandi et al. combined hormonal contraceptives and progestin-only contraceptive were found to be associated with clinically significant reductions in dysmenorrhoea [Citation53]. Postoperative use of contraceptives was significantly associated with preventing the risk of endometriosis recurrence and pain related to endometriosis within our study, which is similar to the findings. A different study conducted by Ghonim observed that among women suffering from uterine fibroids treated by ulipristal acetate, attainment of amenorrhoea was significant, indicating an improvement in symptoms among contraceptive users [Citation54].
Strengths
We initially searched the databases for all studies from their inception. Thus, we included studies with up-to-date contraceptive methods with broad coverage. For each outcome category, our studies covered diseases and conditions commonly seen in women of reproductive age. For some of these outcomes, we found a few studies that yielded larger pooled sample sizes, allowing for higher statistical power, narrower confidence intervals, and more credible results. In addition to the use of contraception for contraceptive purposes, we also explored the effectiveness of contraception as a treatment. This provides additional evidence for the use of contraception as a treatment in clinical settings and for reasonable insurance coverage.
Limitations
The exclusive use of studies that compare hormonal contraceptive users to a placebo group has both clear benefits and limitations. With the varying HC methods and drug dosage options available to women, it is important to identify which regimens work most effectively with minimal side effects, but our analysis does not account for these differences. Some of the regimens used across the studies include ulipristal acetate 5 mg or 10 mg, nomegestrol acetate -E2, etonogestrel-E2, oestradiol valerate/dienogest, levonorgestrel/ethinyl oestradiol, drospirenone/ethinylestradiol, and oestrogen.
The study highlighted several key points. Firstly, it emphasised the need to examine the relationship between hormonal contraceptives and duration of use, as most studies only looked at short-term effects. Longer durations may reveal different outcomes, including potential protective or harmful effects. Secondly, it pointed out limitations in assessing quality-of-life improvements due to inadequate sample sizes and heterogeneous data. Thirdly, it discussed the importance of considering gender inclusivity in research terminology, particularly in studies involving contraceptive use, where not all users identify as women. Overall, the study called for more comprehensive and inclusive research methodologies to better understand the effects of hormonal contraceptives.
Implications for research
The systematic review examined the effects of hormonal contraceptives beyond preventing pregnancy, revealing benefits like alleviating menstrual symptoms and enhancing women’s quality of life. However, it also uncovered potential long-term side effects such as increased risks of cardiovascular disease, breast cancer, and mental health issues, although these weren’t the main focus. With the emergence of new contraceptive methods, more research on their non-reproductive health impacts is crucial for informed clinical decisions. Overall, the review offers valuable insights for clinical practice and policymaking, ensuring women access safe and effective contraceptive options promoting overall health. Further research implications are detailed in Appendix V.
Implications for clinical practice
Healthcare providers should view hormonal contraceptives as safe and effective treatments for heavy menstrual bleeding, endometriosis, uterine fibroids, and premenstrual dysphoric disorder. Patients should be informed about the benefits of hormonal contraception in managing pain, symptoms, and abnormal bleeding associated with these conditions, which greatly affect quality of life. Our review addresses timely issues relevant to gynecological practice, exploring less invasive and cost-effective treatments for conditions like adenomyosis and fibroids, as well as managing endometriosis. While our findings show promising clinical outcomes, some relevant studies were omitted, such as Magalhaes et al. [Citation55] Integrating evidence from these studies could enhance treatment strategies and patient outcomes, emphasising the need for ongoing research and critical evaluation in gynecological care.
Contributions of authors
Conceptualisation: Shayesteh Jahanfar, Ali Moazzam, Julie Mortazavi
Data Curation: Shayesteh Jahanfar, Amy Lapidow, Julie Mortazavi, Jude Al Abosy, Bohang Jiang, Juan Camilo Becerra-Mateus, Ciana Hartman, Cassandra Cu, Katherine Morris
Formal Analysis: Shayesteh Jahanfar, Meredith Steinfeldt, Anjali A Oberoi
Writing – original draft: Shayesteh Jahanfar, Julie Mortazavi, Olivia Maurer, Meredith Steinfeldt, Bohang Jiang
Writing – review, and editing: Shayesteh Jahanfar, Julie Mortazavi, Olivia Maurer, Meredith Steinfeldt, Bohang Jiang, Jude Al Abosy, Moazzam Ali
Disclaimer
The named authors alone are responsible for the views expressed in this publication and do not necessarily represent the decisions or the policies of the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) or the World Health Organisation (WHO).
Ethics approval
Not applicable.
Patient consent for publication
Not applicable.
Supplemental Material
Download MS Word (147.5 KB)Acknowledgements
The authors would like to gratefully acknowledge comments and suggestions from the WHO Technical Advisory Group (TAG) consisting of (listed in Alphabetical order): Dr. Ann Biddlecom; Dr. Harriet Birungi; Professor Herbert Peterson; Dr. Iqbal Shah; Dr. James Kiarie; Professor John Cleland; Dr. John Townsend; Dr. Manala Makua and Professor Sonalde Desai. We would like to specially acknowledge the support and guidance by Dr. James Kiarie (WHO) throughout the process to complete the project. We thank him for his efforts. We acknowledge the support of USAID who provided input on the research questions. USAID did not participate in the data abstraction, analysis or interpretation or the decision to submit it for publication. The analysis, interpretation, write up and decision to submit the paper was coordinated by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, WHO. All authors were consultants and one author is a staff member. Furthermore, we are grateful to Amy Lapidow for her assistance in developing the search strategies and helping us to conduct a literature search that yielded over 7,000 studies. We would also like to thank all at Tufts University School of Medicine who have supported completing this research. Many thanks go to them. A team of researchers from the Cochrane Fertility group contributed intellectually to providing support for this project. The team members are Alison Edelman, Motu Makaplapua, and Jullian Henderson.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No data are available.
Additional information
Funding
References
- Abraham S, Luscombe G, Soo I. Oral contraception and cyclic changes in premenstrual and menstrual experiences. J Psychosom Obstet Gynaecol. 2003;24(3):185–193. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14584305 doi: 10.3109/01674820309039672.
- Kantorová V, Wheldon MC, Ueffing P, et al. Estimating progress towards meeting women’s contraceptive needs in 185 countries: a Bayesian hierarchical modelling study. PLoS Med. 2020;17(2):e1003026. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32069289 doi: 10.1371/journal.pmed.1003026.
- Ejembi CL, Dahiru T, Aliyu A. Contextual factors influencing modern contraceptive use in Nigeria. DHS Work Pap. 2015;120(September):44.
- Carbonell JL, Riverón AM, Leonard Y, et al. Mifepristone 2.5, 5, 10mg versus placebo in the treatment of endometriosis. J Reprod Health Med. 2016;2(1):17–25. doi: 10.1016/j.jrhm.2015.09.001.
- Harada T, Momoeda M, Taketani Y, et al. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583–1588. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18164001 doi: 10.1016/j.fertnstert.2007.08.051.
- Uysal G, Akkaya H, Cagli F, et al. A comparison of two different oral contraceptives in patients with severe primary dysmenorrhoea. J Obstet Gynaecol. 2018;38(6):828–832. doi: 10.1080/01443615.2017.1410533.
- Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70(3):432–439. doi: 10.1016/s0015-0282(98)00208-8.
- Mabrouk M, Frascà C, Geraci E, et al. Combined oral contraceptive therapy in women with posterior deep infiltrating endometriosis. J Minim Invasive Gynecol. 2011;18(4):470–474. doi: 10.1016/j.jmig.2011.04.008.
- Fiscella K, Eisinger SH, Meldrum S, et al. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1381–1387. doi: 10.1097/01.AOG.0000243776.23391.7b.
- Driak D, Sehnal B, Neumannova H, et al. Effect of progestin-dominant combined oral contraception on uterine fibroid development. 2017;4:1077.
- Hernádi L, Marr J, Trummer D, et al. Efficacy and safety of a low-dose combined oral contraceptive containing drospirenone 3 mg and ethinylestradiol 20 mcg in a 24/4-day regimen. Contraception. 2009;80(1):18–24. doi: 10.1016/j.contraception.2009.01.016.
- Harada T, Momoeda M. Efficacy of cyclic and extended regimens of ethinylestradiol 0.02 mg ‐levonorgestrel 0.09 mg for dysmenorrhea: a placebo‐controlled, double‐blind, randomized trial. Reprod Med Biol. 2021;20(2):215–223. doi: 10.1002/rmb2.12373.
- Harada T, Momoeda M, Terakawa N, et al. Evaluation of a low-dose oral contraceptive pill for primary dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2011;95(6):1928–1931. doi: 10.1016/j.fertnstert.2011.02.045.
- Harada T, Momoeda M. Evaluation of an ultra-low-dose oral contraceptive for dysmenorrhea: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2016;106(7):1807–1814. doi: 10.1016/j.fertnstert.2016.08.051.
- Osuga Y, Hayashi K, Kanda S. Evaluation of the efficacy, safety, and clinically recommended dose of dienogest in the treatment of primary dysmenorrhea: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2020;113(1):167–175. doi: 10.1016/j.fertnstert.2019.09.014.
- Parsanezhad ME, Alborzi SA, Namavar Jahromi B. Menstrual abnormalities and pain after five tubal sterilization methods: a randomized controlled trial. Iran J Med Sci. 2015;28(2):51–56.
- Taşkömür AT, Erten Ö. The effect of tubal ligation surgery during cesarean operation on dysmenorrhoea, dyspareunia and menstrual cycle. J Gynecol Obstet Hum Reprod. 2021;50(6):102054. doi: 10.1016/j.jogoh.2020.102054.
- Milsom I, Sundell G, Andersch B. The influence of different combined oral contraceptives on the prevxalence and severity of dysmenorrhea. Contraception. 1990;42(5):497–506. doi: 10.1016/0010-7824(90)90078-a.
- Shy KK, Stergachis A, Grothaus LG, et al. Tubal sterilization and risk of subsequent hospital admission for menstrual disorders. Am J Obstet Gynecol. 1992;166(6 Pt 1):1698–1706. doi: 10.1016/0002-9378(92)91559-s.
- Barati M, Zarei L, Shahbazian N, et al. Use of oral contraceptive pills in the treatment of the endometrial polyps smaller than 1.5 cm. International J of Cancer Research. 2015;11(2):104–108. ():doi: 10.3923/ijcr.2015.104.108.
- Jensen JT, Parke S, Mellinger U, et al. Effective treatment of heavy menstrual bleeding with estradiol valerate and dienogest: a randomized controlled trial. Obstet Gynecol. 2011;117(4):777–787. doi: 10.1097/AOG.0b013e3182118ac3.
- Fraser IS, Römer T, Parke S, et al. Effective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: a randomized, double-blind Phase III trial. Hum Reprod. 2011;26(10):2698–2708. doi: 10.1093/humrep/der224.
- Barlow DH, Lumsden MA, Fauser BCJM, et al. Individualized vaginal bleeding experience of women with uterine fibroids in the PEARL I randomized controlled trial comparing the effects of ulipristal acetate or placebo. Hum Reprod. 2014;29(3):480–489. doi: 10.1093/humrep/det467.
- Capmas P, Brun JL, Legendre G, et al. Ulipristal acetate use in adenomyosis: a randomized controlled trial. J Gynecol Obstet Hum Reprod. 2021;50(1):101978. doi: 10.1016/j.jogoh.2020.101978.
- Davis A, Godwin A, Lippman J, et al. Triphasic norgestimate-ethinyl estradiol for treating dysfunctional uterine bleeding. Obs Gynecol [Internet]. 2000;96(6):913–920. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11084177 doi: 10.1097/00006250-200012000-00009.
- Mehdizadeh Kashi A, Niakan G, Ebrahimpour M, et al. A randomized, double-blind, placebo-controlled pilot study of the comparative effects of dienogest and the combined oral contraceptive pill in women with endometriosis. Int J Gynaecol Obstet. 2022;156(1):124–132. Available from: https://www.ncbi.nlm.nih.gov/pubmed/33728657 doi: 10.1002/ijgo.13677.
- Niakan G, Rokhgireh S, Ebrahimpour M, et al. Comparing the effect of dienogest and OCPS on pain and quality of life in women with endometriosis: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2021;24(9):670–677. doi: 10.34172/aim.2021.96.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. doi: 10.1056/NEJMoa1103182.
- Irahara M, Maejima Y, Shinbo N, et al. Ulipristal acetate for Japanese women with symptomatic uterine fibroids: a double-blind, randomized, phase II dose-finding study. Reprod Med Biol. 2020;19(1):65–74. doi: 10.1002/rmb2.12304.
- Ko JKY, Lao TT, Cheung VYT. Pictorial blood loss assessment chart for evaluating heavy menstrual bleeding in Asian women. Hong Kong Med J. 2021;27(6):399–404. doi: 10.12809/hkmj208743.
- Yu Q, Zhou Y, Suturina L, et al. Efficacy and safety of estradiol valerate/dienogest for the management of heavy menstrual bleeding: a multicenter, double-blind, randomized, placebo-controlled, phase III clinical trial. J Womens Health (Larchmt). 2018;27(10):1225–1232. doi: 10.1089/jwh.2017.6522.
- Engman M, Granberg S, Williams ARW, et al. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod. 2009;24(8):1870–1879. doi: 10.1093/humrep/dep100.
- Comasco E, Kopp Kallner H, Bixo M, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178(3):256–265. doi: 10.1176/appi.ajp.2020.20030286.
- Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85(1):19–27. doi: 10.1016/j.contraception.2011.05.008.
- Yonkers KA, Brown C, Pearlstein TB, et al. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106(3):492–501. doi: 10.1097/01.AOG.0000175834.77215.2e.
- Fox MC, Klipping C, Nguyen AM, et al. A phase 2b multicenter, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of vaginal rings containing nomegestrol acetate or etonogestrel and 17beta-estradiol in the treatment of women with primary dysmenorrhea. Contraception. 2019;99(2):125–130. doi: 10.1016/j.contraception.2018.10.009.
- Hendrix SL, Alexander NJ. Primary dysmenorrhea treatment with a desogestrel-containing low-dose oral contraceptive. Contraception. 2002;66(6):393–399. doi: 10.1016/s0010-7824(02)00414-6.
- Harada T, Kosaka S, Elliesen J, et al. Ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen for the management of endometriosis-associated pelvic pain: a randomized controlled trial. Fertil Steril. 2017;108(5):798–805. Available from: doi: 10.1016/j.fertnstert.2017.07.1165.
- Teixeira MZ, Podgaec S, Baracat EC. Potentized estrogen in homeopathic treatment of endometriosis-associated pelvic pain: a 24-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2017;211:48–55. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28187404 doi: 10.1016/j.ejogrb.2017.01.052.
- Lang J, Yu Q, Zhang S, et al. Dienogest for treatment of endometriosis in Chinese women: a placebo-controlled, randomized, double-blind phase 3 study. J Womens Health (Larchmt). 2018;27(2):148–155. doi: 10.1089/jwh.2017.6399.
- Harrison RF, Barry-Kinsella C. Efficacy of medroxyprogesterone treatment in infertile women with endometriosis: a prospective, randomized, placebo-controlled study. Fertil Steril. 2000;74(1):24–30. doi: 10.1016/s0015-0282(00)00577-x.
- Muzii L, Marana R, Caruana P, et al. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. Am J Obstet Gynecol. 2000;183(3):588–592. doi: 10.1067/mob.2000.106817.
- Chen YJ, Hsu TF, Huang BS, et al. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. Am J Obstet Gynecol. 2017;216(6):582-e1-582–e9. doi: 10.1016/j.ajog.2017.02.008.
- Bagaria M, Suneja A, Vaid NB, et al. Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49(1):77–83. doi: 10.1111/j.1479-828X.2008.00931.x.
- Esteve JLC, Acosta R, Pérez Y, et al. Mifepristone versus placebo to treat uterine myoma: a double-blind, randomized clinical trial. Int J Womens Health. 2013;5:361–369. doi: 10.2147/IJWH.S42770.
- Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431–439. doi: 10.1097/AOG.0000000000002462.
- Carbonell Esteve JL, Riverón AM, Cano M, et al. Mifepristone 2.5 mg versus 5 mg daily in the treatment of leiomyoma before surgery. Int J Womens Health. 2012;4:75–84. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22448109 doi: 10.2147/IJWH.S28103.
- Cucinella G, Granese R, Calagna G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Arch Gynecol Obstet. 2013;288(4):821–827. doi: 10.1007/s00404-013-2841-9.
- Takamura M, Koga K, Osuga Y, et al. Postoperative oral contraceptive use reduces the risk of ovarian endometrioma recurrence after laparoscopic excision. Hum Reprod. 2009;24(12):3042–3048. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19684045 doi: 10.1093/humrep/dep297.
- Wang Y, Yang M, Huang X, et al. Prevention of benign endometrial polyp recurrence using a levonorgestrel-releasing intrauterine system in premenopausal patients: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27(6):1281–1286. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32446971 doi: 10.1016/j.jmig.2019.11.023.
- Iwata M, Oikawa Y, Shimizu Y, et al. Efficacy of low-dose estrogen-progestins and progestins in Japanese women with dysmenorrhea: a systematic review and network meta-analysis. Adv Ther. 2022;39(11):4892–4909. doi: 10.1007/s12325-022-02298-9.
- Lethaby A, Wise MR, Weterings MA, et al. Combined hormonal contraceptives for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;2(2):CD000154. PMID: 30742315; PMCID: PMC6369862. doi: 10.1002/14651858.CD000154.pub3.
- Grandi G, Barra F, Ferrero S, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care. 2019;24(1):61–70. Epub 2019 Jan 21. PMID: 30664383. doi: 10.1080/13625187.2018.1550576.
- Ghonim M, Magdy R, Sabbour M, et al. A systematic review and meta-analysis of ulipristal acetate for symptomatic uterine fibroids. Int J Gynaecol Obstet. 2019;146(2):141–148. Epub 2019 Jun 19. PMID: 31127621. doi: 10.1002/ijgo.12868.
- Magalhaes J, Ferreira-Filho ES, Soares-Junior JM, et al. Uterine volume, menstrual patterns, and contraceptive outcomes in users of the levonorgestrel-releasing intrauterine system: A cohort study with a five-year follow-up. Eur J Obstet Gynecol Reprod Biol. 2022;276:56–62. doi: 10.1016/j.ejogrb.2022.06.029.