Introduction
An effective surgical technique derives from a good knowledge of anatomy. A profound knowledge of surgical anatomy gives surgeons the instruments to accomplish safe, oncologically correct dissections along the embryologic planes, minimizing tissue trauma and bleeding. Laparoscopic surgery, which lacks direct tissue handling, requires an even higher grade of anatomical knowledge to drive instruments along the correct planes and trace structures, especially in those cases where it is difficult to source them, without compromising structures and tissues around them. A good knowledge of anatomy cannot disregard the teachings that come from scientists and scholars of the past, people that highly contributed to progress in medicine and surgery with their in-depth studies. For this reason, we should not be afraid of using eponyms to identify specific anatomical structures: it is not mere culture flaunting, it is a way to be grateful to them and pay tribute to their work.
Technique
Mobilization of the splenic flexure
Although mobilization of the splenic flexure is not always necessary, it is sometimes mandated for a tension-free anastomosis. This is a more complex maneuver because of the proximity of the stomach, spleen, and pancreas, as well as the need to avoid inadvertently transecting the transverse mesocolon, which potentially devascularizes the large intestine to be used for the anastomosis. This is the reason for performing the splenic flexure mobilization as the first operative step of a laparoscopic left colectomy. Splenic flexure mobilization may not be rigorously defined, and the term may be used in several ways by different surgeons. As a rule, however, the term splenic flexure mobilization should not be used to refer to incising the line of Toldt to the level of the splenic flexure but should be reserved for some degree of transection of the distal transverse mesocolon for the purpose of relocating the splenic flexure away from the spleen. The medial-to-lateral (MTL) approach involves placing the patient in maximal reverse Trendelenburg positioning with continued right lateral decubitus tilt ( and Citation2(A)). The midpoint of the gastrocolic ligament is transected at the so-called Bouchet’s area ( and Citation3], a thin and transparent area on the distal side of the gastroepiploic vascular arch, allowing entry into the lesser sac [Citation1]. The remaining gastrocolic ligament is transected to the level of the inferior pole of the spleen, and this point of view allows the surgeon to avoid getting lost and ending up near the splenic hilum, an easy mistake to make in a patient with more visceral obesity. The stomach is then reflected cephalad so as to expose the posterior gastric wall. This also exposes the pancreas, which is important in that the level of mesenteric transection is one centimeter caudal to the inferior border of the pancreas. This is to preserve collateral arterial blood flow, as well as to maximize colonic length. The splenic flexure proper can then be dissected in an MTL direction, dividing the spleno-colic ligament and the spleno-omental ligament, the so-called criminal fold of Morgenstern () [Citation2], with the inferior pole of the spleen, the pancreas, and the transverse mesocolon clearly delineated. The criminal fold of Morgenstern is a constant peritoneal fold attached to the lower pole of the spleen near to the splenic flexure but distinct from the spleno-colic ligament. This peritoneal fold is most commonly responsible for iatrogenic injuries to the splenic lower pole during operation on the left upper quadrant. This is the reason it was labelled as “criminal” by Morgenstern and usually contains one or more small vessels.
Figure 1. (A, B) Reverse-Trendelenburg (head-up tilt) and Trendelenburg positioning are essential for performing a left colectomy through the laparoscopic approach.
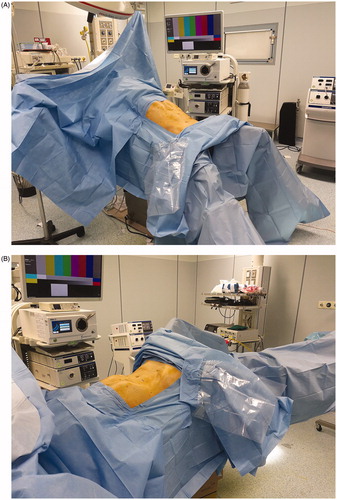
Figure 2. (A) Friederick Trendelenburg (1844–1924), German surgeon. (B Alain Bouchet (1926–), French surgeon and anatomist. (C) Leon Morgenstern (1919–2012) surgeon, scholar and humanist. (D) Carl Toldt (1840–1920), Austrian anatomist, Professor of Anatomy at the University of Prague and Wien. (E) Dimitrie D. Gerota (1867–1939), Romanian Professor of Anatomy and Surgery. (F) Václav Treitz or Wenzel Treitz (1819–1872), Check pathologist, Professor at the University of Prague. (G) Emil Zuckerkandl (1849–1910), Professor of Anatomy at the University of Wien. (H) Wenzel Gruber (1814–1890), Bohemian anatomist and Professor at the University of St. Petersburg. (I) Thomas Ionnesco (1860–1926), Romanian surgeon and anatomist. (J) Harry E. Bacon, professor and chairman at Temple University Hospital. (K) Charles-Pierre Denonvilliers (1808–1872) a French anatomist and surgeon. (L) Henri Mondor (1885–1962), surgeon and literature historian. (M) André Raphael Latarjet Gouy (1877–1947), French anatomist and surgeon. (N) Heinrich Wilhelm Gottfried von Waldeyer-Hartz (1836–1921) German anatomist, Professor at the University of Strasbourg and Berlin. (O) R.J. (Bill) Heald, British surgeon, developer of TME. (P) Paul Hermann Martin Sudeck (1866–1938) German surgeon. (Q) Jean Riolan the Younger (1577 or 1580–1657) French anatomist.

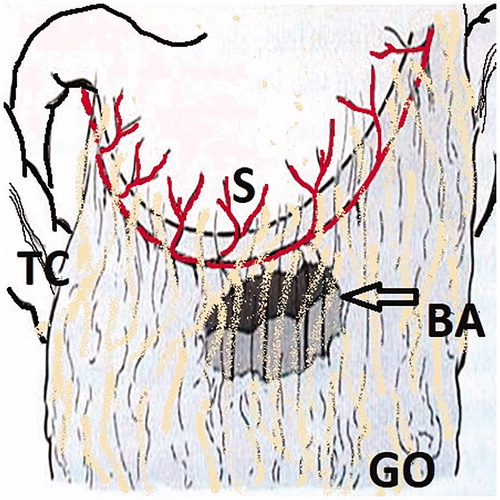
Figure 3. The Bouchet area is a thin and transparent area on the distal side of the gastroepiploic vascular arch. S: Stomach; TC: Transverse Colon; GO: Greater Omentum; BA: Bouchet Area.
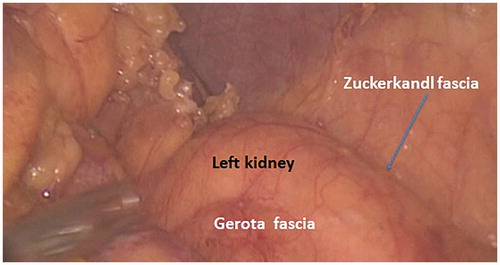
With the patient in reverse Trendelenburg, the colon naturally moves away from the spleen, allowing further exposure of the spleen without retraction. The transection of the gastrocolic and splenocolic ligaments prior to the mesenteric resection allows the colon to be safely retracted caudally without tearing of the splenic capsule: the spleen and colon are no longer attached. Proximal transection of the transverse mesocolon must be carried out carefully: A bleeding vessel can potentially retract into a retropancreatic position, continuing to bleed but not visible without pancreatic mobilization. This procedure provides adequate length for virtually every colorectal anastomosis. The avascular plane between the Toldt fascia and the Gerota fascia is easily visualized and the dissection may be furthered from above to below along this plane (). Toldt () fascia is the continuation of Treitz fascia behind the body of the pancreas [Citation3]. Toldt fascia is a layer of connective tissue containing lymphatic channels. It is found between the two mesothelial layers that separate the mesocolon from the underlying retroperitoneum. Toldt first described this structure as a fascial plane which was formed by the fusion of the visceral peritoneum with the parietal peritoneum. Gerota () fascia is the condensation of the fibroareolar tissue and fat surrounding the kidney to form a sheath for the organ on its anterior aspect [Citation3]. The transverse colon can be mobilized to the midline of the peritoneal cavity, and the main branch of the middle colic artery and the marginal artery (Drummond marginal arcade or artery – ) is preserved [Citation4]; the latter will serve as the main tributary to nourish the descending colon.
Figure 4. (A) The avascular plane between the Toldt fascia and the Gerota fascia is easily visualized and the dissection may be furthered from above to below along this plane. (B) The avascular plane between Toldt and Gerota fasciae is scored with electrical energy or is mechanically separated through tissue distraction. A whitish line marks the correct route to be followed.
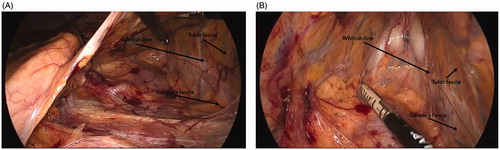
Figure 5. James Lawson Drummond (1783–1853) was an Irish physician, naturalist and botanist. (A) Drummond marginal artery is a continuous arterial arcade along the inner border of the colon formed by the anastomoses of the terminal branches of the superior mesenteric artery (SMA) and the inferior mesenteric artery (IMA). Asterisks mark the critical points of Griffiths at the splenic flexure and the critical point of Sudeck at the recto-sigmoid junction (the site of the anastomosis between the sigmoid and superior rectal arteries). (B) The marginal artery is clearly visible in the intra-operatory backlit picture.
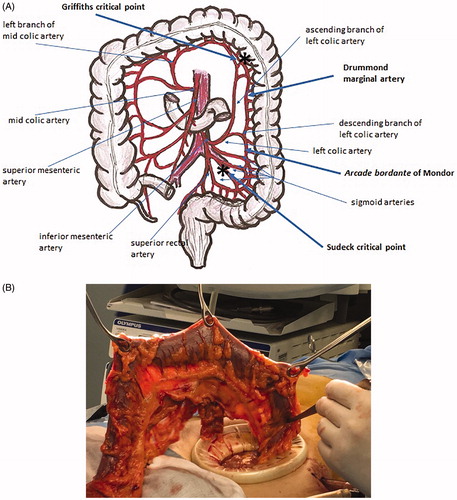
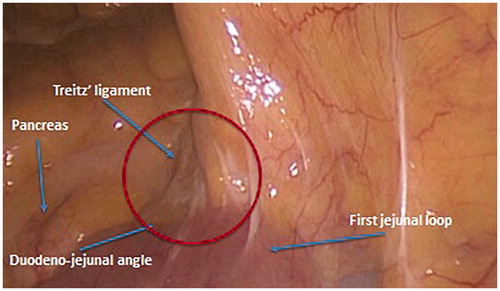
Figure 6. The suspensory muscle of duodenum, also known as the ligament of Treitz, is a thin muscular and fibrous strip of tissue connecting the junction between the duodenum, jejunum, and duodenojejunal flexure to connective tissue surrounding the superior mesenteric artery, the coeliac artery and to the right crus of the median diaphragmatic pillar (esophageal hiatus), although these attachments are quite variable.
1st vascular step: division of the inferior mesenteric vein (IMV)
To accomplish this step, the patients’ positioning is shifted to the supine position with a slight head-down tilt, keeping the rightward rotation. This allows the surgeon to retract the greater omentum, which is brought over the stomach, and the transverse colon upward. Now, the first jejunal loop with the ligament of Treitz and the inferior margin of the pancreas are exposed. The ligament of Treitz ( and Citation6) is dissected by an energy device with maximum care not to damage the jejunum, and the peritoneal leaf overlying the left mesocolon is severed as well. The inferior mesenteric vein (IMV) is easily visualized or, in case of more fatty patients, searched for, right below the inferior margin of the pancreas. The vein is dissected free and lifted. Lifting the arch of IMV allows furthering MTL dissection by opening a window between the Toldt fascia anteriorly and the Gerota fascia posteriorly. The border between the two fasciae, which indicates the embryonic plane of coalescence of posterior mesocolon and retroperitoneum, is whitish (but is not the white line of Toldt): a clear sign which helps the surgeon to keep on the correct dissection plane (). A small piece of gauze may be introduced into this window and left there until the descending colon mobilization will be accomplished. The IMV is then clipped and divided below the inferior margin of the pancreas, thus facilitating the subsequent dissection maneuvers.
Mobilization of the descending colon
The descending colon can be easily mobilized as far distally as the sigmoid colon by continuing the same method of dissection started during the mobilization of the splenic flexure and the first vascular step. The descending colon is retracted toward the anterior abdominal wall and the midline of the peritoneal cavity. The line of fusion between the posterior aspect of the colon mesentery and the retroperitoneum marks the line of dissection, allowing mobilization of the remaining left colon to the midline (embryologic avascular fusion plane between Toldt fascia and Gerota fascia mentioned above). The colon is retracted, and the line of fusion either is scored with electrical energy or is mechanically separated through tissue distraction. Care must be taken to avoid starting the lateral-to-medial (LTM) dissection not too much sideways from the border of the descending colon, with risk for the dissection to be deepened along a wrong plane posterior to the kidney, scoring the Zuckerkandl fascia (the posterior fascial layer of the kidney – and Citation7) [Citation3]. With proper retraction, this is a quick, bloodless process that does not require electrical energy to be performed safely. Descending colon dissection is fully accomplished when the small gauze left in the infra-mesocolic window in the previous step becomes visible and may be removed from the lateral side of colon. If a more extended colectomy on the proximal side is required and having the splenic flexure already fully mobilized, an avascular mesenteric window on either side of the left branch of the mid colic vessels may be further developed and exposed. These windows can then be easily identified and opened, allowing proximal transection of the left branch of the middle colic artery near the inferior border of the pancreas.
2nd vascular step: division of the inferior mesenteric artery (IMA)
Extreme Trendelenburg positioning with rotation to the right is required to expose the root of the sigmoid mesentery (). The jejuno-ileal loops are retracted cephalad and to the right and the right iliac vessels are visualized. The MTL approach brings the inferior mesenteric artery (IMA) easily into view, as well as two avascular mesenteric windows, which are always present immediately cephalad and caudal to the IMA. In addition, it requires only one retracting instrument, thereby allowing less vigorous retraction of the colon and creating less of an opportunity for injury to the specimen. The mesenteric-mesocolic ligament of Gruber, a peritoneal fold stretched from the root of the mesentery to the angle of insertion of the sigmoid mesocolon, is grasped to create tension on the peritoneum which is then incised following a line 1 cm from and over the right iliac vessels ( and ). The dissection of Gruber ligament allows gaining access to the intersigmoid recess, a funnel-shaped area where the coalescence between the posterior aspect of the sigmoid mesentery and the peritoneum overlying the posterior abdomino-pelvic wall is missing () and where the rare retroperitoneal internal intersigmoid hernia named after Jonnesco may occur (). The mesentery can be easily scored along its medial aspect either mechanically or with an energy device, and the sigmoid mesentery can be retracted away from the retroperitoneum while an energy device is used to perform a blunt and bloodless tissue distraction that involves elevating the mesentery en-bloc away from the retroperitoneum. An infra-mesocolic window is created with such maneuvers, bringing into view the left ureter, the left common iliac artery, the gonadic vessels and the psoas muscle, structures that are all covered by the Gerota fascia. This technique can also minimize the extent to which tissue is grasped and exposed to thermal energy, until the left ureter is exposed. The retracting instrument can be inserted into the plane between the mesentery and the retroperitoneum, lifting the mesentery toward the anterior abdominal wall without grasping and tearing tissue.
Figure 8. (A, B) The mesenteric-mesocolic ligament of Gruber is a peritoneal fold stretched from the root of the mesentery to the angle of insertion of the sigmoid mesocolon, the intersigmoid recess is a funnel-shaped area where the coalescence between the posterior aspect of the sigmoid mesentery and the peritoneum overlying the posterior abdomino-pelvic wall is missing.
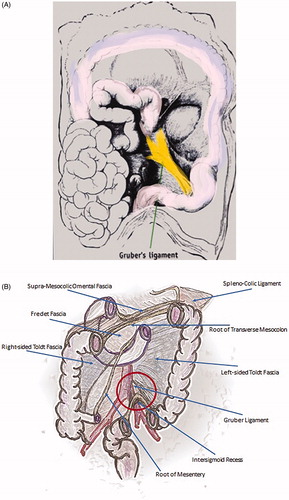
Figure 9. The angle between IMA and the anterior aspect of the aorta is defined as the axilla abdominis of Bacon.
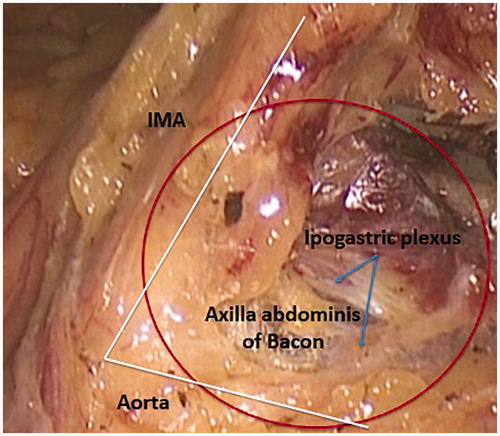
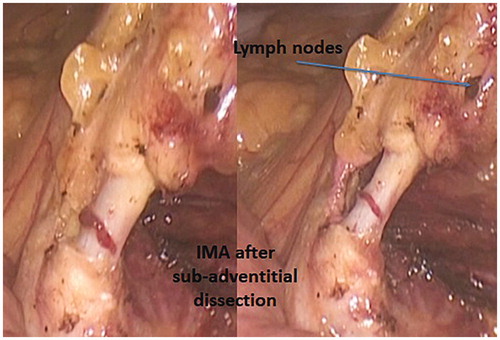
Similarly to what has been described for the first vascular step, a small piece of gauze may be left in this window marking the plane of dissection and protecting the left ureter which, in the native anatomy, is immediately adjacent to IMA, with the vessel lying over the ureter. Cephalad dissection leads directly to IMA origin and the IMA is circumferentially isolated after the hypogastric nerves are clearly identified. The angle between IMA and the anterior aspect of the aorta is defined as the axilla abdominis of Bacon ( and Citation9) [Citation5]. Clearance of the lymphatic and fatty tissue from this area is of utmost relevance in radical surgery. For this reason, in order to achieve an adequate lymph node yield in cases of cancer, authors do prefer the sub-adventitial route to IMA dissection (). Such a technique entails two advantages: the lengthening of the vessel which gives more room for clipping and distances the hypogastric nerves, and the guarantee of a complete lymph node clearance. For colonic malignancies, the IMA, skeletonized as described, should be ligated proximal to its origin to remove the entire lymph node cache by transecting the artery flush the aorta. Conversely, for benign diseases, the IMA may be ligated more distally so as to reduce the risk of urinary and sexual dysfunction from damage to the autonomic nerves in the para-aortic region.
Mobilization of the sigmoid colon
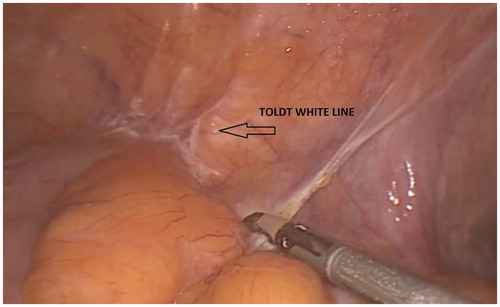
The patient is kept positioned in an extreme Trendelenburg position and rotated to the right (). Whether an LTM or an MTL approach is used to complete the sigmoid colon mobilization is a matter of preference. The most important feature of colon mobilization is identification of the interface between the colonic mesentery and the retroperitoneum. This tissue interface is not the white line of Toldt, though the Toldt line must be incised to expose the mesocolon-retroperitoneum interface: Toldt white line is intended as either the whitish line of lateral peritoneal reflection along the outer edge of the ascending and descending colon () or the junction of the parietal peritoneum with Denonvilliers fascia (). Denonvillers first described the retroprostatic fascia, a part of the pelvic fascia that separates the prostate and the vesiculae from the rectum, consisting of several fibro-muscular layers that are fused together in a single structure covering the posterior aspect of the prostate and surrounding the seminal vesicles. This structure is of utmost relevance in surgery of the rectum.
Figure 11. Toldt white line is intended as either the whitish line of lateral peritoneal reflection along the outer edge of the ascending and descending colon (see picture) or the junction of parietal peritoneum with Denonvilliers fascia. This picture shows the whitish line of incision of the peritoneal reflection along the descending colon.
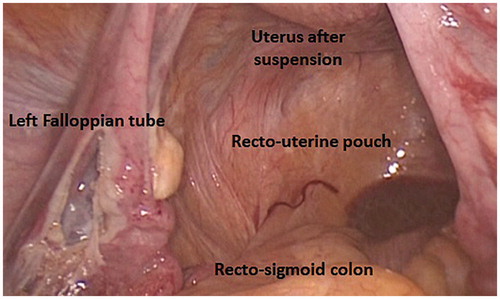
The LTM dissection of the line of Toldt with an energy device usually starts at the peritoneal reflection with the mesentery of the sigmoid colon, right over the left iliac vessels, and proceeds cephalad and medially along the fusion plane between Toldt and Gerota fasciae till the dissection plane of descending colon mobilization and the MTL sigmoid colon dissection plane are joined and the small gauze, previously inserted into the infra-mesocolic window, is brought into view. This technique removes the diseased and thickened mesentery that must be maneuvered over or around in constructing a tension-free colorectal anastomosis. Furthermore, this prevents devascularization of the colon through distal transection of its mesentery, and provides maximal colonic length for a proper anastomosis. At this point the left colon is fully mobilized and still well nourished by the blood supply through the Drummond marginal artery and the “arcade bordante” of Mondor (an arterial segment connecting the descending branch of the left colic artery and the ascending branch of the sigmoid artery – and ). The dissection should now be furthered as far caudally as below the promontorium in benign cases and descending colon malignancies, and the deflection of the peritoneal fold over the upper rectum in sigmoid colon malignancies (cul-de-sac of the recto-uterine pouch or Douglas’ pouch in women – ).
Figure 12. The recto-uterine pouch, a peritoneal space formed by peritoneal deflection between rectum and uterus, and the septum formed by the union of Rathke’s folds, forming the rectum of the fetus, are named after James Douglas. James Douglas (1675–1742) was a Scottish physician and anatomist. Douglas worked as an obstetrician and was the Physician Extraordinary to Queen Caroline wife of King George II of Great Britain.
Intraperitoneal rectal dissection and rectal transection
Posterior dissection is carried on after prolonging the line of the peritoneal incision caudally, on both sides of the upper rectum, toward the peritoneal reflection. The dissection plane follows the course of the superior rectal artery, extension of IMA, which run within the pelvic mesentery and, first, cross the left iliac vessels, then and below the promontorium, heads toward the posterior aspect of the rectum where it splits into two terminal branches (hilum of Mondor with lymphatic nodes draining into the axilla abdominis of Bacon). On the posterior floor of the dissection plane the superior hypogastric plexus (Latarjet presacral nerve – ) is visible, which, below the promontorium, splits into the right and left hypogastric nerves covered by the presacral fascia (Waldeyer fascia – ) [Citation6]. Furthering the posterior dissection along the correct plane, the avascular space between the mesorectal fascia and the parietal presacral fascia (Heald “holy plane”, retrorectal space – ) is entered. On the anterior side, the peritoneal refection may be incised as well, thus having fully mobilized the whole upper rectum.
A critical vascular point at the rectosigmoid junction was described by Sudeck in 1907 as the point of origin of the last sigmoid arterial branch, originating from IMA ( and ). According to Sudeck, a marginal anastomosis between the last sigmoid artery and the superior rectal artery is missing in most cases, therefore, a ligature above the Sudeck’s point may compromise the vascularization of the distal stump [Citation7,Citation8]. At present, experimental ligation studies, arteriograms, roentgenograms, and surgical procedures showed that a marginal anastomosis does exist, thus denying the functional importance of Sudeck’s point, which exists from a gross anatomical point. However, surgeons need to be aware of a small percentage of cases (around 4.7%) where the anastomosis is absent and where it is of great importance to retain Sudeck’s point during colorectal resections [Citation7–9].
Once the rectal cylinder is fully dissected, the application of an endostapler to the distal resection margin is carried out. Every effort should be made to transect as much mesentery as possible toward the bowel wall before stapler application: this will prevent bleeding after stapling. The endostaplers must be placed straight across the bowel, not at an angle; angled placement creates zones of ischemia, as well as a higher risk of inadequate margins of resection for cancers. A reverse V-shaped line of transection should be preferred in patients with a narrow pelvis.
Exteriorization of specimen, and construction of anastomosis
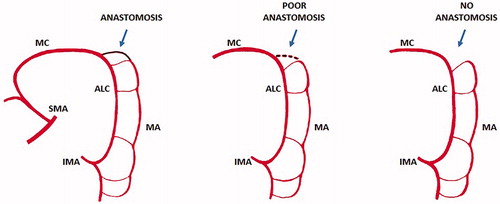
Specimen exteriorization and creation of an anastomosis is self-explanatory. A suprapubic or a right lower quadrant mini-laparotomy close to the groin is created by extending the laparoscopic incision in this area. Such mini-laparotomy should be as short as to allow specimen retrieval without any damage or squeezing. A wound protector/retractor device must be inserted mandatorily to avoid contamination or, even worse, peritoneal seeding by cancer. After extraction of the mobilized colon and rectum, the left colectomy is completed outside the abdominal cavity including the vascular pedicle. The level of proximal colonic transection – and the extent of colonic resection – depends on the distance from the tumor and the vascularization of the colonic stump. The blood supply of the colonic stump is assured by the Drummond marginal artery. The role and even the existence of the Arc of Riolan is controversial (). The Arc of Riolan (Riolan’s arcade, Arch of Riolan, Haller’s anastomosis), also known as the “meandering mesenteric artery”, was supposed to be another vascular arcade present in the colonic mesentery, that connects the proximal middle colic artery with a branch of the left colic artery [Citation10,Citation11]. This artery was found by Riolan low in the mesentery, near its root. More recent studies showed that no distinct identity can be ascribed to this arcade and that the so–called “meandering mesenteric artery” represents a hypertrophied marginal artery and/or the ascending branch of the left colic artery [Citation11]. Infrequently, a centrally located, communicating artery may be found [Citation10]. Villemin and Huard first described such an arcade in 1924 [Citation12]. The Villemin arcade, which is present in 12–18% of cases, is a branch of IMA originating at the point where the artery crosses the IMV, running along the vein and connecting to the superior mesenteric artery [Citation10–12]. The most important critical point in left colonic vascularization is the Griffiths’ point ( and ). It is defined as the site of both the communication of the ascending left colic artery with the marginal artery of Drummond, and the anastomotic bridging between the right and left terminal branches of the ascending left colic artery at the splenic flexure of the colon [Citation13–15]. This critical point is of utmost significance following surgical ligation of IMA: it is upon this critical point that collateral circulation between the superior mesenteric artery and the marginal artery branch of the inferior mesenteric artery supplying the descending colon is dependent. Analysis of arteriographic studies shows that anastomosis at Griffiths’ point is present in 48%, poor or tenuous in 9%, and absent in 43% [Citation13]. The absence of the anastomosis severely impairs the trophism of the colonic stump, jeopardizing the subsequent anastomosis.
Figure 13. John Daniel Griffiths (1926–2001) was a leading cancer surgeon and senior consultant both at Barts and Royal Marsden hospitals in London. In the late 1940s Griffiths carried out research into the blood supply of the colon and circulating cancer cells at the time he spent two years as an anatomy demonstrator under A J E Cave, before becoming registrar and starting his surgical education and training. The Griffiths’ point (or Griffiths’ critical point) refers to the site of watershed anastomosis between the ascending left colic artery and the marginal artery of Drummond occurring in the region of the splenic flexure: the anastomosis may be substantial, tenuous or absent. SMA: superior mesenteric artery; IMA: inferior mesenteric artery; MC: mid colic artery; ALC: ascending left colic artery; MA: marginal artery (modified from Meyers MA).
After colonic transection and fixation of the EEA (end-to-end anastomosis) stapler’s anvil into the colonic stump, with caution not to twist it while handling and bringing the colonic stump down to the pelvis, a tension-free double-stapling anastomosis is constructed under laparoscopic guidance
Applied technologies
Laparoscopic left colectomy requires both tissue dissection and tissue connection technologies. The application of powered dissection and the use of mechanical suturing devices are time-saving and make the procedure safer. The development of these technologies has been of utmost relevance for the implementation of laparoscopic colorectal procedures.
Surgical staplers have been industrialized in 1967 [Citation16]. Since then, their features have changed thanks to continuous improvements. Both circular and linear disposable staplers have been introduced into the clinical practice, the former designed for end-to-end anastomoses, the latter for side-to-side anastomoses and tissue transection. Circular staplers make low colorectal anastomoses easier with leakage rates similar to those of hand-sewn anastomoses. Combining the use of linear and circular staplers according to the Knight-Griffen technique allows performing colorectal anastomosis fast, with minimal tissue handling and does not require high skills [Citation16]. This double stapling technique is widely applied to perform the colorectal anastomosis in laparoscopic left colectomies.
At present, advanced circular and linear staplers are powered. They have been designed mainly to distribute compression throughout the anastomosis without compromising perfusion and provide increased stability by reducing movements at the distal tip. Power circular staplers (Echelon Circular™ powered stapler – Ethicon EndoSurgery Inc. Cincinnati, OH, US) feature 3 D stapling technology with offset closure of the stapler legs. More recently, a smart linear stapling system has been introduced (Signia™ stapling system – Covidien Medtronic, Minneapolis, MN, US), which provides fully powered rotation, articulation, and firing, delivering consistent staple lines by adapting to variable tissues and adjusting firing speed based on tissue variability and thickness.
Powered stapling devices, which have been designed to and may reduce anastomotic leaks, have the added benefit of standardizing performance across users.
Multifunctional energized devices are the other key technology required to perform laparoscopic left colectomies with improved safety and enhanced dexterity. Usually, they combine tissue dissection with coagulation and cutting functions. In some instances and with some limitations, they can be used as grasping forceps. Ultrasonically activated shears were the first such devices introduced into the clinical practice and then employed in laparoscopic left colectomies [Citation17–18]. The ideal energy form for use in laparoscopic surgery should provide controlled, hemostatic cutting, minimal thermal injury to surrounding tissue, minimal smoke production. Most of these instruments use ultrasonic and bipolar energy (Harmonic™ACE – Ethicon Endosurgery Inc, Cincinnati, OH, USA; Caiman® – Braun Aesculap, Tuttlingen, Germany), or hybrid energy (bipolar sealing combined with ultrasonic dissection – Thunderbeat® – Olympus Medical Systems, Hamburg, Germany [Citation19]; bipolar tissue fusion combined with monopolar dissection – LigaSure Advanced™ – Medtronic, Minneapolis, MI, USA). Most present devices feature software controlled adaptive technology with tissue impedance feedback to optimize energy delivery in response to the changes during use, contributing to control temperature and minimizing tissue damage. All of them may be employed in laparoscopic left colectomies: their tips are mostly curved and designed to provide fine dissection or uniform compression and allow vessel sealing up to 7 mm.
Authorship
Cristiano G.S. Hüscher, Marco Maria Lirici, John H. Marks, and Giovanni Dapri contributed equally to the conception of this work and the acquisition of data and drafted the manuscript. Ermanno Ancona critically revised it for intellectual content.
Acknowledgements
Professor Sir Alfred Cuschieri is acknowledged for his advice in the choice of the title.
Declaration of interest
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Bouchet A, Cuilleret J. Anatomie topographique, descriptive et fonctionelle. Tome 4. Villeurbanne (France): Edition SIMEP; 1975.
- Morgenstern L, Skandalakis JE. Anatomy and embriology of the spleen. In: Hiatt JR, Phillips EH, Morgenstern L, editors. Surgical diseases of the spleen. Berlin, Heidelberg: Springer-Verlag; 1997. p. 15–24.
- Testut L, Jacob O. Traite d’anatomie topographique avec applications medico-chirurgicales. Tome second: Abdomen – Bassin – Membres. Paris: Octave Doin; 1906.
- Drummond H. The arterial supply of the rectum and pelvic colon. Br J Surg. 1913;1(4):677.
- Bacon HE, Smith CH. The arterial supply of the distal colon pertinent to abdominoperineal proctosigmoidectomy, with preservation of the sphincter mechanism. Ann Surg. 1948;127(1):28.
- Winkelmann A. Wilhelm von Waldeyer-Hartz (1836–1921): an anatomist who left his mark. Clin Anat. 2007;20(3):231–234.
- van Tonder JJ, Boon JM, Becker JH, et al. Anatomical considerations on Sudeck’s critical point and its relevance to colorectal surgery. Clin Anat. 2007;20(4):424–427.
- Sudeck P. Über die Gefässversorgung des Mastdarmes in Hinsicht auf die operative Gangrän. München Med Wchnschr. 1907;54:1314.
- Howat D. Paul Sudeck-his contribution to anaesthesia. Anaesthesia. 1989;44(10):847–850.
- Tubbs RS, Shoja MM, Loukas M, editors. Bergman’s comprehensive encyclopedia of human anatomic variation. Hoboken (NJ): John Wiley & Sons, Inc; 2016.
- Lange JF, Komen N, Akkerman G, et al. Riolan’s arch: confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am J Surg. 2007;193(6):742–748.
- Villemin F, Huard P. La constitution de l’arc de Treitz. CR Ass Anat Strasbourg. 1924;1:263–267.
- Meyers MA. Griffiths’ point: critical anastomosis at the splenic flexure. Significance in ischemia of the colon. AJR Am J Roentgenol. 1976;126(1):77–94.
- Griffiths JD. Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl. 1956;19(4):241–256.
- Griffiths JD. Extramural and intramural blood-supply of colon. Br Med J. 1961;1(5222):323–326.
- Lirici MM, Hüscher C. Techniques and technology evolution of rectal cancer surgery: a history of more than a hundred years. Minim Invasive Ther Allied Technol. 2016;25:226–233.
- Amaral JF. Ultrasonic dissection. Endosc Surg Allied Technol. 1994;2(3–4):181–185.
- Amaral JF. The experimental development of an ultrasonically activated scalpel for laparoscopic use. Surg Laparosc Endosc. 1994;4(2):92–99.
- Ceccanti S, Falconi I, Frediani S, et al. The THUNDERBEAT system for tissue dissection and vascular control in laparoscopic splenectomy. Minim Invasive Ther Allied Technol. 2017;26:249–252.