Abstract
Aims
To identify areas that are difficult to access by the single scope at the time of endoscopic submucosal dissection (ESD) and examine the effectiveness, en-bloc, R0 resection, and perforation rate after changing to multibending scope at the same site.
Material and methods
When the direct visualization of the submucosal layer became impossible with Q260J or in the position where the device became vertical and peeling became impossible in parallel, we decided to change to the multibending 2TQ260M scope to record the position where the change was effective and the perforation rate.
Results
A total of 315 lesions were studied. Of the 12 sites, ESD was completed using the Q260J alone at four sites. The 2TQ260M scope was used with greater frequency at the fornix (88.9%) and on the line of the lesser curvature of the stomach (37.1%). In the cases with observed perforations (0.9%), the submucosal layer was not elevated due to the adhesion caused by strong fibrosis. None of the cases involving the change to 2TQ260M was ineffective, nor were perforations observed, and all resected specimens were en-bloc and R0 resections.
Conclusions
The success rate of this scope may help clinicians perform ESD with greater understanding.
Introduction
Endoscopic submucosal dissection (ESD) was introduced to facilitate en-bloc resection of early gastrointestinal neoplasms, allowing precise, histopathological diagnosis, and minimizing the likelihood of recurrence [Citation1,Citation2]. The popularity of ESD has rapidly increased in Asia and the rest of the world. Guidelines for ESD have been developed recently in Europe and the USA [Citation3,Citation4]. Compared to conventional endoscopic treatment, the technique is extremely difficult. Complications, such as perforation, are frequently observed [Citation5–11]. This is largely influenced by the blind approach of devices when the dissecting submucosal layer is not visible. Therefore, to enhance vision in the submucosal layer, emphasis has been placed on the development of the traction devices used to facilitate therapeutic procedures, and on the knives used for excision [Citation12–14]. However, there have been no reports examining the choice of endoscope for a given purpose, based on different sites and situations. A broader understanding and the optimal instrument choice will prevent the risk of perforation and help to achieve the best possible treatment outcomes.
Unlike ESD in other organs, the stomach is not only difficult to approach but can also have complicating elements, such as hypervascularity, fibrosis, and scarring. Furthermore, the location of the lesion is an important consideration. In many reports, regions of the stomach are classified simply as upper, middle, and lower [Citation15–17]. Other reports further subclassify by anterior and posterior wall location [Citation15,Citation18]. For greater precision, we have previously proposed a new classification system based on 12 locations and multiple criteria, such as the condition of the surrounding mucosa, vascularity of the lesion, and presence of ulceration, fibrosis, scarring, and submucosal fat [Citation19].
To apply traction by grasping a lesion from one of two forceps channels, and to maintain an appropriate distance from the lesion, use of the R scope with the multibending function has been reported [Citation20–22]. These articles note the usefulness of grasping with the forceps from the other channel to achieve counter-traction, as well as the effectiveness of the multibending capability, when the approach would be difficult with a conventional scope. However, these reports do not detail the gastric location and in which situations the multibending scope is useful. In fact, the multibending scope is mostly used to facilitate access to the lesion, as in orienting the device vertically to approach in a horizontal manner, rather than being used for grasping the lesion by two forceps channels.
Now, an R-scope launched as 2T-Q260M (Olympus, Tokyo, Japan), is available. Although the usefulness of this scope is widely known on a global scale, no reports have specifically analyzed the recommended gastric sites for which to use the multibending scope.
We believe that acquiring evidence for the characteristics important in multibending scope use, such as site of involvement, has important clinical implications. With these factors in mind, we conducted a study to clarify the correct use.
Material and methods
Target lesion
We collected the clinical information of patients with gastric cancer who had indications for ESD. Specifically, we identified patients included in hospital endoscopic resection databases from 1 April 2009 to 31 July 2014, who were appropriate candidates for ESD according to the Japanese guidelines and who had undergone the procedure [16]. We collected data from 348 lesions in 335 patients. Differentiated, undifferentiated, and mixed-type (differentiated and undifferentiated) lesions with undifferentiated components, <20 mm in size, satisfied the new criteria for ESD [Citation16,Citation23,Citation24].
Potentially cancerous adenomas were included based on the histopathological examination of resected specimens, as well as those lesions that could become cancerous (>20 mm in size, lesions with a depression, lesions with rapid growth in a short time, and lesions diagnosed by biopsy as having high-grade atypia) [Citation25–27]. For improved reliability of data, non-curative resections were excluded. Ultimately, only patients undergoing curative en-bloc and R0 resection were studied.
Endoscope features and use
In this study, we used endoscope units made by Olympus, the only company currently manufacturing a multibending scope. We used three types of endoscopes: the single-bending GIF Q260J, GIF Q260, and the multi-bending GIF 2TQ260M model (Olympus, Tokyo, Japan). The GIF Q260J model is most commonly used in ESD. It has a water-jet function, is light in weight, and has excellent maneuverability.
Despite these features, vertical positioning makes the dissection procedure difficult. The submucosal layer becomes invisible if the lesions are separated even if the scopes are collimated. The GIF 2TQ260M has a multibending function that allows a horizontal approach, allowing appropriate viewing of a distant lesion; however, the disadvantage of using this scope is that it is large and heavy (.
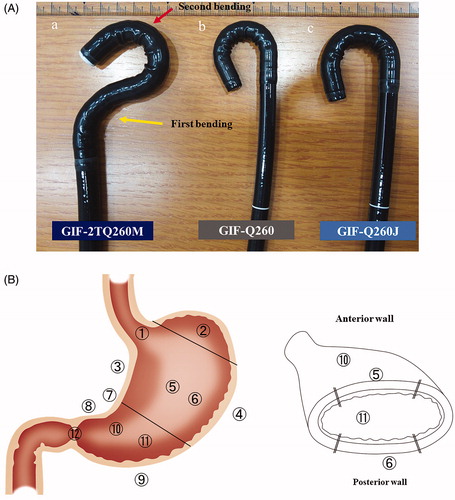
Figure 1. (a) Endoscope structure comparison. GIF Q260J: Scope most commonly used in endoscopic submucosal dissection. It has a water-jet function, is light in weight, and has excellent maneuverability. Outside tip diameter is 9.9 mm and weight is 1.3 kg. GIF Q260: Most flexible model because of the thin diameter, permitting reverse maneuvers; does not have a water-jet function. Outside tip diameter is 9.2 mm and weight is 1.2 kg. GIF 2TQ260M: Multibending scope with two bends (yellow arrow shows first bending point, red arrow shows second bending point). The two bends enable a better approach to the lesion and allow dissection to be performed in a horizontal manner; it has a water-jet function and two-channel forceps. Outside tip diameter is 9.2 mm and weight is 1.5 kg. (b) Treatment area classification of gastric lesion location: Coronal section of stomach showing the 12 lesion locations defined as follows: 1) across the esophagogastric junction, 2) the fornix, 3) lesser curvature of the body, 4) greater curvature of the body, 5) anterior wall of the body, 6) posterior wall of the body, 7) across the angle area, 8) lesser curvature of the antrum, 9) greater curvature of the antrum, 10) anterior wall of the antrum, 11) posterior wall of the antrum, and 12) across the pyloric ring. Inset: Horizontal cross-section through stomach.
Clinical study of ESD
ESD was performed by 14 endoscopists who had completed the training system advocated by Tsuji et al. [Citation28] and had experience with ten or more cases utilizing a porcine model. The lesion classification was based on our reported system [Citation19]. A total of 12 locations in the stomach were identified: 1) across the esophagogastric junction (AEGJ), 2) the fornix, 3) lesser curvature of the body, 4) greater curvature of the body, 5) anterior wall of the body, 6) posterior wall of the body, 7) across the angle area, 8) lesser curvature of the antrum, 9) greater curvature of the antrum, 10) anterior wall of the antrum, 11) posterior wall of the antrum, and 12) across the pyloric ring (APR) (. We used the Q260J as the standard scope at the start of ESD and changed scopes when operation with this scope became difficult. For APR (number 12) in all the cases, we used the Q260 because this scope has the greatest flexibility when turning around. Within the narrow duodenal bulb, this scope is the most suitable. Furthermore, to minimize contact with the opposite intestinal wall and to secure the working space, we did not use the tip hood.
After circumference incision of the lesion, when the submucosal layer became vertical with Q260J and parallelization became impossible, or when the lesion became distant and the submucosal layer was not visible, and the device encountered a blind approach, the scope was changed to 2TQ260M. When the submucosal layer became visible and parallel by the change, it was recorded as effective, and the location, frequency, perforation rate, and the situation were verified. Finally, all specimens were pathologically assessed as curative, en-bloc, and R0 resections.
ESD procedure
Pethidine, midazolam, or propofol was used as a sedative with close monitoring of the cardiopulmonary functions. When the target lesion was of the undifferentiated and/or mixed type, the marking for the incisional line was approximately 10 mm from the lesion. The marking was done 5 mm outside the lesion in other cases. The basic technique began with a precut in the mucosa using a dual knife (KD-650; Olympus, Tokyo, Japan). Then, a mucosal circumference incision was made using the dual knife or insulation-tipped knife 2 (KD-611L; Olympus, Tokyo, Japan). Normal saline or hyaluronic acid was injected locally as needed, and submucosal dissection using the insulation-tipped knife 2 and/or dual knife (particularly if a dual knife was used in the case of significant fibrosis) was performed. If prominent, thick blood vessels were observed, or when there was active bleeding during treatment, hemostasis and prevention of hemostasis were performed using the Coagrasper (FD-410LR; Olympus, Tokyo, Japan). For the precutting, incision, and dissection procedures, a high-frequency electrosurgical unit for cutting and coagulation (Erbotom VIO300D; ERBE, Tübingen, Germany) was employed.
Definitions
Curative resection was defined as resection that satisfied the expanded criteria of ESD [Citation16,Citation23,Citation24] in the case of en-bloc and R0 resection. The morphology of the tumor was described using the Paris classification [Citation29]. Pathological findings were described using the Vienna classification [Citation30]. Perforation was defined as the endoscopic observation of serosal tissue intraoperatively, and free air was confirmed by postoperative computed tomography and X-ray.
Study approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Juntendo University Hospital.
Statistical analysis
Data were analyzed using the Fisher’s exact test or the χ2 test. Statistical significance was defined as p<.05. All statistical analyses of recorded data were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Lesion details
Of the 348 cases, 33 cases were non-curative resections (lesions invading more than 50 µm into the submucosa and/or positive lymphovascular invasion [Citation16]). Three hundred and fifteen lesions were curative, en-bloc and R0 resections. Macroscopically, 46.0% and 50.5% of the lesions were of the flat and depressed types, respectively, whereas the protruded type accounted for only 3.5% of all lesions. The median tumor diameter was 11 mm, and the median size of the resected specimen was 34 mm. The most common histological tumor type was differentiated adenocarcinoma (82.9%). Mixed types containing differentiated and (≤20 mm) undifferentiated types accounted for 4.1% of the lesions, and the undifferentiated type accounted for only 1.6% of the total number of lesions. Adenomas comprised 11.4% of the lesions ().
Table 1. Baseline characteristics of patients, gastric tumors, and complications.
Study summary
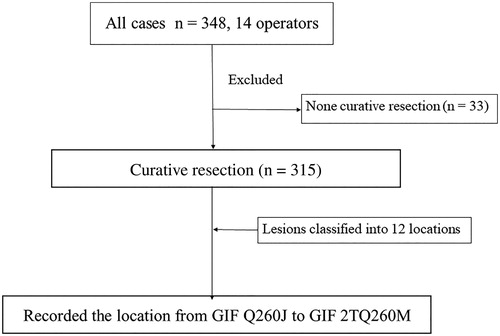
A total of 14 endoscopists participated in the study, and 348 lesions were analyzed. Among the cases in which ESD was performed, 33 non-curative resections were excluded. Therefore, a total of 315 lesions treated by curative resection were included in the analysis ().
Figure 2. Study outline. A total of 14 endoscopists participated in the study, and 348 lesions were analyzed. Among the cases in which en-bloc resections were performed, 33 non-curative resections were excluded. A total of 315 lesions treated by curative resection (en-bloc and R0 resection) were included in the analysis. They were divided into 12 locations and the rate of change from Q-260J to 2T Q-260M and perforation were recorded.
Lesion location and characteristics
The breakdown of the number of cases according to the 12-location classification is shown in . The lesions were most commonly found on the posterior wall of the gastric body (n = 48, 15.2%), whereas those extending to the pyloric ring were least frequent (n = 4, 1.3%). The clinicopathological characteristics according to the lesion location are listed in . No significant between-group difference was observed with respect to age (p=.37). A significant difference was observed in the tumor diameter between various locations (p<.05).
Table 2. Number of lesions at each location and rate of changing the scope from Q260J at each location.
Endoscope use by location
The locations at which the scope was changed from the GIF-Q260J to the GIF-Q260 or GIF-2TQ260M are shown in . The 2TQ260M was used in 17.1% of all cases. The success rate of the GIF-2TQ260M was 100% for all cases of difficult operation and impossibility in the GIF-Q260J, and there were no failures. The most frequent case types are presented in . Of the 12 sites, we were able to complete ESD using the GIF-Q260J alone at only site numbers 4, 9, 10, and 11. For APR, we switched to the Q260 in all cases.
Figure 3. Sites where the Q-260J was changed to 2TQ260M. Case 1: Location No. 3; Lesser curvature of the body (0-IIa 20-mm T1a well-differentiated adenocarcinoma). (a) Q260J scope showing marking around the lesion. (b) The scope could not go any closer to the lesion, so the submucosal layer could not be checked visually. Therefore, we might have to operate the scope in a blinded manner during dissection, leading to possible perforation. Switching to a multibending scope allows direct visual observation of the submucosal layer by going closer to the lesion. This reduced the risk of perforation because the direction of dissection could be checked properly. Case 2: Location No. 8; Lesser curvature of the antrum (0-IIc 12-mm T1a well-differentiated adenocarcinoma). (d) Q260J scope facing the lesion perpendicularly. (e) Q260J scope; circumferential incision was possible, but yellow arrow indicates the submucosal layer could not be made horizontal approach. (f) Red arrow indicates after changing to the 2TQ260M scope, enabling a horizontal approach to the submucosal layer dissection.
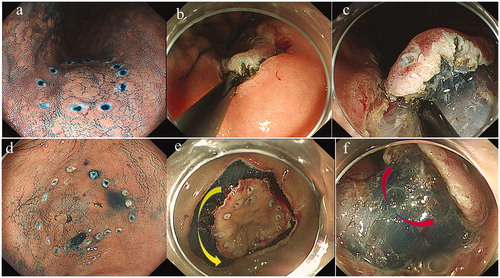
Although there was a difference in frequency, we needed to switch to the GIF-2TQ260M in some cases at the rest of the sites. Among these sites, the frequency was relatively higher at site numbers 2 (88.9%), 4 (52.1%), 3 (34.3%), 1 (20.0%), and 7 (20.0%). Thus, the GIF-2TQ260M scope was used with greater frequency on the line of the lesser curvature of the stomach (location numbers 3, 7, and 8; 37.1%) and the fornix (number 2).
Complications, background factors, and endoscope type
Perforations occurred in 0.9% (3) of cases. Reviews of those cases are shown in .
Table 3. Patients with perforation.
Of the three patients in whom perforations occurred, all cases had strong fibrosis and adhesion, so the submucosal layer could not be lifted by injection. The Q260J model was used in all the cases, and the scope was not changed during the procedure. The locations involved were number 6 (posterior wall of the body) and number 7 (angle). No cases of perforation while using the GIF-2TQ260M were observed at the location where the lift was sufficiently obtained.
Discussion
In this study, we examined the effectiveness of the multibending scope to access recommended locations that are difficult to reach in ESD. The perforation rate, a complication of ESD, en-bloc, and R0 resection rate were also examined.
The median tumor size was 11 mm, which was slightly small. It was suggested that 1.6% of the patients had undifferentiated type and 4.1% had undifferentiated mixed type. But differentiated type was marked on the 5 mm outside and the undifferentiated type was marked on the 10 mm outside. Therefore, when a safety margin is included, EMR is likely to result in segmental resection. In the report to avoid tumor recurrence due to incomplete resection, Hoteya et al. reported lesions >5 mm [Citation31], Watanabe et al. reported 10 mm [Citation32], Shimura et al. reported 11 mm [Citation33]. Therefore, it seemed to be appropriate to treat ESD even in our median size of 11 mm.
Thus, we were able to clarify which sites are difficult to approach with the commonly used GIF Q260J endoscope and identify the appropriate locations for the GIF2TQ260M.
The lesser curvature of the body was the most common location that was difficult to approach, necessitating a change of the scope from the GIF Q260J to the GIF 2TQ260M in 37.1% of cases. The reason for this finding was that the shape of the stomach varies widely in this region, and there is considerable individual variation. When dissecting the submucosa in the area, GIF Q260J has difficulty approaching lesions, the devices making a blind approach when dissecting the submucosal layer. However, a multibending scope that can bend in two places enables a horizontal approach close to the lesion ().
The situation was the same at the anterior (4.3% of cases) and posterior (4.1%) walls of the body. The scope was prone to facing lesions perpendicularly in the fornix (88.9% of cases) and lesser curvature of the antrum (52.1%), which was considered a result of the vertical orientation of the device stand. In these cases, the circumferential incision was accomplished easily, but for submucosal dissection, it was often difficult to approach the lesion horizontally with the GIF Q260J, as the device often ended up facing perpendicular to the lesion (). The angle has a sharp anatomical bend, and, therefore, it must be approached from various directions. It is also a common site for ulcers and scars that cause adhesions resulting in poor lifting, making the dissection difficult. Therefore, this location resulted in a scope change rate of 20.0%. The scope change rate at the esophagogastric junction was also high. In these cases, a different scope was used to achieve an appropriate distance because the scope was too close to the lesion when its orientation was altered.
As demonstrated in the analysis, the scope was changed to the 2TQ260M model with the multibending function in such locations. The 2TQ260M scope enables the lesion to be approached and the dissection to be performed in a horizontal manner, thereby facilitating the procedure. Thus, if the location is difficult to approach using a conventional scope, such as the Q260J model, we recommend immediately switching to a multibending scope. Moreover, since the 2TQ260M endoscope has two-channel forceps, the circumference incision, and dissection can be performed through one channel, while injections and hemostatic forceps can be maneuvered through the other channel [Citation20–22], reducing the procedure time. However, this scope is heavy and thick, causing physical strain on the operator when used for long periods. Once treatment is complete, we recommend returning to the Q260J.
In addition to the regions involved, other factors influence endoscope choice. The desirable features for ESD include the following: lightweight, water-jet capability, and adequate forceps channel size (minimum 3.2 mm for aspiration). A hooded tip is recommended to counteract the tendency of the endoscope to dive under the mucosa and cause counter-traction.
Complications involving perforation occurred in only three cases. In other reports, the rates are generally 2–4% [Citation2,Citation5–11,Citation23]. These results are very few in contrast to those reported elsewhere. The Q260J was used in all three cases of perforation, but these were due to dissection under the condition that submucosal layer did not lift even if sufficient injection was provided due to strong adhesion and fibrosis. Thus, they were perforated by direct visualization without the blind approach of the device, so it was considered that the perforations were not caused by the type of scope but due to the adhesion and fibrosis of the lesion. The perforation was also minute, and no large accident was caused by the suture contraction of the clip. The incidence of perforation was neither observed in cases without fibrosis nor in cases using the multibending scope, which may provide evidence for the importance of using this scope effectively. In addition, uncontrolled hemorrhage did not occur as the submucosal layer was constantly visible.
The major reasons for the difficulty of ESD and the high risk of perforation include poor visibility in close proximity of the submucosal layer and poor parallel manipulation at the time of dissection. The risk would be greatly reduced if the submucosal layer could be visualized reliably and approached in close proximity and parallel. In treatment centers having a high perforation rate, ESD may be performed in situations where the use of the multibending scope is not fully understood or when a multibending scope is not available. It may be no exaggeration to say that a multibending scope is an essential tool to prevent perforation and to carry out ESD safely. Considering the possibility of using this scope in ESD is important for the prevention of perforations, connected to the treatment.
As a limitation of this study, multibending scopes are currently manufactured only by Olympus (Tokyo, Japan). Hence, our results cannot be used when performing ESDs with scopes from other companies. Additionally, this scope has large handles, and is thick, imposing a large burden on the operator when it is used for a long time. Currently, ESD is an increasingly widespread therapy worldwide. As we have seen in this study, this scope is essential for ESD, and if a product, manufactured by other companies, that is made lighter and smaller in diameter is developed, ESD can be expected to be more widely used as a safe therapy.
Preparing a suitable multibending scope for the treatment in advance leads to the prevention of an incidental event. Therefore, we especially focused on the usability of the multibending scope at the site based on our new classification of the location. This study is the first to reveal the characteristics of the multibending scope, based on a more detailed classification of lesion location. The results of this study may help us perform ESD with greater understanding. We believe that further development of new approaches, devices, and scopes based on these results will contribute to making the ESD procedure safer and easier.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41(10):929–942.
- Muto M, Miyamoto S, Hosokawa A, et al. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37(2):178–182.
- Kantsevoy SV, Adler DG, Conway JD, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68(1):11–18.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47:829–854.
- Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45(1):30–36.
- Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23(1):73–77.
- Lee JH, Hong SJ, Jang JY, et al. Outcome after endoscopic submucosal dissection for early gastric cancer in Korea. World J Gastroenterol. 2011;17(31):3591–3595.
- Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26(9):2456–2464.
- Ono S, Ono M, Nakagawa M, et al. Delayed bleeding and hemorrhage of mucosal defects after gastric endoscopic submucosal dissection on second-look endoscopy. Gastric Cancer. 2016;19(2):561–567.
- Chun HJ, Keum B, Kim JH, et al. Current status of endoscopic submucosal dissection for the management of early gastric cancer: a Korean perspective. World J Gastroenterol. 2011;17(21):2592–2596.
- Ahn JY, Choi KD, Choi JY, et al. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73(5):911–916.
- Matsumoto K, Nagahara A, Sakamoto N, et al. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the “medical ring. Endoscopy. 2011;43(S 02)UCTN:E67–68.
- Matsumoto K, Nagahara A, Ueyama H, et al. Development and clinical usability of a new traction device “medical ring” for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2013;27(9):3444–3451.
- Teoh AY, Chiu PW, Hon SF, et al. Ex vivo comparative study using the Endolifter® as a traction device for enhancing submucosal visualization during endoscopic submucosal dissection. Surg Endosc. 2013;27(4):1422–1427.
- Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38(10):987–990.
- Association JGC. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123.
- Goto O, Fujishiro M, Kodashima S, et al. Is it possible to predict the procedural time of endoscopic submucosal dissection for early gastric cancer? J Gastroenterol Hepatol. 2009;24(3):379–383.
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69(7):1228–1235.
- Konuma H, Matsumoto K, Ueyama H, et al. Procedure time for gastric endoscopic submucosal dissection according to location, considering both mucosal circumferential incision and submucosal dissection. Gastroenterol Res Pract. 2016;2016:1.
- Neuhaus H, Costamagna G, Devière J, et al. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy. 2006;38(10):1016–1023.
- Spaun GO, Zheng B, Martinec DV, et al. Bimanual coordination in natural orifice transluminal endoscopic surgery: comparing the conventional dual-channel endoscope, the R-Scope, and a novel direct-drive system. Gastrointest Endosc. 2009;69(6):e39–45.
- Moyer MT, Haluck RS, Gopal J, et al. Transgastric organ resection solely with the prototype R-scope and the self-approximating transluminal access technique. Gastrointest Endosc. 2010;72(1):170–176.
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3(4):219–225.
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12(3):148–152.
- Nakamura K, Sakaguchi H, Enjoji M. Depressed adenoma of the stomach. Cancer. 1988;62(10):2197–2202.
- Tamura G, Sakata K, Nishizuka S, et al. Allelotype of adenoma and differentiated adenocarcinoma of the stomach. J Pathol. 1996;180(4):371–377.
- Tamura G. Molecular pathogenesis of adenoma and differentiated adenocarcinoma of the stomach. Pathol Int. 1996;46(11):834–841.
- Tsuji Y, Ohata K, Sekiguchi M, et al. An effective training system for endoscopic submucosal dissection of gastric neoplasm. Endoscopy. 2011;43:1033–1038.
- Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy. 2002;34(2):163–168.
- Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47(2):251–255.
- Hoteya S, Iizuka T, Kikuchi D, et al. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24(6):1102–1106.
- Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63(6):776–782.
- Shimura T, Sasaki M, Kataoka H, et al. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22(6):821–826.
