Abstract
Objective
To investigate the safety and oncological prognosis of laparoscopic seromuscular dissection (LSD) in the treatment of gastric stromal tumors.
Material and methods
From June 2016 to July 2022, 67 patients with gastric stromal tumors underwent laparoscopic seromuscular dissection (LSD), and 58 patients underwent non-LSD surgery during the same period (52 patients underwent laparoscopic gastric wedge resection (LWR), two patients underwent proximal gastrectomy, three patients underwent total gastrectomy, one patient underwent distal gastrectomy and partial hepatectomy). Gastric stromal tumor patients were compared to compare the surgical results, tumor relapse rate, and survival rate of the two groups.
Results
The results of the two groups were compared. For gastric stromal tumors, especially gastric stromal tumors located at ‘difficult sites’, LSD can reduce the amount of bleeding and the number of cutting staplers used during the operation, reduce the incidence of postoperative complications, shorten the postoperative hospitalization time, reduce the hospitalization cost and improve the quality of life of patients without increasing the operation time.
Conclusion
Laparoscopic seromuscular dissection for gastric stromal tumors is safe and technically feasible in the hands of experienced laparoscopic surgeons.
Introduction
Gastrointestinal stromal tumor (GIST) is the most prevalent mesenchymal tumor of the digestive tract [Citation1]. The standard treatment for gastrointestinal stromal tumor is surgical resection. Surgical procedures are frequently chosen based on the individual situations of patients and surgeons. Various surgical procedures are used for gastrointestinal stromal tumor of varying diameters. Endoscopic therapy is commonly utilized for smaller or partly endogenous tumors. Laparoscopic or open surgery is frequently required for tumors unsuited for endoscopic therapy or exogenous tumors, and total tumor excision must be ensured [Citation2]. When endoscopy cannot manage stomach stromal tumors, laparoscopic wedge wesection (LWR) is frequently employed in clinical practice. The technological requirements are uncomplicated and safe [Citation3]. Studies have found that this surgical approach has advantages over radical gastrectomy, including less trauma, faster recovery, and a better oncological prognosis [Citation4,Citation5]. With the introduction of new medical equipment and the constant improvement of surgeons’ surgical capabilities in recent years, the treatment of GIST has progressively shown a trend towards minimally invasive procedures, and numerous new surgical techniques have evolved. Among these, laparoscopic seromuscular dissection (LSD) is a new surgical technique that is gradually being used in gastric stromal tumor surgery. LSD removes the tumor without harming the stomach mucosa and prevents tumor rupture. It can also decrease the damage to normal gastric mucosa around the tumor, increase organ function preservation, and improve the patient’s quality of life. The Japanese team of Kazuaki Tanabe [Citation6] reported the feasibility of LSD technology. On the safety of LSD and its influence on tumor prognosis, however, there is no clear conclusion and consensus. This retrospective study examined the clinical data of patients with gastric stromal tumors at our institution in order to determine the safety and efficacy of LSD.
Material and methods
Patient selection
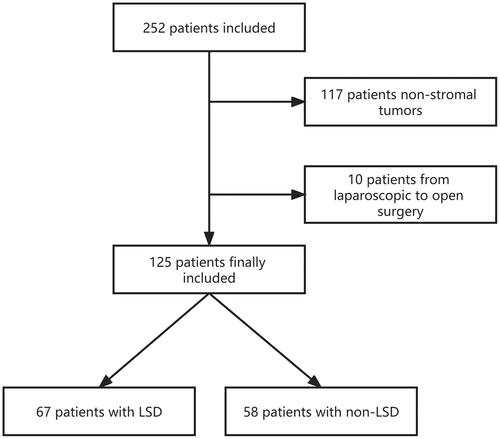
All enrolled patients were identified from the Center’s prospectively collected database between 2016 and 2022. Since LSD can be considered as a modified laparoscopic gastric wedge resection (LWR), whether or not patients take LSD depends on the way of randomization grouping at the time of prospective patient data collection at our center and patients will be advised to sign an informed consent form to obtain their consent before surgery. Finally, 67 patients with gastric stromal tumors who underwent LSD in our center were compared to 58 patients with gastric stromal tumors who underwent non-LSD surgery (52 patients underwent LWR, two patients underwent proximal gastrectomy, three patients underwent total gastrectomy, one patient underwent distal gastrectomy and partial hepatectomy). The preoperative, perioperative, and postoperative information of all patients involved in this study was obtained by hospital information system (HIS), SMS, and telephone follow-up. shows the inclusion and exclusion criteria.
Surgical procedure
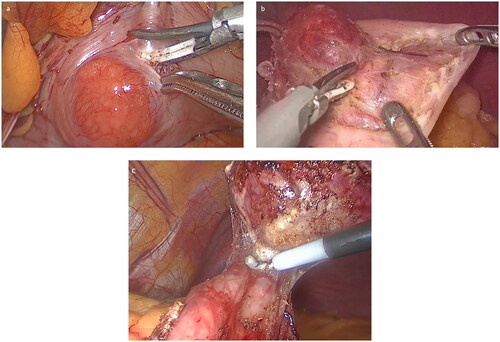
The patients in both groups were in the supine position with their legs apart. The omentum and mesentery around the gastric wall where the tumor was located were dissected with an ultrasonic scalpel in the LWR group, the gastric wall where the tumor was located was lifted with intestinal forceps, and the gastric wall was clipped with a cutting stapler 1 cm from the lesion. Following stimulation, the wedge resection of the gastric wall was completed, and the bleeding of the cutting wound decided whether 3/0 absorbable barbed wire was added. In the LSD group, the serosa was lifted 0.5 cm from the gastric wall where the tumor was located, and the seromuscular layer of the local gastric wall was cut along the tumor with an ultrasonically activated scalpel. The tumor was gradually pulled out of the gastric wall by incision of the seromuscular layer of the gastric wall due to relaxation of the gastric wall mucosa and submucosa. If the tumor had not invaded the gastric mucosa, it could be completely removed from the mucosa; if the tumor had invaded a portion of the mucosa of the local gastric wall, the mucosa of the tumor’s pedicle could be severed using a cutting stapler, or the gastric wall mucosa connected to the bottom of the tumor could be severed directly using an ultrasonically activated scalpel, and finally, the seromuscular and mucosal layers could be constantly sutured using absorbable barbed wire under laparoscopy (). Distal gastrectomy, total gastrectomy or proximal gastrectomy was performed according to the conventional method to complete tumor resection and digestive tract reconstruction. After surgery, there was no need for a gastric tube, and patients were able to eat and get out of bed on the first day.
Statistical analysis
SPSS 26.0 software was used for statistical analysis. Categorical variables were reported as frequency (%), and quantitative variables were reported as mean ± SD except where otherwise noted. Categorical variables were analyzed with the χ2 or Fisher exact test. Quantitative variables were analyzed with t test or a Wilcoxon rank-sum test. Statistical significance was assumed when the p value was <0.05.
Results
This study comprised 252 patients with a preoperative diagnosis of gastric stromal tumors who were getting surgical therapy from June 2016 to July 2022. One hundred twenty-seven patients were ruled out (). Eventually, the research covered 125 patients. The LSD group had 67 patients, whereas the non-LSD group had 58. and show the clinical data and perioperative conditions of the two groups.
Table 1. Comparative analysis of clinical and pathological features between LSD group and non-LSD group.
Table 2. Comparative analysis of perioperative data features between LSD group and non-LSD group.
In terms of clinical and pathological characteristics, the number of cases recruited, the mean age, the mean maximum diameter of the tumor, the proportion of gender, and the proportion of tumors situated in ‘difficult sites’ were generally matched between the two groups, ensuring data comparability. In terms of intraoperative blood loss, the number of cutting staplers used, post-operative hospital stays, and the incidence of postoperative complications, the LSD group outperformed the non-LSD group. The difference in operating time between the two groups was not statistically significant.
In terms of safety and prognosis, neither group experienced a tumor rupture during the procedure. One patient in the non-LSD group had incomplete intestinal obstruction one month after proximal gastrectomy, but improved with symptomatic therapy after admission. After surgery, neither group of patients with stomach stromal tumors experienced a recurrence that resulted in rehospitalization or death.
The separate investigation of 67 patients with stomach stromal tumors at ‘difficult sites’ revealed that the LSD group was superior to the non-LSD group in terms of intraoperative blood loss, number of cutting staplers used, postoperative hospital stays, and incidence of postoperative problems. When compared to non-difficult places, the LSD group showed more significant benefits ().
Table 3. Comparative analysis of LSD and non-LSD in the treatment of gastric stromal tumors located in ‘difficult sites’.
Discussion
For patients with gastrointestinal stromal tumors, surgical resection can achieve radical resection if the tumor cannot be completely removed with endoscopy. Due to the characteristics of gastrointestinal stromal tumors, such as expansive growth and non-lymph node metastasis, the Chinese Society of Clinical Oncology (CSCO) guidelines [Citation7] and another expert consensus [Citation8] propose that lymph node dissection may not be performed during surgery for patients with gastrointestinal stromal tumors; only complete resection of the tumor is required to ensure that the margin is negative [Citation9]. Even if the tumor margin is positive, tumor R1 resection will not alter the prognosis of gastrointestinal stromal tumors, according to recent clinical studies [Citation10,Citation11]. In addition, as gastrointestinal stromal tumors originate from interstitial cells of Cajal in the smooth muscle layer of the digestive tract rather than the mucosal layer [Citation12], there are clear boundaries and pseudocapsules around the tumor [Citation13] for expansion growth. The unique growth pattern and oncological characteristics of gastrointestinal stromal tumors provide a theoretical basis for laparoscopic seromuscular dissection (LSD).
The results of this research indicate that LSD can minimize intraoperative blood loss and the number of cutting staplers used, as well as shorten post-operative hospital stays and reduce patients’ hospitalization costs, without increasing operation time. On the one hand, when using LSD, the surgeon applies an ultrasonic scalpel to accurately remove the tumor and normal mucosa. Its precise stripping operation minimizes the need for cutting staplers significantly. In addition, stripping reduces the severity of gastric wall injury, the damage to adjacent tissue, and the amount of blood loss during the procedure. On the other hand, due to the use of precise stripping and less cutting stapler, the volume of the normal gastric tissue cut is comparatively reduced; however, the volume of the remaining stomach is increased [Citation13]. In addition, the mucosal layer is not directly harmed during the operation of the cutting stapler, and the gastrointestinal function is only minimally affected. Because there is no indwelling gastric tube after surgery, early postoperative feeding can accelerate the recovery of gastrointestinal function, which is conducive to the recovery of patients after surgery, reducing the incidence of complications, thereby reducing the hospitalization time of patients and reducing their hospitalization costs. When compared to patients with ‘non-difficult sites’, patients with ‘difficult sites’ in the LSD group showed greater benefits. Gastric mesenchymal tumors in “difficult sites” are those located in the esophagogastric junction, the gastric lesser curvature near the cardia, the pylorus, the posterior wall of the stomach, and the gastric sinus. Compared with the greater curvature and the body of the stomach, these sites are difficult to resect either endoscopically or surgically, which will prolong the operation time and increase the incidence of complications [Citation14]. When wedge resection is not performed correctly, there is a danger of tumor rupture, which can lead to tumor dissemination and the loss of the importance of partial gastrectomy due to the unique placements and limited working spaces involved [Citation15], LSD uses precise stripping method, which can effectively avoid this situation. Not only that, LSD may produce the impact of ‘function preservation’ based on ensuring safety, accelerating the recovery of patients following the operation, and enhancing the quality of life following surgery. Within the indications, the author believes that LSD should be the treatment of choice for stomach stromal tumors in ‘difficult parts’.
Regarding surgical trauma, one patient with a gastric stromal tumor in the non-LSD group was re-admitted one month after proximal gastrectomy due to the complication of intestinal obstruction and recovered after symptomatic therapy. In the LSD group, there were no complications such as bleeding, blockage, or anastomotic leaking. According to a South Korean study [Citation16], gastric wedge resection for stomach submucosal tumors near the gastroesophageal junction is associated with a significant incidence of postoperative gastroesophageal reflux disease. The results of this study demonstrate once again the function preservation benefits of LSD surgery, i.e. LSD is a safe and effective method for treating stomach stromal tumors. It can accomplish complete excision of the tumor, preserve the pyloric and cardiac function of the stomach [Citation17], prevent postoperative reflux, gastroparesis and other complications, and improve patients’ quality of life.
In terms of surgical safety and oncological prognosis, neither group had intraoperative tumor rupture leading to tumor dissemination. Over the time of follow-up, neither group had hospitalization or death owing to recurrence. On the one hand, gastrointestinal stromal tumor is nearly devoid of lymph node metastases [Citation18]; along with the surgeon’s skillful operation technique, the tumor can be totally excised to achieve a radical cure. On the other hand, for patients with postoperative pathology suggesting intermediate risk or above, postoperative use of imatinib for targeted therapy is routine [Citation19]. This comprehensive treatment mode of surgery combined with drugs significantly reduces the relapse rate of patients, improves the survival cycle of patients, and benefits patients. However, for surgeons, the surgical indications and contraindications of LSD must be strictly controlled. For example, ulcerative stromal tumors are contraindications of LSD surgery because LSD not only fails to achieve the purpose of radical cure, but also leads to tumor dissemination and disease progression. In addition, the surgeon must also master the technology of LSD, follow the principle of ‘non-contact’ of the tumor [Citation20], and avoid tumor rupture, which might result in tumor dissemination or severe damage to the surrounding tissue, with irreversible consequences [Citation21].
In conclusion, the safety and prognosis of LSD technology in treating gastric stromal tumors is comparable to radical gastrectomy and LWR technology. However, there are areas for improvement in this study: this is a single-center study with limited patient data, and the results may be subject to bias. The center will maintain the number of instances of gastric stromal tumors, incorporating patient data from multi-centers, increase the sample size, and incorporate additional comparative factors. Further clinical observation will be conducted.
Conclusion
Considering its biological behavior, partial gastrectomy is the most common surgical treatment for gastric stromal tumor. Laparoscopic seromuscular dissection (LSD) is oncologically and technically possible and is safe for experienced laparoscopic surgeons.
Declaration of interest
No potential conflict of interest was reported by the author(s).
References
- Blay JY, Kang YK, Nishida T, et al. Gastrointestinal stromal tumors. Nat Rev Dis Primers. 2021;7(1):22. doi: 10.1038/s41572-021-00254-5.
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(1):20–33. doi: 10.1016/j.annonc.2021.09.005.
- Stevanović D, Stojanović D, Jasarović D, et al. Laparoscopic gastric wedge resection as the method of choice in the treatment of gastrointestinal stromal tumors–a case report. Srp Arh Celok Lek. 2016;144:211–214.
- Zhang M, Cai X, Liang C, et al. Single-Incision laparoscopic intragastric surgery for gastric submucosal tumors located near the esophagogastric junction. J Laparoendosc Adv Surg Tech A. 2022;32(4):360–365. doi: 10.1089/lap.2021.0186.
- Mueller CL, Braun J, Leimanis ML, et al. Application of an individualized operative strategy for wedge resection of gastric gastrointestinal stromal tumors: Effectiveness for tumors in difficult locations. Surgery. 2016;160(4):1038–1048. doi: 10.1016/j.surg.2016.06.004.
- Tanabe K, Urabe Y, Tokumoto N, et al. A new method for intraluminal gastrointestinal stromal tumor resection using laparoscopic seromuscular dissection technique. Dig Surg. 2010;27(6):461–465. doi: 10.1159/000320458.
- CSCO Guidelines for the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. People’s Health Publishing House. 2021.
- Hui C, Zhidong G, Yulong H, et al. Chinese expert consensus on standardized surgical treatment of gastrointestinal stromal tumors (2018 edition). Chin J Pract Surg. 2018;(9):965–973.
- Wada H, Murakawa K, Ono K, et al. Laparoscopic ultrasound guided wedge resection of the stomach: a novel procedure for gastric submucosal tumor. Updates Surg. 2022;74(1):367–372. doi: 10.1007/s13304-021-01024-4.
- Hølmebakk T, Bjerkehagen B, Hompland I, et al. Relationship between R1 resection, tumour rupture and recurrence in resected gastrointestinal stromal tumour. Br J Surg. 2019;106(4):419–426. doi: 10.1002/bjs.11027.
- Liu L, Xu X, Wang Q, et al. An evaluation of the use of double-curved endoscopes for gastric gastrointestinal stromal tumors. Minim Invasive Ther Allied Technol. 2023;32(3):112–118. doi: 10.1080/13645706.2023.2186182.
- Nishida T, Yoshinaga S, Takahashi T, et al. Recent progress and challenges in the diagnosis and treatment of gastrointestinal stromal tumors. Cancers. 2021;13(13):3158. doi: 10.3390/cancers13133158.
- Kanehira E, Kanehira AK, Tanida T, et al. CLEAN-NET: a modified laparoendoscopic wedge resection of the stomach to minimize the sacrifice of innocent gastric wall. Surg Endosc. 2020;34(1):290–297. doi: 10.1007/s00464-019-06765-3.
- Wang W, Liu CX, Niu Q, et al. OTSC assisted EFTR for the treatment of GIST: 40 cases analysis. Minim Invasive Ther Allied Technol. 2022;31(2):238–245. doi: 10.1080/13645706.2020.1781190.
- Kawamura H, Shibasaki S, Yoshida T, et al. Strategy of laparoscopic partial resection for gastric gastrointestinal stromal tumors according to the growth pattern. Surg Laparosc Endosc Percutan Tech. 2015;25(6):e175–e179. doi: 10.1097/SLE.0000000000000212.
- Ko SY, Lee JS, Kim JJ, et al. Higher incidence of gastroesophageal reflux disease after gastric wedge resections of gastric submucosal tumors located close to the gastroesophageal junction. Ann Surg Treat Res. 2014;86(6):289–294. doi: 10.4174/astr.2014.86.6.289.
- Lee JS, Kim JJ, Park SM. Laparoscopic gastric wedge resection and prophylactic antireflux surgery for a submucosal tumor of gastroesophageal junction. J Gastric Cancer. 2011;11(2):131–134. doi: 10.5230/jgc.2011.11.2.131.
- Khoshnood A. Gastrointestinal stromal tumor - A review of clinical studies. J Oncol Pharm Pract. 2019;25(6):1473–1485. doi: 10.1177/1078155219846955.
- Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol. 2019;10(1):144–154. doi: 10.21037/jgo.2018.08.20.
- Kiyozaki H, Saito M, Chiba H, et al. Laparoscopic wedge resection of the stomach for gastrointestinal stromal tumor (GIST): non-touch lesion lifting method. Gastric Cancer. 2014;17(2):337–340. doi: 10.1007/s10120-013-0272-8.
- Zhou P, Zhong Y, Li Q. Chinese consensus on endoscopic diagnosis and management of gastrointestinal submucosal tumor (version 2018)[J]. Chin J Gastrointest Surg. 2018;21:841–852.