Abstract
Background
The indication of laparoscopic liver resection (LLR) for treating large hepatocellular carcinoma (HCC) is controversial. In this study, we compared the short-term and long-term outcomes of LLR and open liver resection (OLR) for large HCC.
Material and methods
We searched eligible articles about LLR versus OLR for large HCC in PubMed, Cochrane Library, and EMBASE and performed a meta-analysis.
Results
Eight publications involving 1,338 patients were included. Among them, 495 underwent LLR and 843 underwent OLR. The operation time was longer in the LLR group (MD: 22.23, 95% CI: 4.14–40.33, p = 0.02). but the postoperative hospital stay time was significantly shorter (MD : −4.88, CI: −5.55 to −4.23, p < 0.00001), and the incidence of total postoperative complications and major complications were significantly fewer (OR: 0.49, 95% CI:0.37–0.66, p < 0.00001; OR: 0.54, 95% CI:0.36 − 0.82, p = 0.003, respectively). Patients in the laparoscopic group had no significant difference in intraoperative blood loss, intraoperative transfusion rate, resection margin size, R0 resection rate, three-year overall survival (OS) and three-year disease-free survival (DFS).
Conclusion
LLR for large HCC is safe and feasible. This surgical strategy will not affect the long-term outcomes of patients.
Introduction
Primary hepatocellular carcinoma (HCC) is a common malignancy with a poor prognosis. In 2020, there were 905,677 newly diagnosed cases and 830,180 deaths of HCC, ranking seventh and second, respectively, among all cancers worldwide [Citation1]. To date, operative resection is the most effective method for HCC. For resectable HCC, hepatectomy is the preferred treatment [Citation2], with 5-OS ranging from 33.6% to 76.4% and 5-DFS ranging from 24% to 63% [Citation3–5]. Nowadays, more and more patients with liver cancer are undergoing laparoscopic liver resection (LLR). Studies have found that LLR has comparable oncologic outcomes to open liver resection (OLR) and superior short-term results compared to OLR [Citation6,Citation7]. However, these studies treated all resectable lesions as a single homogeneous group, which may lead to potential bias and inaccurate conclusions. Therefore, further studies are warranted to explore the optimal patient selection for LLR and to compare its long-term outcomes with OLR.
The current clinical practice has identified tumor size and location as crucial factors in determining the indication for LLR in patients with HCC [Citation8]. Undoubtedly, it is well-established that the difficulty and risk of the surgical operation increase with an increase in tumor diameter [Citation9]. Additionally, a tumor diameter > 5 cm has been identified as an independent risk factor for the recurrence of liver cancer [Citation10]. There is currently no consensus on whether LLR for large HCC can achieve comparable safety and efficacy as OLR. Furthermore, no meta-analysis has been conducted to assess this issue. Therefore, this systematic review and meta-analysis aims to accumulate current knowledge and evaluate the short- and long-term outcomes of LLR vs. OLR for large HCC.
Material and methods
This study was conducted following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation11] and the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) checklist (PROSPERO Register Number: CRD42023421780).
Inclusion and exclusion criteria
Inclusion criteria
Study type: randomized controlled trials or comparative cohort studies
Study participants: individuals with a pathologic diagnosis of primary HCC with a minimum diameter > 5 cm
Intervention: LLR vs. OLR
Outcome indicators:
surgical outcome indicators: operation time, intraoperative blood loss, intraoperative transfusion rate, resection margin size, R0 resection rate, total complication rate, major complication rate (Clavien-Dindo classification of grade III or above) and postoperative hospital stay time
long-term outcome indicators: 3-year OS and 3-year DFS
Exclusion criteria
Irrelevant literature
Reviews, meta-analyses or repeated published studies
Case reports
Data that is incorrect or statistical methods that are unreasonable
Literature search strategy
Three distinct databases were searched for potentially eligible studies between January 1998 and April 2023: PubMed, Embase, and Cochrane library. The search formula included: ((laparoscopic [Title/Abstract]) AND (open [Title/Abstract])) AND ((((big [Title/Abstract]) OR (huge [Title/Abstract])) OR (large [Title/Abstract])) AND (((liver cancer [Title/Abstract]) OR (hepatocellular carcinoma [Title/Abstract])) OR (("Carcinoma, Hepatocellular"[Mesh]) OR "Liver Neoplasms"[Mesh]))).
Literature screening and data extraction
Two independent investigators conducted literature screening, data extraction, and cross-validation. Initially, title and abstract screening were performed, followed by a full-text review to determine the final selection of literature. Controversial literature was discussed to determine inclusion or non-inclusion. The extracted data included
basic information about the study: name of the first author, type of study, year of publication, and country or region
baseline characteristics of the study population and interventions
relevant prognostic indicators.
In addition, for the propensity score matching (PSM) correction studies, we collected patient information after correction.
Literature quality assessment
Two reviewers independently assessed the risk of bias in the included literature and performed cross-validation by The Newcastle-Ottawa Scale (NOS) [Citation12]. Literature with a score of ≥ 7 was defined as high-quality.
Statistical analysis
Rev Man 5.4 software was used for meta-analysis. Odds ratio (OR) and 95% confidence interval (95%CI) were used to evaluate categorical variables, 3-OS rate and 3-DFS rate. Mean differences (MD) and 95%CI were used to evaluate continuous variables. Data reported as medians and range or interquartile range were tested for skewness and, if not skewed, were converted to means and standard deviations using the models of Luo et al. [Citation13] and Wan et al. [Citation14], respectively. I2 quantitation was used to determine inter-study heterogeneity. The fixed-effects model was used for meta-analysis if there was no inter-study heterogeneity (I2 < 50%, p > 0.10). If significant inter-study heterogeneity was present (I2 > 50%, p < 0.10), the source of heterogeneity was further analyzed, and subgroup analysis or sensitivity analysis was used for processing. If heterogeneity could not be removed, the random effects model was used for meta-analysis. A difference of p < 0.05 was considered statistically significant.
Results
Literature screening process
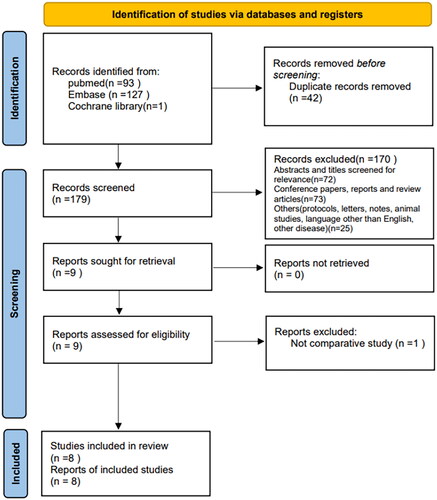
shows the literature screening strategy and selection process. A total of 221 manuscripts were retrieved from the database, among which 42 were duplicates. Through screening the titles and abstracts, 72 documents were excluded because they were inconsistent with the study content, 73 were conference reports, case reports, and reviews, and 25 were experimental protocols, dealt with other diseases, and non-English literature. The remaining nine documents were obtained in full text and read; one non-comparative study was excluded. Finally, eight publications (NOS score ≥7) were included [Citation8, Citation9, Citation15–20] ().
Table 1. The Newcastle-Ottawa scale (NOS) score of the literature.
Basic characteristics of the included literature
Eight articles were included for analysis, comprising of one prospective study [Citation8] and seven [Citation9, Citation15–20] retrospective studies. Of these, six articles [Citation8, Citation15–17, Citation19, Citation20] were from various hospitals in mainland China, one [Citation9] from Taiwan, China, and one [Citation18] from Korea. Four papers [Citation17–20] utilized propensity-matched scoring methods (PSM) to control confounding factors. The studies included a total of 1,338 cases, with 495 patients (37%) undergoing LLR, and 843 patients (63%) undergoing OLR.
All eight papers [Citation8, Citation9, Citation15–20] reported the gender distribution of patients. The proportion of male patients in the laparoscopic and open groups was 82% and 84%, respectively, with no statistically significant difference between the two groups (OR = 0.84, 95% CI: 0.62 to 1.13, p = 0.25) and no significant heterogeneity between studies (I2 = 8%, p = 0.37). Seven publications [Citation8, Citation9, Citation15–18, Citation20] reported patient age characteristics, of which six publications [Citation8, Citation9, Citation15, Citation17, Citation18, Citation20] could be included in the analysis, with no statistically significant difference between the two groups (MD = 0.08, 95% CI: −1.44 to 1.59, p = 0.92). Heterogeneity testing showed no significant heterogeneity (I2 = 0%, p = 0.94). Seven publications [Citation8, Citation9, Citation15–19] reported tumor diameter, of which four publications [Citation8, Citation9, Citation15, Citation17] were included in the analysis, with no statistically significant difference between the two groups, but significant between-study heterogeneity was found (MD = −0.80, 95% CI: −1.62 to 0.02, p = 0.05; I2 = 90%, p < 0.00001). Eight publications [Citation8, Citation9, Citation15–20] reported liver cirrhosis background in the laparoscopic and open groups, with no statistically significant difference between the two groups (70% vs 69%, OR = 0.99, 95% CI: 0.77 to 1.28, p = 0.95; I2 = 0%, p = 0.92). Seven publications [Citation8, Citation9, Citation15–19] reported hepatitis B virus (HBV) carriage in the laparoscopic and open groups, with no statistically significant difference between the two (78% vs 77%, OR = 1.00, 95% CI: 0.75 to 1.33, p = 0.99; I2 = 0%, p = 0.63). Six publications [Citation8, Citation9, Citation15, Citation17–19] reported Child-Pugh grading of patients, and the percentage of grade A cases has no statistically significant difference between laparoscopic group and open group (85% vs 84%, OR = 0.97, 95% CI: 0.68 to 1.39, p = 0.87; I2 = 0%, p = 0.62) ().
Table 2. The baseline characteristics quality assessment of the included studies.
Meta-analysis results
Surgical outcome indicators
Six publications [Citation8, Citation9, Citation15–18] reported the operative time for both groups of patients. A total of three papers [Citation9, Citation15, Citation18] were included in the meta-analysis, and the heterogeneity test showed no significant heterogeneity (I2 = 0%, p = 0.39). Meta-analysis showed that the operative time was significantly longer in the laparoscopic group than in the open group using a fixed effects model (MD = 22.23, 95% CI: 4.14 to 40.33, p = 0.02) ().
Figure 2. Forest plots of the effect of LLR vs. OLR on (A) operation time, (B) intraoperative blood loss, (C) intraoperative transfusion rate, (D) resection margin size, (E) R0 resection rate. Mean difference (MD) and odds ratios (OR) shown with 95% confidence intervals.
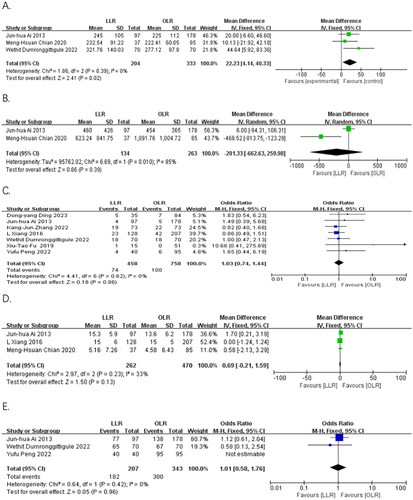
Intraoperative blood loss was reported in seven publications [Citation8, Citation9, Citation15–18, Citation20]. Only two publications [Citation9, Citation15] were included in the analysis and significant heterogeneity was detected by the heterogeneity test (I2 =85%, p = 0.01). Meta-analysis using a random effects model revealed no statistically significant difference between the two groups (MD = −201.33, 95%CI: −662.63 to 259.98, p = 0.39) ().
Seven papers [Citation8, Citation15–20] reported the intraoperative transfusion rate. The heterogeneity test indicated no significant heterogeneity (I2 = 0%, p = 0.62). Meta-analysis using a fixed-effects model revealed that the transfusion rate has no statistically significant difference between the laparoscopic group and the open group (16% vs 13%, OR = 1.03, 95% CI: 0.74 to 1.44, p = 0.86) ().
Six papers [Citation8, Citation9, Citation15–18] reported the resection margin size. Three publications [Citation8, Citation9, Citation15] were included in the analysis, and no significant heterogeneity was detected (I2 = 33%, p = 0.23). The meta-analysis using a fixed effects model showed no significant difference between the two groups (MD = 0.69, 95% CI: −0.21 to 1.59, p = 0.13) ().
Three papers [Citation15, Citation17, Citation18] reported the R0 resection rate. The heterogeneity test indicated no significant heterogeneity (I2 = 0%, p = 0.42). A fixed-effects model was used for meta-analysis, which showed no statistically significant difference between the laparoscopic group and the open group (88% vs 87%, OR = 1.01, 95% CI: 0.58 to 1.76, p = 0.96) ().
Six publications [Citation8, Citation15, Citation17–20] reported the incidence of total postoperative complications. The heterogeneity test indicated no significant heterogeneity (I2 = 18%, p = 0.29). A meta-analysis using a fixed-effects model demonstrated that the incidence of complications was 18% in the laparoscopic group, which was significantly lower than that in the open group (30%). There was a statistically significant difference between the two groups (OR = 0.49, 95% CI:0.37 to 0.66, p < 0.00001) ().
Figure 3. Forest plots of the effect of LLR vs. OLR on (A) total postoperative complication rate and (B) major complication rate (≥ grade III). Odds ratios (OR) shown with 95% confidence intervals.
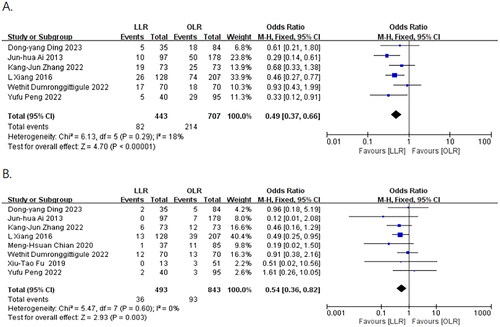
All publications [Citation8, Citation9, Citation15–20] reported the incidence of major postoperative complications. Heterogeneity tests showed no significant heterogeneity (I2 = 0%, p = 0.60). A meta-analysis using a fixed-effects model showed a complication rate of 7.3% in the laparoscopic group, which was significantly lower than the complication rate in the open group (11%). There was a statistically significant difference between the two groups (OR = 0.54, 95% CI:0.36 to 0.82, p = 0.003) ().
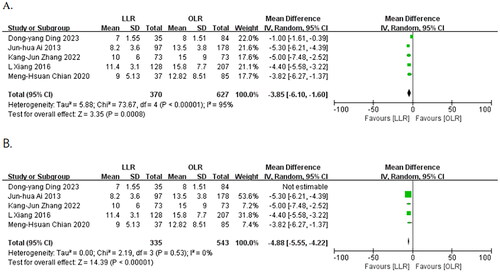
Eight publications [Citation8, Citation9, Citation15–20] reported postoperative hospital stay time. Five publications [Citation8, Citation9, Citation15, Citation19, Citation20] were included in the analysis, and the heterogeneity test showed significant heterogeneity (I2 = 95%, p < 0.00001). The postoperative hospital stay time was significantly shorter in the laparoscopic group compared to the open group (MD = −3.85, 95% CI: −6.10 to −1.60, p = 0.0008). After excluding the study by Dong-yang Ding et al. there was no significant heterogeneity between the two groups (I2 = 0%, p = 0.53). The results still show that postoperative hospital stay time was significantly shorter in the laparoscopic group (MD = −4.88, 95% CI: −5.55 to −4.22, p < 0.00001) using a fixed effects model for meta-analysis ().
2.3.2. Oncological outcome
Six papers [Citation8, Citation15, Citation16, Citation18–20] reported 3-year OS after surgery, and the heterogeneity test indicated no significant heterogeneity (I2 = 0%, p = 0.79). A meta-analysis using a fixed-effects model showed a slightly lower 3-year OS in the laparoscopic group than in the open group. However, the difference between the two groups was not statistically significant (77.5% vs. 80.1%, OR = 0.84, 95% CI: 0.62 to 1.15, p = 0.28) ().
Figure 5. Forest plots of the effect of LLR vs. OLR on A) 3-year OS rate, B) 3-year DFS rate. Odds ratios (OR) shown with 95% confidence intervals.
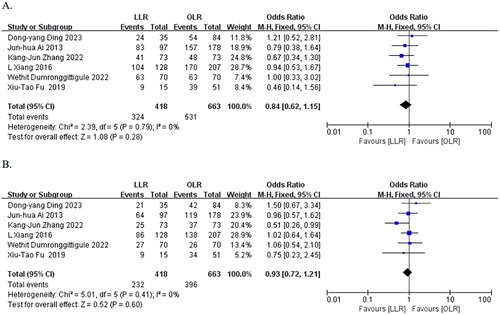
Six papers [Citation8, Citation15, Citation16, Citation18–20] reported 3-year DFS, and heterogeneity test suggested no significant heterogeneity (I2 = 0%, p = 0.41). The meta-analysis using a fixed-effect model showed a slightly lower 3-year DFS in the laparoscopic group compared to the open group, but the difference between the two groups was not statistically significant (55.5% vs. 59.7%, OR = 0.93, 95% CI:0.72 to 1.21, p = 0.60) ().
Discussion
This meta-analysis indicates that LLR for large HCC prolongs operative time compared to the OLR, but results in lower rates of overall postoperative and major complications, and shorter hospital stay time. There was no difference in intraoperative blood loss, intraoperative transfusion rate, resection margin size, R0 resection rate, 3-year OS and 3-year DFS between the two groups. Due to the limited number of included studies and statistical heterogeneity, particularly in terms of intraoperative blood loss and postoperative hospital stay time, the results should be interpreted with caution.
As the number of LLR cases increases, attention has been given to the indications for the procedure. In 2008, an expert consensus [Citation21] in Louisville, USA, concluded that the best indication for LLR is a single lesion < 5 cm in diameter. Several studies have confirmed this conclusion [Citation22, Citation23]. Although studies have reported that surgical resection of HCC > 5 cm in diameter can be equally beneficial to patients [Citation24], which is consistent with the oncologic prognosis of small HCC, there is no consensus on the suitability of laparoscopic techniques for large HCC. Some scholars believe that tumor diameter alone is no longer a contraindication to LLR [Citation25], and have reported the experience of LLR for large HCCs [Citation26–29]. However, there is a lack of high-quality randomized controlled studies and meta-analyses for this issue.
Peng Zhu et al. [Citation30] used propensity-matched scores to compare the operative time of minimally invasive hepatectomy (including laparoscopic and robotic-assisted surgery) with that of open hepatectomy. They found that the surgery duration was significantly longer in the minimally invasive group than in the open group. Those findings are consistent with our results, which may be attributed to the following reasons. First, lens contamination is a common problem in laparoscopic surgery and extra time has to be spent cleaning the lens [Citation17]. Second, the operator would operate more carefully to avoid uncontrollable bleeding. All of this will prolong the operation time.
We analyzed two studies and found that intraoperative blood loss between the two groups had no significant differences, although there exists significant heterogeneity. This result remains consistent with the previous study [Citation22]. The magnification effect of laparoscopy helps identify small vessels and manage them in advance to avoid uncontrollable bleeding. Intraoperative ultrasound also enables the operator to forecast the vascular course and manage them in advance. In addition, the low central venous pressure technique and the hemostatic effect of pneumoperitoneum on the compression of small veins are also beneficial in reducing intraoperative bleeding [Citation31]. Studies have demonstrated that massive blood loss resulting in transfusion is positively associated with poorer short-term outcomes and higher postoperative recurrence rates in patients with liver tumors [Citation32, Citation33]; therefore, we compared the transfusion rates between the two groups and found no significant difference between them, which also echoes the fact that laparoscopic hepatectomy does not increase intraoperative blood loss.
Sufficient margin distance and R0 resection are strongly associated with postoperative recurrence and long-term survival of HCC [Citation34, Citation35]. Our study demonstrated that the resection margin size and R0 resection rate in the laparoscopic group did not differ from those in the open group, which is consistent with the results of a previous meta-analysis [Citation36]. Several studies have demonstrated that LLR reduces the incidence of postoperative complication [Citation37, Citation38], which consistent with our results.
In this study, we found that the laparoscopic group had a significantly shorter postoperative hospital stay time than the open group. However, there was significant heterogeneity among the five studies. After excluding the study by Ding, D. Y. et al. the heterogeneity disappeared, and the results remained consistent. We speculate that this study was a heterogeneous source and it may be due to general advances in liver surgery techniques, equipment improvements, and the application of the concept of enhanced recovery after surgery.
The Balliol IDEAL classification view recommended to use long-term oncologic outcomes to assess the value of minimally invasive techniques in the treatment of liver cancer and to evaluate whether they can become standard options [Citation39]. Due to variability follow-up periods in the included publications, we only analyzed 3-year OS and 3-year DFS and found that they were not statistically different between the two groups. This conclusion is consistent with the results of another meta-analysis [Citation40].
It is worth noting that as the tumor volume increases, the chance of major vascular invasion inevitably rises. In order to achieve R0 resection, the choice of combined vascular resection and reconstruction becomes necessary. However, hepatic resection with vascular resection and reconstruction is challenging [Citation41], especially during laparoscopy. Macroscopic vascular invasion has been considered an absolute contraindication for liver resection in many Western centers [Citation42]. Furthermore, vascular invasion is a poor prognostic factor for liver cancer [Citation43]. The publications included in this study screened cases without major vascular invasion as the research subjects. Therefore, for patients with large HCC complicated by major vascular invasion, whether laparoscopy still demonstrates superior clinical outcomes compared to open surgery requires further evaluation through randomized controlled trials (RCTs).
This study also has some limitations. The included studies were mainly retrospective cohort studies, and there were variations in the inclusion criteria among different study centers. Although some studies adopted PSM scores to adjust for group differences, this method cannot completely eliminate the impact of confounding factors. Also, some variable information can be extracted from most literatures, but cannot be included in the meta-analysis, because they do not meet the transformation requirements. This lost information would affect the judgment of the final results. Finally, subgroup analysis could explain significant heterogeneity, but this was not possible due to insufficiently detailed data in the included studies. Despite these limitations, this study provides a preliminary result for selecting the optimal surgical strategy for large HCC and lays the foundation for conducting high-quality randomized controlled studies.
Conclusions
This study shows that LLR yields superior surgical outcomes in terms of postoperative complications and postoperative hospital stay time compared to OLR for patients with large HCCs and there was no significant difference in 3-year OS and 3-year DFS. Therefore, LLR should be a selection of the cases of large HCC excluding the large majority of cases requiring complex vascular thrombectomy or vascular resection.
Disclosure /statement
No potential conflicts of interest are reported by the authors.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. 15 doi: 10.1016/S0140-6736(22)01200-4.
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873. doi: 10.1093/annonc/mdy510.
- Coelho FF, Kruger JA, Fonseca GM, et al. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg. 2016;8(1):5–26. doi: 10.4240/wjgs.v8.i1.5.
- Han HS, Shehta A, Ahn S, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63(3):643–650. doi: 10.1016/j.jhep.2015.04.005.
- Kabir T, Tan ZZ, Syn NL, et al. Laparoscopic versus open resection of hepatocellular carcinoma in patients with cirrhosis: meta-analysis. Br J Surg. 2021;109(1):21–29. doi: 10.1093/bjs/znab376.
- Ivanics T, Claasen MP, Patel MS, et al. Long-term outcomes of laparoscopic liver resection for hepatocellular carcinoma: a propensity score matched analysis of a high-volume North American center. Surgery. 2022;171(4):982–991. doi: 10.1016/j.surg.2021.10.017.
- Xiang L, Li J, Chen J, et al. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(13):1895–1901. doi: 10.1002/bjs.10294.
- Chiang MH, Tsai KY, Chen HA, et al. Comparison of surgical outcomes for laparoscopic liver resection of large hepatocellular carcinomas: a retrospective observation from single-center experience. Asian J Surg. 2021;44(11):1376–1382. doi: 10.1016/j.asjsur.2021.03.027.
- Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z.
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135.
- Ai JH, Li JW, Chen J, et al. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5-10 cm. PLoS One. 2013;8(8):e72328. doi: 10.1371/journal.pone.0072328.
- Fu XT, Tang Z, Shi YH, et al. Laparoscopic versus open left lateral segmentectomy for large hepatocellular carcinoma: a propensity score-matched analysis. Surg Laparosc Endosc Percutan Tech. 2019;29(6):513–519. doi: 10.1097/SLE.0000000000000723.
- Peng Y, Li B, Xu H, et al. Is the anterior approach suitable for laparoscopic right hemihepatectomy in patients with large HCC (5-10 cm)? a propensity score analysis. Surg Endosc. 2022;36(8):6024–6034. doi: 10.1007/s00464-022-09119-8.
- Dumronggittigule W, Han HS, Komoltri C, et al. Laparoscopic versus open hepatectomy for large hepatocellular carcinoma: a single center propensity-score-matching comparative analysis of perioperative outcomes and long-term survival. Surg Endosc. 2023;37(4):2997–3009. doi: 10.1007/s00464-022-09812-8.
- Zhang KJ, Liang L, Diao YK, et al. Short- and long-term outcomes of laparoscopic versus open liver resection for large hepatocellular carcinoma: a propensity score study. Surg Today. 2023;53(3):322–331. doi: 10.1007/s00595-022-02576-7.
- Ding DY, Liu L, Lin KY, et al. Perioperative and long-term survival outcomes of laparoscopic versus open hepatectomy for BCLC stage a large hepatocellular carcinoma patients in difficult segments: a two-centre, propensity score matching analysis. Front Oncol. 2023;13:1095357. doi: 10.3389/fonc.2023.1095357.
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg. 2009;250(5):825–830. doi: 10.1097/sla.0b013e3181b3b2d8.
- Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(7):871–880. doi: 10.1002/bjs.10137.
- El-Gendi A, El-Shafei M, El-Gendi S, et al. Laparoscopic versus open hepatic resection for solitary hepatocellular carcinoma less than 5 cm in cirrhotic patients: a randomized controlled study. J Laparoendosc Adv Surg Tech A. 2018;28(3):302–310. doi: 10.1089/lap.2017.0518.
- Yip VS, Gomez D, Tan CY, et al. Tumour size and differentiation predict survival after liver resection for hepatocellular carcinoma arising from non-cirrhotic and non-fibrotic liver: a case-controlled study. Int J Surg. 2013;11(10):1078–1082. doi: 10.1016/j.ijsu.2013.10.001.
- Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol. 2014;20(17):4892–4899. doi: 10.3748/wjg.v20.i17.4892.
- Kwon Y, Han HS, Yoon YS, et al. Are large hepatocellular carcinomas still a contraindication for laparoscopic liver resection? J Laparoendosc Adv Surg Tech A. 2015;25(2):98–102. doi: 10.1089/lap.2014.0226.
- Gil E, Kwon CHD, Kim JM, et al. Laparoscopic liver resection of hepatocellular carcinoma with a tumor size larger than 5 cm: review of 45 cases in a tertiary institution. J Laparoendosc Adv Surg Tech A. 2017;27(8):799–803. doi: 10.1089/lap.2016.0575.
- Levi Sandri GB, Spoletini G, Vennarecci G, et al. Laparoscopic liver resection for large HCC: short- and long-term outcomes in relation to tumor size. Surg Endosc. 2018;32(12):4772–4779. doi: 10.1007/s00464-018-6225-x.
- Shelat VG, Cipriani F, Basseres T, et al. Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol. 2015;22(4):1288–1293. doi: 10.1245/s10434-014-4107-6.
- Zhu P, Liao W, Zhang WG, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic, or open liver resection for patients with BCLC stage 0-a hepatocellular carcinoma. Ann Surg. 2023;277(1):e103–e111. doi: 10.1097/SLA.0000000000005380.
- Wei Chieh AK, Chan A, Rotellar F, et al. Laparoscopic major liver resections: current standards. Int J Surg. 2020;82s:169–177. doi: 10.1016/j.ijsu.2020.06.051.
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–407. doi: 10.1097/00000658-200210000-00001.
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860–870. doi: 10.1097/01.SLA.0000072371.95588.DA.
- Lee W, Han HS, Ahn S, et al. Correlation between resection margin and disease recurrence with a restricted cubic spline model in patients with resected hepatocellular carcinoma. Dig Surg. 2018;35(6):520–531. doi: 10.1159/000485805.
- Huan-Wei C, Shan L, Wan-Yee L, et al. Prognostic impact of hepatic resection for hepatocellular carcinoma: the role of the surgeon in achieving R0 resection–a retrospective cohort study. Int J Surg. 2015;13:297–301. doi: 10.1016/j.ijsu.2014.12.017.
- Yin Z, Fan X, Ye H, et al. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20(4):1203–1215. doi: 10.1245/s10434-012-2705-8.
- Witowski J, Rubinkiewicz M, Mizera M, et al. Meta-analysis of short- and long-term outcomes after pure laparoscopic versus open liver surgery in hepatocellular carcinoma patients. Surg Endosc. 2019;33(5):1491–1507. doi: 10.1007/s00464-018-6431-6.
- Shehta A, Han HS, Yoon YS, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc. 2016;30(2):638–648. doi: 10.1007/s00464-015-4253-3.
- Hirst A, Philippou Y, Blazeby J, et al. No surgical innovation without evaluation: Evolution and further development of the IDEAL framework and recommendations. Ann Surg. 2019;269(2):211–220. doi: 10.1097/SLA.0000000000002794.
- Mirnezami R, Mirnezami AH, Chandrakumaran K, et al. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB (Oxford). 2011;13(5):295–308. doi: 10.1111/j.1477-2574.2011.00295.x.
- Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. Biosci Trends. 2022;16(3):178–188. doi: 10.5582/bst.2022.01245.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018.
- Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;19(6):1370. doi: 10.3390/cells9061370.