Abstract
Objective
Robotic surgery is being increasingly used for colorectal cancer surgery. However, its utility versus laparoscopic surgery in older patients is unclear. We systematically examined evidence to assess the differences in short-term outcomes of robotic versus laparoscopic surgery for colorectal cancer in older patients.
Material and methods
Comparative studies published on PubMed, Web of Science, Embase, and CENTRAL databases were searched up to August 30th, 2023.
Results
Seven studies totaling 14,043 patients were included. Meta-analysis showed no difference in the operation time between the robotic and laparoscopic groups. Meta-analysis of ClavienDindo complications showed no difference between the robotic and laparoscopic groups for grades I and II or grades III and IV complications. Similarly, conversion to open surgery, reoperation rates and length of hospital stay were not significantly different between the two groups. Readmission rates and mortality rates were significantly lower with robotic surgery.
Conclusion
This first meta-analysis comparing outcomes of robotic and laparoscopic surgery in older colorectal cancer patients shows that both approaches result in no difference in operating time, complication rates, conversion to open surgery, reoperation rates, and LOS. Scarce data shows that mortality and readmission rates may be lower with robotic surgery.
Introduction
Colorectal cancer is amongst the most prevalent malignancies worldwide being the third most common cancer in men and the second most common cancer in women [Citation1]. A large proportion of colorectal cancer patients are of the older age group [Citation2]. Statistics suggest that more than half of these cases are seen in patients aged ≥65 with the median age at diagnosis being 67 years [Citation3]. As the life span of humans continues to increase thanks to improved healthcare, clinicians are likely to be confronted with a large proportion of older patients with colorectal cancer; these patients need specialized treatment protocols owing to several physiological changes associated with ageing. Older patients often have age-related decline in organ function and in host immunity. Depleted physiological reserves owing to poor nutrition lead to reduced resilience and adaptive capacity in these individuals [Citation4]. Older people are frequently frail and have multiple comorbidities, especially metabolic diseases which complicate cancer management [Citation5].
Surgery is usually the primary treatment for colorectal cancer, and minimally invasive approaches have gained popularity in recent times [Citation6]. Laparoscopic surgery has been adopted worldwide resulting in reduced perioperative morbidity without affecting survival outcomes of colorectal cancer [Citation7]. Escalating the technology further, robotic surgery has also found ground in colorectal surgery since its first description in 2002 [Citation8]. Robotic surgery offers improved dexterity and accessibility which can circumvent the limitations of conventional laparoscopic surgery [Citation9]. While there are several reviews which have compared the outcomes of robotic and laparoscopic colorectal surgery [Citation10–12], to date none have focused on older patients.
Since older patients constitute a special group owing to several differences in physiology and coexisting comorbidities, there is a need to generate quality evidence on the surgical treatment approach to be adopted for such patients. Hence, the purpose of this review is to conduct a literature search and collate data to examine whether short-term outcomes of robotic surgery are better when compared to laparoscopic surgery in older colorectal cancer patients.
Material and methods
Inclusion criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyes (PRISMA) guidelines [Citation13] during the design, execution, and presentation of this review and meta-analysis. Pre-registration was completed on International Prospective Register of Systematic Reviews (PROSPERO) before beginning the literature search (CRD42023454580). The review question was: “Does the use of robotic surgery result in improved outcomes as compared to laparoscopic surgery in elderly colorectal cancer patients?” As ≥65 years is one of the widely used definitions for being older, the same was adopted for this review [Citation14, Citation15].
For this question, the PICOS inclusion criteria were as follows:
Population: Older patients undergoing colorectal cancer surgery.
Intervention: Robotic colorectal surgery.
Comparison: Laparoscopic colorectal surgery.
Outcome: Operating time, complication rates, readmission, reoperation, mortality, length of hospital stay (LOS).
Study type: All types of comparative studies.
Excluded were studies not restricted to older patients, single-arm studies, and studies comparing older vs younger populations. Similarly, unpublished studies, case series, and editorials were not considered.
Search
We identified literature by scanning the databases of PubMed, Web of Science, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL). The search was conducted without date or language restriction up to August 30th, 2023. Two reviewers were involved in the search (XW, RM). The keywords consisted of: ’elderly’, ‘aged’, ‘octogenarian’, ‘robotic’, ‘laparoscopic’, ‘colorectal’, ‘rectal surgery’, and ‘colon surgery’. These keywords were combined with ‘AND’ and ‘OR’ to generate multiple search strings, which were entered into the database website.
All search results were downloaded in reference software and deduplicated to generate a list of unique studies. These were then screened by the reviewers (XW, RM) based on the given inclusion criteria. After initial title and abstract screening, selected articles underwent full-text review for final inclusion. The third reviewer (TH) was called for deliberation and reaching a consensus in case of an inconsistency in study selection. An additional manual screening of the reference lists for relevant original and review articles was conducted as a supplement.
Extracted data and study quality
Extracted data from studies included details of the primary author, year of publication, study location, the definition of “elderly”, sample size, age, male gender in the sample, American Association of Anesthesiologists (ASA) grades III and IV patients, diabetes, hypertension, synchronous tumor, mean tumor size, poorly differentiated tumor, T3-T4 stage, TNM stage, perineural invasion, and adjuvant chemotherapy. Two reviewers (XW, RM) were involved in data collection, and all data were cross-checked again with the primary article in case of discrepancies in data collection.
Two reviewers (HX, CZ) assessed the methodological quality of the observational studies using the Newcastle Ottawa Scale (NOS) [Citation16]. Points were awarded for representativeness of the study cohort, comparability of groups, and measurement of outcomes. Points were combined to give a total score ranging from 0 to 9.
Statistical analysis
Quantitative synthesis was carried out by “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014). We initially extracted all outcome data in tabular form. Outcomes with at least three studies were subjected to quantitative synthesis. All continuous variables were combined using mean difference (MD) with 95% confidence intervals (CI). Data not amenable to synthesis in the meta-analysis were converted to mean and standard deviation by methods of Wan et al. [Citation17]. Dichotomous variables were combined to obtain risk ratio (RR) and 95% CI. Complication data were extracted based on the Clavien-Dindo score and a separate analysis was conducted for grades I and II and III and IV complications. Forest plots were produced in the software by using the random-effect meta-analysis model. Heterogeneity between studies was examined by I2 statistic with a value of >50% meaning substantial heterogeneity. Owing to the limited number of publications, funnel plots were not generated.
Results
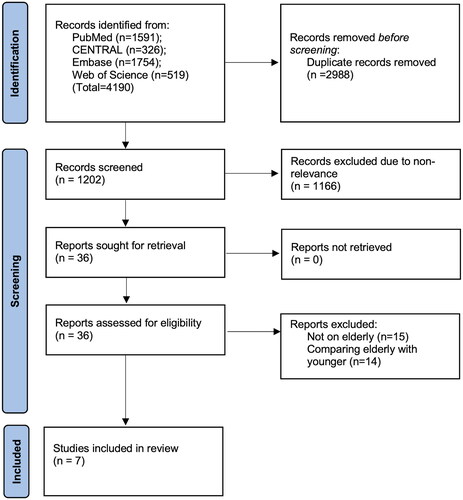
A combined search of the three databases retrieved 4,190 articles. Duplicates were removed and 1,202 articles underwent screening. Of the 36 articles selected for full-text review, seven studies were included [Citation18–24] ().
Extracted data from the studies are shown in . Data were published between 2018 and 2023 and originated from only a few countries, namely the USA, Australia, Italy, France, and China. Four studies included patients >80 years of age, two studies included those >70 years of age and one study defined being older as >65 years of age. Only two studies used propensity score matching for baseline differences between study groups. The combined sample size of the studies was 14,043. Of these, 3,627 patients underwent robotic surgery, while 10,416 patients underwent laparoscopic surgery. All studies were retrospective cohort studies either from a national registry or from a single hospital database. Most studies did not report specific details such as tumor size, histology, cancer stage, perineural invasion, and use of adjuvant therapy. The NOS score of the studies was 6 or 8.
Table 1. Details of included studies.
presents the data on outcomes reported by the studies. There was much variation in the outcomes reported by the studies, which limited the scope of the meta-analysis. On a pooled analysis of three studies, it was noted that there was no difference in the operation time between the robotic and laparoscopic groups (MD: 15.99, 95% CI: −39.89, 71.88, I2 = 94%) (). On a meta-analysis of Clavien-Dindo complications, there were no differences between the robotic and laparoscopic groups for grades I and II (RR: 1.06, 95% CI: 0.54, 2.09, I2 = 72%) or grades III and IV complications (RR: 0.73 95% CI: 0.42, 1.27, I2=0%) (). Similarly, conversion to open surgery (RR: 0.60 95% CI: 0.05, 7.89, I2 = 48%) and reoperation rates (RR: 0.74 95% CI: 0.23, 2.33, I2 = 0%) were not significantly different between the two groups (). The pooled analysis showed a significant reduction in the risk of readmission (RR: 0.58, 95% CI: 0.48, 0.69, I2 = 0%) and mortality (RR: 0.82 95% CI: 0.68, 0.99 I2=0%) with robotic surgery in older patients (). Readmission rates were reported as 30-day readmissions in all analyzed studies, except for de’Angelis et al. [Citation23] who reported 60-day readmission rates. On pooled analysis of LOS, it was noted that patients undergoing robotic surgery had reduced LOS, but this was not statistically significant (MD: −0.56, 95% CI: −1.27, 0.15, I2 = 72%) ().
Figure 2. Meta-analysis of operation time between robotic and laparoscopic colorectal surgery in older patients.

Figure 3. Meta-analysis of Clavien–Dindo complication rates, conversion to open surgery, reoperation, readmission, and mortality rates between robotic and laparoscopic colorectal surgery in older patients.
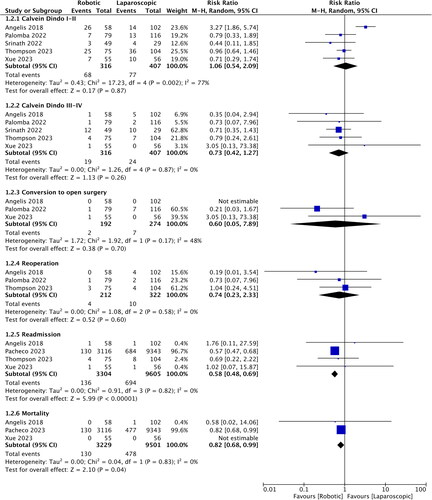
Figure 4. Meta-analysis of the length of hospital stay between robotic and laparoscopic colorectal surgery in older patients.
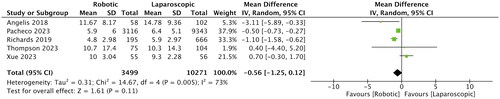
Table 2. Outcomes reported by included studies.
Discussion
The current study presents the first meta-analysis in the literature comparing outcomes of robotic and laparoscopic surgery in older patients. After a comprehensive literature search, seven studies were identified with a cumulative sample size of 14,043. Meta-analysis of short-term outcomes showed that older patients undergoing robotic or laparoscopic colorectal cancer surgery do not differ in terms of operating time, Clavien-Dindo complications (all grades), conversion to open surgery or reoperation rates. There was, however, a reduction in the risk of mortality and readmission in patients undergoing robotic surgery. Also, LOS was shorter in the robotic group, but the difference failed to reach statistical significance. The lack of substantial differences suggests that both techniques have equivalent safety in older patients, and it is up to the surgeon to choose the treatment modality based on expertise, availability of equipment, and cost of treatment.
Older patients with colorectal cancer are frequently undertreated owing to concerns regarding failure and the potential inability of older patients to handle the adverse effects of aggressive therapy [Citation25]. Indeed, older patients represent a special cohort which can have several comorbid conditions, higher incidence of frailty, poor nutrition, reduced ability to tolerate surgical stress and a high risk of surgical and chemotherapy-related complications [Citation26]. The International Society of Geriatric Oncology has suggested that all older patients with colorectal cancer should undergo thorough geriatric evaluation to confirm fitness for surgical intervention and adjuvant chemotherapy [Citation27]. However, studies have shown that older patients undergoing surgery for colorectal cancer have similar overall survival and disease-free survival rates as their younger counterparts and that age does not represent an independent factor for survival [Citation28,Citation29]. Also, elective surgical intervention carries a lower risk compared to emergency treatment, therefore a large proportion of older patients can undergo surgery for colorectal cancer safely [Citation30]. Hence, there is a need to optimize the treatment modality and select the most appropriate surgical approach to achieve the best outcomes for older patients.
Laparoscopic surgery for colorectal cancer has been successful in reducing the invasiveness of the procedure, thereby reducing surgical complications and improving outcomes for all age groups [Citation31]. Seishima et al. [Citation7] have shown that laparoscopic surgery significantly reduces perioperative mortality and postoperative complications in older patients when compared to open procedures, without any difference in long-term survival. Given such results, the use of robotic surgery is postulated to further improve outcomes for older patients. Robotic surgery offers a three-dimensional high-definition view with its stable camera, provides better control of instruments, and improves operability and comfort during surgery [Citation32]. A large multicentric randomized controlled trial (RCT) conducted in China has shown that robotic surgery results in better oncological quality of resection, with less surgical trauma, and better postoperative recovery, as compared to laparoscopic surgery for middle and low rectal cancer [Citation33]. However, such advantages have not been replicated in all studies. A recent systematic review and meta-analysis of six RCTs comparing robotic and laparoscopic surgery for colorectal cancer found that both approaches had similar rates of complications, blood loss, conversion to open surgery, time of first flatus, number of lymph nodes harvested and LOS. The only difference noted was in the operating time and costs, which were higher with robotic surgeries [Citation34]. Nevertheless, such evidence may not be generalizable to older patients. RCTs have strict inclusion criteria and may not be representative of the real-world population, which includes patients with comorbidities and vulnerabilities of age [Citation35]. The lack of external validity of such studies limits treatment decisions for subgroups such as older patients.
In this context, the current review provides important evidence regarding the minimally invasive surgical approach to be chosen for older patients. The lack of differences in short-term results, namely Clavien-Dindo grade I-II and III-IV complications, conversion to open surgery, and reoperation rates, suggests that both robotic and laparoscopic approaches have similar degrees of surgical safety. Operating time was also not significantly different between the two approaches. However, operating time greatly depends on the skill and training of the surgeon and is highly subjective. LOS was noted to be decreased with robotic surgery suggesting a positive effect of the approach in improving results with a MD of −0.56 days. However, the 95% CI were wide with the upper end just crossing zero, indicating no significant difference. An interesting finding of the meta-analysis was a significant reduction in the readmission and mortality rates associated with robotic surgery. However, these results were highly influenced by the large study of Pacheco et al. [Citation21] which demonstrated a significant reduction in the risk of readmission and mortality in octogenarians with the use of robotic surgery. In both outcomes, this study had the largest weight overshadowing the results of other studies. The authors used the USA National Cancer Database, which has no data on patient comorbidities and cancer characteristics. Also, reasons for death or readmission were not noted. The lack of such important variables limits the interpretation of the results. Secondly, given the scarce data available for meta-analysis these results should be interpreted with caution.
The current review focused only on the short-term outcomes of the two approaches. On the other hand, oncological safety and survival are equally important outcomes. However, only a limited number of studies have assessed long-term outcomes. Xue et al. [Citation18] have shown that three-year overall survival and disease-free survival rates do not differ when comparing robotic and laparoscopic surgery in older patients. Similarly, Thompson et al. [Citation20] have also noted no difference in survival rates between the two. Further studies are needed to improve evidence on long-term outcomes of the two approaches for colorectal cancer surgery in older patients.
There are some limitations of the review which warrant attention. The limited number of studies and variability of outcome data meant that very few outcomes could be quantitatively analyzed. The number of studies in each meta-analysis was not high. The lack of baseline matching for confounders is another drawback that downgrades the evidence quality. Retrospective studies are prone to selection bias, and adjusting confounders is important to derive quality evidence. A major limitation of the review was the heterogeneity in defining the older population. While there is no consensus on the exact definition of being older, being ≥65 years old is considered by many to be part of the older segment of the population[Citation14, Citation15]. However, as noted in the review, some authors [Citation24] consider an age of >70 years as being older, while others consider the cut-off to be 80 years [Citation20]. Such variation can have important implications on the results. However, due to limited data, we were unable to conduct a subgroup analysis based on different age cut-offs. Most studies in our review focused on the octogenarian population while others used cut-offs of 70 and 65 years for defining being older. Patients aged >80 years can be considered more fragile than those >65 years of age. Future studies must segregate data of octogenarians from those aged >70 and >65 years for better results. Also, there is a need for a worldwide consensus in the definition of being older for a better interpretation of future studies. Lastly, data came from a few select countries and may not be generalizable yet.
Conclusions
This first meta-analysis comparing outcomes of robotic and laparoscopic surgery in older colorectal cancer patients shows that both approaches result in no differences in operating time, complication rates, conversion to open surgery, reoperation rates, and LOS. Robotic surgery does not seem to improve the outcomes of colorectal cancer in older patients as compared to laparoscopic surgery. Scarce data show that mortality and readmission rates may be lower with robotic surgery. The limited number of studies and selection bias are important limitations of the review. Further studies with propensity score matching are needed to improve evidence.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by XW, RM, TH, HX, and CZ. The first draft of the manuscript was written by CZ and CY, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Declaration of interest
The authors declare no competing interests.
Additional information
Funding
References
- Pérez-Escalante E, Cariño-Cortés R, Fernández-Martínez E, et al. Colorectal cancer: causes and evidence of chemopreventive treatments. Curr Pharm Biotechnol. 2018;19(14):1135–1155. doi: 10.2174/1389201020666181226112712.
- Harfouch RM, Alkhaier Z, Ismail S, et al. Epidemiology and risk factors of colorectal cancer in syria: a single-center retrospective study. Eur Rev Med Pharmacol Sci. 2022;26(13):4654–4658. doi: 10.26355/eurrev_202207_29187.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/CAAC.21492.
- Cai M, Gao Z, Liao J, et al. Frailty affects prognosis in patients with colorectal cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:1017183. doi: 10.3389/FONC.2022.1017183.
- Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. doi: 10.1016/j.jgo.2015.12.002.
- Walshaw J, Huo B, McClean A, et al. Innovation in gastrointestinal surgery: the evolution of minimally invasive surgery-a narrative review. Front Surg. 2023;10:1193486. doi: 10.3389/fsurg.2023.1193486.
- Seishima R, Okabayashi K, Hasegawa H, et al. Is laparoscopic colorectal surgery beneficial for elderly patients? A systematic review and meta-analysis. J Gastrointest Surg. 2015;19(4):756–765. doi: 10.1007/s11605-015-2748-9.
- Weber PA, Merola S, Wasielewski A, et al. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45(12):1689–1696. discussion 16956 doi: 10.1007/s10350-004-7261-2.
- Kim MJ, Park SC, Park JW, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg. 2018;267(2):243–251. doi: 10.1097/SLA.0000000000002321.
- Tschann P, Szeverinski P, Weigl MP, et al. Short- and long-term outcome of laparoscopic- versus robotic-Assisted right colectomy: a systematic review and meta-analysis. J Clin Med. 2022;11(9):2387. doi: 10.3390/jcm11092387.
- Seow W, Dudi-Venkata NN, Bedrikovetski S, et al. Outcomes of open vs laparoscopic vs robotic vs transanal total mesorectal excision (TME) for rectal cancer: a network meta-analysis. Tech Coloproctol. 2023;27(5):345–360. doi: 10.1007/s10151-022-02739-1.
- Solis-Pazmino P, Oka K, La K, et al. Robotic right versus left colectomy for colorectal neoplasia: a systemic review and meta-analysis. J Robot Surg. 2023;17(5):1907–1915. doi: 10.1007/s11701-023-01649-0.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906.
- Orimo H, Ito H, Suzuki T, et al. Reviewing the definition of “elderly. Geriatr Gerontol Int. 2006;6:149–158. doi: 10.1111/j.1447-0594.2006.00341.x.
- Singh S, Bajorek B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm Pract (Granada). 2014;12(4):489. doi: 10.4321/s1886-36552014000400007.
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 30 Oct 2020.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135.
- Xue Y, Li S, Guo S, et al. Evaluation of the advantages of robotic versus laparoscopic surgery in elderly patients with colorectal cancer. BMC Geriatr. 2023;23(1):105. doi: 10.1186/s12877-023-03822-4.
- Srinath H, Kim T-J, Mor IJ, et al. Robot-assisted vs laparoscopic right hemicolectomy in octogenarians. J Am Med Dir Assoc. 2022;23(4):690–694. doi: 10.1016/j.jamda.2022.01.080.
- Thompson HM, Williams H, Omer DM, et al. Comparison of short-term outcomes and survival between minimally invasive colectomy and open colectomy in patients 80 years of age and older. J Robot Surg. 2023;17(4):1857–1865. doi: 10.1007/s11701-023-01575-1.
- Pacheco F, Harris-Gendron S, Luciano E, et al. Robotic versus laparoscopic colectomy outcomes in Colon adenocarcinoma in the elderly population: a propensity-score matched analysis of the national cancer database. Int J Colorectal Dis. 2023;38(1):183. doi: 10.1007/s00384-023-04481-y.
- Palomba G, Dinuzzi VP, Capuano M, et al. Robotic versus laparoscopic colorectal surgery in elderly patients in terms of recovery time: a monocentric experience. J Robot Surg. 2022;16(4):981–987. doi: 10.1007/s11701-021-01332-2.
- de’Angelis N, Abdalla S, Bianchi G, et al. Robotic versus laparoscopic colorectal cancer surgery in elderly patients: a propensity score match analysis. J Laparoendosc Adv Surg Tech A. 2018;28(11):1334–1345. doi: 10.1089/lap.2018.0115.
- Richards CR, Steele SR, Lustik MB, et al. Safe surgery in the elderly: a review of outcomes following robotic proctectomy from the nationwide inpatient sample in a cross-sectional study. Ann Med Surg (Lond). 2019;44:39–45. doi: 10.1016/j.amsu.2019.06.004.
- Di Capua B, Bellieni A, Fusco D, et al. Perspectives and limits of cancer treatment in an oldest old population. Aging Clin Exp Res. 2021;33(10):2831–2837. doi: 10.1007/s40520-021-01821-2.
- Hayes L, Forrest L, Adams J, et al. Age-related inequalities in colon cancer treatment persist over time: a population-based analysis. J Epidemiol Community Health. 2019;73(1):34–41. doi: 10.1136/jech-2018-210842.
- Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: international society of geriatric oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26(3):463–476. doi: 10.1093/annonc/mdu253.
- Sueda T, Tei M, Nishida K, et al. Evaluation of short- and long-term outcomes following laparoscopic surgery for colorectal cancer in elderly patients aged over 80 years old: a propensity score-matched analysis. Int J Colorectal Dis. 2021;36(2):365–375. doi: 10.1007/s00384-020-03770-0.
- Oh BY, Huh JW, Kim HC, et al. Oncologic outcome of colorectal cancer patients over age 80: a propensity score-matched analysis. Int J Colorectal Dis. 2018;33(8):1011–1018. doi: 10.1007/s00384-018-3028-4.
- Park EJ. Tailoring strategies for colorectal cancer screening and treatment based on age in colorectal cancer patients. Ann Coloproctol. 2022;38(3):181–182. doi: 10.3393/ac.2022.00395.0056.
- Kiyozumi Y, Yamaguchi T, Ichikawa N, et al. Endoscopic surgical skill qualification system: propensity-score matched cohort analysis of accredited supervisors in laparoscopic rectal cancer surgery. Br J Surg. 2023;110(12):1834–1839. doi: 10.1093/bjs/znad282.
- Liu H, Xu M, Liu R, et al. The art of robotic colonic resection: a review of progress in the past 5 years. Updates Surg. 2021;73(3):1037–1048. doi: 10.1007/s13304-020-00969-2.
- Feng Q, Yuan W, Li T, et al. Robotic versus laparoscopic surgery for Middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(11):991–1004. doi: 10.1016/S2468-1253(22)00248-5.
- Yang L, Fang C, Bi T, et al. Efficacy of robot-assisted vs. laparoscopy surgery in the treatment of colorectal cancer: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2023;47(7):102176. doi: 10.1016/j.clinre.2023.102176.
- Tan YY, Papez V, Chang WH, et al. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3(10):e674–89–e689. doi: 10.1016/S2666-7568(22)00186-6.