Abstract
Objectives: Hyperprolactinemia is a common adverse event associated with psychotropic medications (mainly antipsychotics) used in the management of schizophrenia and bipolar disorders. The aim of this study was to estimate the prevalence of hyperprolactinemia in psychiatric patients and to evaluate its association with various psychiatric diagnoses and the use of various psychotropic medications.
Methods: A cross-sectional observational study was conducted between July 2012 and June 2014. Patients were recruited from a number of hospitals located in the five regions of Saudi Arabia. Hyperprolactinemia was defined as blood prolactin levels >25 ng/mL in females and >20 ng/mL in males, regardless of the presence of symptoms.
Results: A total of 997 patients (553 males and 444 females) were included in the current analysis. The average blood prolactin level was 32.6 ± 44.1 ng/mL, with higher levels among females than males (42.9 ± 61.3 versus 24.4 ± 18.6, p < .001). The prevalence of hyperprolactinemia was 44.3%, with no significant gender difference (41.9% in females versus 46.3% in males, p = .164) but with huge variability according to individual antipsychotic and other psychotropic medications. In the multivariate analysis adjusted for demographic and clinical characteristics, hyperprolactinemia was independently and positively associated with using antipsychotic medications (OR = 2.08, 1.26–3.42, p = .004). Additionally, previous hospitalisation, diabetes and hypothyroidism were positively associated, whereas having primary depressive disorders was negatively associated.
Conclusions: We report a high prevalence of hyperprolactinemia among a large sample of psychiatric patients in Saudi Arabia, which was linked to the use of antipsychotic medications. Routine measurement of blood prolactin levels for all patients maintained on antipsychotic agents is recommended, regardless of symptoms.
Introduction
Prolactin is a polypeptide hormone secreted by the anterior pituitary gland to induce lactation and other reproductive functions (Halbreich, Kinon, Gilmore, & Kahn, Citation2003). Several endogenous substances have been found to affect the prolactin level, with dopamine inhibiting and serotonin stimulating its release (Voicu, Medvedovici, Ranetti, & Rădulescu, Citation2013). Additionally, physiological conditions (such as pregnancy, lactation and sleep), neurological diseases, hormonal disorders, systemic diseases and medications have been associated with elevated prolactin levels (Holt, Citation2008; Peuskens, Pani, Detraux, & De Hert, Citation2014). Hyperprolactinemia is defined as elevated prolactin levels above gender-specific normal levels, which have been variably defined in previous studies (Halbreich et al., Citation2003; Peuskens et al., Citation2014). Hyperprolactinemia can affect sexual and reproductive functions in both genders, and the clinical presentation generally depends on the magnitude of prolactin elevation (Serri, Chik, Ur, & Ezzat, Citation2003). Additionally, chronic hyperprolactinemia has been linked to an increased risk of osteoporosis and probably breast cancer (Kohen & Wildgust, Citation2008; Misra, Papakostas, & Klibanski, Citation2004).
The prevalence of hyperprolactinemia, defined as >600 mU/L, among the general population ranges between 0.7% in men and 2.5% in women (Vanderpump, French, Appleton, Tunbridge, & Kendall-Taylor, Citation1998). The prevalence has been shown to be much higher (ranging between 10% and 55%) among women and men with reproductive disorders (Holt, Citation2008). Interestingly, hyperprolactinemia is a common adverse event associated with psychotropic medications (mainly antipsychotics) used in the management of schizophrenia and bipolar disorders (Halbreich et al., Citation2003; Hummer & Huber, Citation2004; O’Keane, Citation2008). The prevalence of hyperprolactinemia among psychiatric patients receiving antipsychotic medications was estimated to be between 30% and 70% in Western populations and in Japan (Bushe, Shaw, & Peveler, Citation2008; Kikuchi et al., Citation2012; Kinon, Gilmore, Liu, & Halbreich, Citation2003a, Citation2003b; Meaney et al., Citation2004; Montgomery et al., Citation2004; Smith, Wheeler, Murray, & O’Keane, Citation2002). However, such data are lacking in Saudi Arabia and nearby countries. Moreover, an investigation of the most significantly associated psychotropic medications and/or psychiatric diseases has never been attempted. The objective of the current study was to estimate the prevalence of hyperprolactinemia in psychiatric patients and to assess its association with various psychiatric diagnoses and the use of various psychotropic medications.
Methods
Setting
The current study was conducted among patients seeking psychiatric advice at a number of hospitals located in Central, Eastern, Western, Northern and Southern regions of Saudi Arabia. We aimed to choose the largest mental hospital in each of the five major regions of Saudi Arabia. However, because of logistic difficulties, not all included hospitals eventually, were the biggest in that region. To compensate for that, two hospitals were chosen from the central region. The hospitals included are King Saud University Medical City in Riyadh and Zulfi General hospital (Central region), Jeddah mental Health hospital (Western region), Al Amal Complex for Mental Health – Dammam (Eastern region), Aljouf mental Health hospital (Northern region) and Abha mental Health hospital (Southern region). King Saud University Medical City is a University-affiliated governmental hospital, whereas the other hospitals are governmentally funded under the authority of the Ministry of Health. All included hospitals provide free psychiatric inpatient and outpatient healthcare services.
Study design
A cross-sectional observational study was conducted between July 2012 and June 2014. The study obtained all required ethical approvals from the institutional review board at Faculty of Medicine at King Saud University in Riyadh and administrative approvals from the respective hospitals.
Population
Consecutive male and female patients seeking psychiatric help in the included hospitals during the study period were asked to join the study. Those who signed the informed consent irrespective of the type of psychiatric diagnosis, the duration of disease, and recent use of psychotropic medications were included. Patients whose records and interview indicated an absence of psychiatric diseases (n = 59), no blood prolactin measurement (n = 209), women who were pregnant or lactating at the time of the study (n = 16), those who had a pituitary tumour (n = 3), and those who had hypothalamic disease (n = 2) were excluded. Therefore, 997 of the 1264 patients who were initially reviewed were included in the current analysis.
Data collection
The data collected included socio-demographic characteristics, medical history, current psychiatric diagnoses (made by the attending psychiatrist using DSM-IV-TR criteria) and recent use of psychotropic medications. Data were obtained primarily by reviewing the patients’ charts. Unclear or missing information was verified by interviewing the patient and/or his or her family. Trained psychiatric residents and staff were responsible for the chart review and for conducting interviews with the patients and/or their families.
Diagnosis of hyperprolactinemia
Blood samples were obtained to measure blood prolactin levels. Hyperprolactinemia was defined as blood prolactin levels above 25 ng/mL in females and above 20 ng/mL in males, regardless of the presence of symptoms (Halbreich et al., Citation2003; Peuskens et al., Citation2014; Peveler et al., Citation2008). The increase in blood prolactin levels was further categorised as mild increase (up to 50 ng/mL), moderate increase (50–100 ng/mL) or marked increase (>100 ng/mL) (Serri et al., Citation2003).
Classification of psychiatric diagnoses
For the purpose of analysing the data, the psychiatric diagnoses of the studied patients were classified under seven categories: primary psychotic disorders, primary bipolar disorders, primary depressive disorders, primary anxiety disorders, personality disorders, secondary psychiatric disorders and other disorders.
Classification of psychotropic medications
Both individual psychotropic medications and pharmacological groups were used in the analysis. These included antipsychotics (low potency first generation, high potency first generation and second generation), antidepressants (selective serotonin reuptake inhibitors ‘SSRIs’, tricyclics and others), mood stabilisers and antianxiety.
Statistical analysis
Data were presented as frequencies and percentages for categorical data and mean and standard deviation (SD) for continuous data. Significant differences between those with and without hyperprolactinemia in terms of demographics, clinical characteristics, diagnoses and medications were tested using chi-square test or Fisher’s exact test (as appropriate) for categorical data and Student’s t-test for continuous data. Independent associations of hyperprolactinemia with various psychiatric diagnoses and psychotropic medications after adjusting for relevant demographic and clinical characteristics were evaluated using multivariate logistic regression models with stepwise backward elimination. All p values were two-tailed. p Value <.05 was considered significant. SPSS software (release 20.3, IBM Corp, Armonk, NY) was used for all statistical analyses.
Results
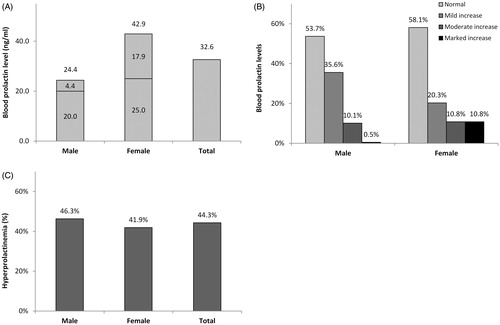
A total of 997 patients (553 males and 444 females) were included in the current analysis. As shown in , the average blood prolactin level was 32.6 ± 44.1 ng/mL for the entire sample, with a significant difference (p < .001) according to gender that was higher among females (42.9 ± 61.3) than males (24.4 ± 18.6). Females had a higher frequency of markedly increased (>100 ng/mL) blood prolactin levels than males (p < .001). Using the gender-specific cut-off points for the diagnosis of hyperprolactinemia (>20 ng/mL for males and >25 ng/mL for females), the prevalence of hyperprolactinemia was 44.3% (442/997) for the entire sample, with a slightly higher prevalence among males (46.3%) than females (41.9%). However, the gender difference was not statistically significant (p = .164).
Figure 1. Blood prolactin levels (A), the degree of prolactin increase (B) and the prevalence of hyperprolactinemia (C) according to gender among psychiatric patients (N = 997). Using gender-specific cut-off points of high blood prolactin level (>25 ng/mL in females and >20 ng/mL in males), mild increase up to 50 ng/mL, moderate increase 50–100 ng/mL and marked increase >100 ng/mL.
Demographic characteristics are shown in . The majority of patients were currently unmarried (59.1%), were illiterate or had less than secondary education (86.7%), were non-working (70.8%), had a family income of 6000 SR (1600 US$) or less per month (62.6%). The prevalence of hyperprolactinemia was higher among single (p = .005) and non-working (p = .011) patients. On the other hand, there were no differences according to age, gender or other demographic characteristics.
Table 1. Demographic characteristics according to hyperprolactinemia status among psychiatric patients (N = 997).
The clinical characteristics of the included patients are shown in . Common medical comorbidities included diabetes, hypertension and hypothyroidism. The symptoms (and associated frequencies) commonly associated with hyperprolactinemia included menstrual irregularities (30.9%), decreased libido (21.2%), gynecomastia (5.4%) and galactorrhea (4.0%). The prevalence of hyperprolactinemia was higher among inpatients (p < .001), those who were diagnosed at a younger age (p < .001), those with a longer disease duration (p = .005), those with a previous hospitalisation (p < .001), and those with a history of diabetes (p = .013) or hypothyroidism (p = .003), but not those complaining of decreased libido (p = .004).
Table 2. Clinical characteristics according to hyperprolactinemia status among psychiatric patients (N = 997).
The association of hyperprolactinemia with various psychiatric diagnoses and the use of various psychotropic medications is shown in . The prevalence of hyperprolactinemia was higher among patients with primary psychotic disorders and primary bipolar disorders (p < .001 for each) but lower among patients with primary depressive disorders and primary anxiety disorders (p < .001 for each). Additionally, the prevalence of hyperprolactinemia was higher among patients using multiple medications (p = .030), including antipsychotics (p < .001), mood stabilisers (p < .001) and antianxiety medications (p = .002) but not antidepressants (p < .001). As shown in , the highest prevalence of hyperprolactinemia (>60%) was observed in patients using lithium, clonazepam, risperidone, fluphenazine, paliperidone or haloperidol, whereas the lowest prevalence of hyperprolactinemia (<30%) was observed in patients using amitriptyline, paroxetine, escitalopram, venlafaxine or mirtazapine.
Figure 2. The prevalence of hyperprolactinemia according to the use of individual psychotropic medications among psychiatric patients (N = 997). Using gender-specific cut-off points for high blood prolactin level (>25 ng/mL in females and >20 ng/mL in males). Those with small users (N < 10) were excluded.
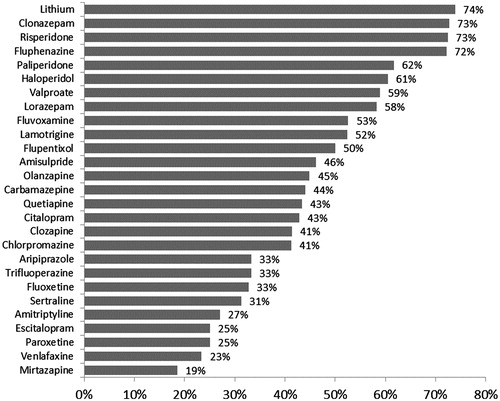
Table 3. Psychiatric diagnoses and use of psychotropic medications according to hyperprolactinemia status among psychiatric patients (N = 997).
shows the multivariate logistic regression analysis of the associations with hyperprolactinemia. The model tested the association of hyperprolactinemia with various psychiatric diagnoses and the use of various psychotropic medications after adjusting for significant potential demographic and clinical characteristics ( and ), including age, gender, marital status, working status, care type, age at disease onset, disease duration, previous hospitalisation and medical history (diabetes and hypothyroidism). In the fully adjusted model, hyperprolactinemia was independently and positively associated with using any antipsychotic medications (OR = 2.08, 1.26–3.42, p = .004) but independently and negatively associated with having primary depressive disorders (OR = 0.28, 0.17–0.48, p < .001). Other characteristics that were also independently and positively associated with hyperprolactinemia included previous hospitalisation (p < .001), having a history of diabetes (p = .032) and, to a lesser extent, hypothyroidism (p = .074).
Table 4. Multivariate logistic regression analysisTable Footnotea for the association of hyperprolactinemia among the studied patients (N = 854).
Discussion
We report a 44% prevalence of hyperprolactinemia among a large sample of psychiatric patients in both inpatient and outpatient settings in Saudi Arabia. The current prevalence was generally similar to the prevalence of hyperprolactinemia reported in several studies mainly performed in the US, UK and Japan, where 30–70% of schizophrenic patients receiving antipsychotic medications had hyperprolactinemia (Bushe et al., Citation2008; Kikuchi et al., Citation2012; Kinon et al., Citation2003a, Citation2003b; Meaney et al., Citation2004; Montgomery et al., Citation2004; Smith et al., Citation2002). However, the comparison of the current prevalence with that of previous studies is complicated by the considerable variability in the age, gender and comorbidity of the patients selected; the type, duration and adherence to the medications used; and the cut-off point used to define hyperprolactinemia in both sexes. For example, the patients included in the aforementioned international studies were mainly schizophrenic patients receiving antipsychotic medications, while those in our study were patients diagnosed with various psychiatric diseases and treated with various psychotropic medications. Re-analysis of our data to include only patients who were receiving antipsychotic but not antidepressant medications increased the prevalence from 44% to 54%. Additionally, we used the cut-off point of >20 ng/mL for males and >25 ng/mL for females, which were commonly used in the literature to define hyperprolactinemia. However, the huge variability in the cut-off points used in various studies (11–35 ng/mL for males and 20–50 ng/mL for females) (Peuskens et al., Citation2014) can markedly affect the calculated percentage of hyperprolactinemia and undermine any comparison.
The current study did not find any age differences in the prevalence of hyperprolactinemia among the study participants. Several studies found that patients of all ages demonstrated sensitivity to increased prolactin and that they sustained the effect of hyperprolactinemia over time (Kinon et al., Citation2003a, Citation2003b). However, some studies suggested that hyperprolactinemia is more prevalent in premenopausal than in postmenopausal women (Kinon et al., Citation2003a, Citation2003b; Pigato et al., Citation2015).
While there were no significant gender differences in the prevalence of hyperprolactinemia among our study participants, females clearly had higher levels of prolactin and a higher frequency of severe degrees of hyperprolactinemia. Gender-specific differences in the prevalence of hyperprolactinemia have been reported in both the general population and in patients with non-psychiatric diseases (Holt, Citation2008; Vanderpump et al., Citation1998). The same finding has also been observed in several studies among psychiatric patients, with up to a 40% higher prevalence in females than males (Bushe et al., Citation2008). This difference is believed to be related to the ability of oestrogens to raise blood prolactin levels and to enhance the responsiveness of lactotrophic cells of the anterior pituitary to prolactin-releasing stimuli (Peuskens et al., Citation2014). However, some studies failed to detect any gender difference in the prevalence of hyperprolactinemia among psychiatric patients (Kikuchi et al., Citation2012; Meaney et al., Citation2004; Montgomery et al., Citation2004). Additionally, some researchers are doubtful about the presence of genuine gender differences in the prevalence of hyperprolactinemia and explained the higher prevalence in women by the earlier manifestation and consequent detection of high prolactin levels in women than in men (Holt, Citation2008). Furthermore, some of the detected difference could be related to methodological errors (Smith et al., Citation2002). For example, in the study that showed a 40% higher prevalence of hyperprolactinemia among women than men, the researchers used a single cut-off point to define hyperprolactinemia for both sexes (>480 mIU/L), which may have reduced the prevalence in men but increased the prevalence in women (Smith et al., Citation2002).
Hyperprolactinemia in the current study was independently and positively associated with the use of antipsychotic medications. This finding was consistent with the accumulating evidence from clinical trials (De Hert, Dobbelaere, Sheridan, Cohen, & Correll, Citation2011; Kinon et al., Citation2003a, Citation2003b) and cross-sectional studies (Bushe et al., Citation2008; Kikuchi et al., Citation2012; Kinon et al., Citation2003a, Citation2003b; Meaney et al., Citation2004; Montgomery et al., Citation2004; Smith et al., Citation2002) showing hyperprolactinemia to be a consequence of (or associated with) the use of antipsychotics. The prolactin-raising effect of antipsychotics is largely mediated through blocking dopamine (D2) receptors in the anterior pituitary gland, thereby blocking the inhibitory effect of dopamine on the lactotroph cells (Haddad & Wieck, Citation2004; Holt & Peveler, Citation2011; Voicu et al., Citation2013). This effect was shown to cause sustained increases in the prolactin level up to 10-fold above pretreatment values (Haddad & Wieck, Citation2004; Holt & Peveler, Citation2011; Voicu et al., Citation2013). Similar to previous studies, there was considerable variability in the prolactin-raising effect of various antipsychotics used in the current study (Bushe et al., Citation2008; Holt & Peveler, Citation2011; Peuskens et al., Citation2014). For example, with exception of risperidone and paliperidone, second generation antipsychotics in the current study tended to be associated with a lower prevalence of hyperprolactinemia than conventional antipsychotics. This variability is believed to reflect the differential D2 receptor-binding affinity in various antipsychotics (Holt & Peveler, Citation2011). Another hypothesis relates to variability in relative occupancy of D2 receptors within the brain and the pituitary gland which located outside of the blood–brain barrier (Ajmal, Joffe, & Nachtigall, Citation2014; Peuskens et al., Citation2014; Voicu et al., Citation2013). Positron emission tomography studies showed different brain/plasma concentration occupancy ratios of antipsychotic medications in brain parenchyma and pituitary D2 receptor sites. This ratio, indicating the penetrating capability across the blood–brain barrier is a good distinguishing biomarker of each antipsychotic drug for the risk of hyperprolactinemia at therapeutic dose (Arakawa et al., Citation2010). Most second generation antipsychotics showed greater occupancy of brain receptors compared with pituitary receptors, whereas conventional antipsychotics poorly cross the blood–brain barrier and bind more to D2 receptors at the pituitary site (Ajmal et al., Citation2014; Madhusoodanan, Parida, & Jimenez, Citation2010). However, since risperidone was the most commonly (37%) used antipsychotic in our patients, the difference in the prevalence of hyperprolactinemia between second generation (52%) and conventional (58%) antipsychotics in the current study was lower than that previously reported (Bushe et al., Citation2008; Holt & Peveler, Citation2011). Interestingly, the high and sustained prolactin-raising effect of risperidone and its metabolite paliperidone was shown to be mediated by the high binding affinity to D2 receptor and the reduced ability to cross the blood–brain barrier, thus increasing the exposure of pituitary D2 receptors (Holt & Peveler, Citation2011; Voicu et al., Citation2013).
Antidepressants in the current study were associated with a lower prevalence of hyperprolactinemia in crude but not adjusted analyses. The association is probably confounded by the fact that almost all patients who were using antidepressants were also using antipsychotics. The prolactin-raising effect of antidepressants is known to be milder and more transient than that of antipsychotics (Voicu et al., Citation2013). Similarly, the higher prevalence of hyperprolactinemia among those who were using mood stabilisers and antianxiety medications in crude but not adjusted analyses may reflect the concomitant use of antipsychotics (Voicu et al., Citation2013).
Hyperprolactinemia in the current study was determined using blood prolactin levels, irrespective of symptoms. Additionally, fewer than half of our hyperprolactinaemic patients were symptomatic. Despite the high prevalence of hyperprolactinemia in the current and previous studies, the exact prevalence of drug-induced or antipsychotic-induced hyperprolactinemia is believed to be underestimated in psychiatric clinics (Haddad & Wieck, Citation2004; Voicu et al., Citation2013). This underestimation may be due to the frequent lack of externally visible symptoms, patients’ reluctance to report sexual and other embarrassing symptoms, and lack of physician awareness. The finding may emphasise the need of routine measurement of blood prolactin levels for all patients maintained on antipsychotic agents, regardless of symptoms (Wong-Anuchit, Citation2016). This recommendation becomes even more critical when we know that more than 80% of our psychiatric patients were using antipsychotics. When hyperprolactinemia is confirmed to be related to antipsychotic used, there will be a need to consider changing the treatment plan depending on several clinical factors (duration of treatment, risk of relapse, etc.). It is recommended in such conditions to decrease the dose of the prolactin-raising agent, switching to a prolactin-sparing antipsychotic like aripiprazole, using a dopamine agonist agent or using hormonal therapy with oestrogens and progestogens (Milano, Colletti, & Capasso, Citation2017; Montejo, Citation2008; Wong-Anuchit, Citation2016).
The current study has several strengths; it is the first local study to estimate the prevalence of hyperprolactinemia and to determine its independent risk factors among psychiatric patients. It is a multicentre study with large sample size, which is almost double that of any previous international cross-sectional studies (Bushe et al., Citation2008). Unlike previous studies, which focused mainly on schizophrenic patients receiving antipsychotic medications, the current study examined the crude and adjusted prolactin-raising potential of various groups of psychiatric medications as our patients were typical outpatients/inpatients diagnosed with various psychiatric diseases and treated with various psychiatric medications. Nevertheless, we acknowledge a number of limitations. First, because of the use of convenience sampling, our results should be cautiously generalised to all psychiatric patients in Saudi Arabia. Being a cross-sectional study makes it difficult to ascertain causality between psychiatric medications and hyperprolactinemia. Finally, although we collected very comprehensive medication data, we did not have a tool to verify the patient compliance with intake.
In conclusion, we report a high prevalence of hyperprolactinemia among a large sample of psychiatric patients in Saudi Arabia, which was independently and positively associated with the use of antipsychotic medications. Routine measurement of blood prolactin levels for all patients maintained on antipsychotic agents is recommended, regardless of symptoms. Further studies need to be conducted to investigate all factors associated with hyperprolactinemia individually and collectively and to determine the suitable procedures to prevent and reduce this clinical problem.
Acknowledgements
The authors would like to acknowledge the College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Kingdom of Saudi Arabia for supporting this study. Furthermore, the authors express their gratitude to Dr. Aiman El-Saed for his assistance in data analysis and manuscript writing and to Ms. Fatima Jama for her assistance in data entry.
Ethics approval and consent to participate: The study obtained all required ethical approvals from the institutional review board at Faculty of Medicine at King Saud University in Riyadh and administrative approvals from the respective hospitals. Consecutive male and female patients seeking psychiatric help in the included hospitals during the study period were asked to join the study. Those who signed the informed consent irrespective of the type of psychiatric diagnosis, the duration of disease, and recent use of psychotropic medications were included.
Availability of data and materials: All data generated or analysed during this study are included in this published article.
Disclosure statement
The authors have no conflicts of interest, and the work was not supported or funded by any drug company.
References
- Ajmal, A., Joffe, H., & Nachtigall, L. B. (2014). Psychotropic-induced hyperprolactinemia: A clinical review. Psychosomatics, 55, 29–36. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24140188
- Arakawa, R., Okumura, M., Ito, H., Takano, A., Takahashi, H., Takano, H., … Suhara, T. (2010). Positron emission tomography measurement of dopamine D2 receptor occupancy in the pituitary and cerebral cortex: Relation to antipsychotic-induced hyperprolactinemia. The Journal of Clinical Psychiatry, 71, 1131–1137. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20361897
- Bushe, C., Shaw, M., & Peveler, R. C. (2008). A review of the association between antipsychotic use and hyperprolactinaemia. Journal of Psychopharmacology (Oxford, England), 22, 46–55. doi:10.1177/0269881107088435
- Haddad, P. M., & Wieck, A. (2004). Antipsychotic-induced hyperprolactinaemia: Mechanisms, clinical features and management. Drugs, 64, 2291–2314. doi:10.2165/00003495-200464200-00003
- Halbreich, U., Kinon, B. J., Gilmore, J. A., & Kahn, L. S. (2003). Elevated prolactin levels in patients with schizophrenia: Mechanisms and related adverse effects. Psychoneuroendocrinology, 28 Suppl 1, 53–67. doi:10.1016/S0306-4530(02)00112-9
- De Hert, M., Dobbelaere, M., Sheridan, E. M., Cohen, D., & Correll, C. U. (2011). Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. European Psychiatry, 26, 144–158. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21295450
- Holt, R. I. (2008). Medical causes and consequences of hyperprolactinaemia. A context for psychiatrists. Journal of Psychopharmacology (Oxford, England), 22, 28–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18477618
- Holt, R. I., & Peveler, R. C. (2011). Antipsychotics and hyperprolactinaemia: Mechanisms, consequences and management. Clinical Endocrinology, 74, 141–147. doi:10.1111/j.1365-2265.2010.03814.x
- Hummer, M., & Huber, J. (2004). Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Current Medical Research and Opinion, 20, 189–197. doi:10.1185/030079903125002865
- Kikuchi, T., Iwamoto, K., Sasada, K., Aleksic, B., Yoshida, K., & Ozaki, N. (2012). Sexual dysfunction and hyperprolactinemia in Japanese schizophrenic patients taking antipsychotics. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 37, 26–32. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22172534
- Kinon, B. J., Gilmore, J. A., Liu, H., & Halbreich, U. M. (2003a). Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology, 28 Suppl 2, 55–68. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12650681
- Kinon, B. J., Gilmore, J. A., Liu, H., & Halbreich, U. M. (2003b). Hyperprolactinemia in response to antipsychotic drugs: Characterization across comparative clinical trials. Psychoneuroendocrinology, 28, 69–82. doi:10.1016/S0306-4530(02)00128-2
- Kohen, D., & Wildgust, H. J. (2008). The evolution of hyperprolactinaemia as an entity in psychiatric patients. Journal of Psychopharmacology, 22, 6–11. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18477616
- Madhusoodanan, S., Parida, S., & Jimenez, C. (2010). Hyperprolactinemia associated with psychotropics – A review. Human Psychopharmacology, 25, 281–297. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20521318
- Meaney, A. M., Smith, S., Howes, O. D., O’Brien, M., Murray, R. M., & O’Keane, V. (2004). Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. British Journal of Psychiatry, 184, 503–508. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15172944
- Milano, W., Colletti, C., & Capasso, A. (2017). Hyperprolactinemia induced by antipsychotics: From diagnosis to treatment approach. Endocrine, Metab Immune Disord - Drug Targets [Internet], 17, 38–55. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28440197
- Misra, M., Papakostas, G. I., & Klibanski, A. (2004). Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. The Journal of Clinical Psychiatry, 65, 1607–1618, 1760–1761. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15641865
- Montejo, A. L. (2008). Prolactin awareness: An essential consideration for physical health in schizophrenia. European Neuropsychopharmacology, 18 Suppl 2, S108–S114. doi:10.1016/j.euroneuro.2008.02.004
- Montgomery, J., Winterbottom, E., Jessani, M., Kohegyi, E., Fulmer, J., Seamonds, B., & Josiassen, R. C. (2004). Prevalence of hyperprolactinemia in schizophrenia: Association with typical and atypical antipsychotic treatment. Journal of Clinical Psychiatry, 65, 1491–1498. Retrieved from http://www.psychiatrist.com/JCP/article/Pages/2004/v65n11/v65n1108.aspx
- O’Keane, V. (2008). Antipsychotic-induced hyperprolactinaemia, hypogonadism and osteoporosis in the treatment of schizophrenia. Journal of Psychopharmacology, 22, 70–75. doi:10.1177/0269881107088439
- Peuskens, J., Pani, L., Detraux, J., & De Hert, M. (2014). The effects of novel and newly approved antipsychotics on serum prolactin levels: A comprehensive review. CNS Drugs, 28, 421–453. doi:10.1007/s40263-014-0157-3
- Peveler, R. C., Branford, D., Citrome, L., Fitzgerald, P., Harvey, P. W., Holt, R. I., … O’Keane, V., et al. (2008). Antipsychotics and hyperprolactinaemia: Clinical recommendations. Journal of Psychopharmacology (Oxford, England), 22, 98–103. doi:10.1177/0269881107087346
- Pigato, G., Piazzon, G. V. M., Di Florio, A., Ermani, M., Toffanin, T., & Perini, G. I. (2015). Early hyperprolactinaemia in acute psychiatric inpatients: A cross-sectional study Iperprolattinemia precoce in pazienti ricoverati in sPdc: Uno studio trasversale. Journal of Psychopathology, 21, 226–230.
- Serri, O., Chik, C. L., Ur, E., & Ezzat, S. (2003). Diagnosis and management of hyperprolactinemia. CMAJ, 169, 575–581. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12975226
- Smith, S., Wheeler, M. J., Murray, R., & O’Keane, V. (2002). The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. Journal of Clinical Psychopharmacology, 22, 109–114. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11910254
- Vanderpump, M. P., French, J. M., Appleton, D., Tunbridge, W. M., & Kendall-Taylor, P. (1998). The prevalence of hyperprolactinaemia and association with markers of autoimmune thyroid disease in survivors of the Whickham Survey cohort. Clinical Endocrinology, 48, 39–44. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9509066
- Voicu, V., Medvedovici, A., Ranetti, A. E., & Rădulescu, F. Ş. (2013). Drug-induced hypo- and hyperprolactinemia: Mechanisms, clinical and therapeutic consequences. Expert Opinion on Drug Metabolism & Toxicology, 9, 955–968. Retrieved from http://www.tandfonline.com/doi/full/10.1517/17425255.2013.791283
- Wong-Anuchit, C. (2016). Clinical management of antipsychotic-induced hyperprolactinemia. Perspectives in Psychiatric Care, 52, 145–152. doi:10.1111/ppc.12111
