Abstract
Frailty occurs in aging males for a variety of reasons. It is less common in males than females. Diseases which are particularly associated with frailty are diabetes mellitus, atherosclerosis, anemia and chronic obstructive pulmonary disease. Insulin resistance syndrome plays a pathogenetic role in the “fat-frail” syndrome. Sarcopenia occurs predominantly because of hormone deficiency and cytokine excess. Pain and anorexia are also associated with frailty. Stem cell research represents a potential promise for the treatment of frailty.
Introduction
Over the last decade there has been an increasing enthusiasm to understand the pathophysiology of frailty and potential approaches to its prevention. Frailty represents a syndrome that is easily recognizable by most clinicians but has proved difficult to define. Frailty can be considered as a syndrome when deterioration in a person's basic physiological mechanisms places them at increased risk for developing disability, even when exposed to a minor degree of stress. Attempts to objectively define frailty have been fraught with difficulty. Recently, the definition of Fried et al. Citation[1] has been widely accepted as a working model (). This definition is predictive of subsequent disability and mortality Citation[2]. Using this model, the prevalence of frailty is 6.7%, with females being more likely to be frail than males. This is in keeping with the well-recognized phenomenon that while females live longer than males, males spend less of their life with a functional impairment than do women.
Table I. Objective definition of frailty* as proposed by Fried et al. Citation[1]
The reason(s) for males being less likely to become frail over their lifespan is multifactorial. Firstly, males tend to have a higher peak bone mineral density and muscle mass. Also, there is a suggestion that males have a slower rate of loss of muscle and bone. The possible reasons for this are unclear but can include a tendency for males to do more recreational exercise than females. Increased arthritis and pain syndromes in females lead to disuse of muscles or genetic factors. When examining world records for athletic events over the lifespan, females show an accelerated rate of decline compared to males, supporting the greater rate of physiological decline in females compared to males Citation[3].
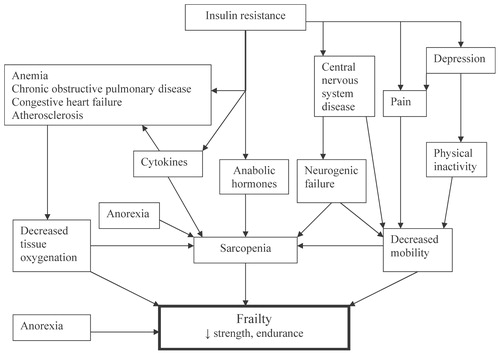
The causes of frailty are multifactorial. They include pain, diseases such as congestive heart failure and diabetes mellitus, alterations in central nervous system function, poor nutritional intake and physical inactivity. Many of these conditions lead to deterioration in the VO2max, making it an excellent measure of physiological compared to chronological aging Citation[4]. Many of the physiological deteriorations produced by disease processes are mediated by proinflammatory cytokines. Physiological deterioration also occurs due to the decline in hormones, such as testosterone, and insulin growth factor-1. An overview of the factors involved in the pathophysiology of frailty is given in. Recently, it has been postulated that the insulin resistance syndrome is a key factor in the pathogenesis of frailty Citation[4],Citation[5].
Of worms and frailty
The nematode, Caenorhabditis elegans, has a decrease in its body movement over its lifespan Citation[6]. This decline in body movement is related to muscle deterioration Citation[7]. This muscle deterioration resembles that which is histologically present in humans with sarcopenia. There is no deterioration in neurons. This decline in older animals is associated with an impairment of the behavioral response to sensory cues and is a predictor of lifespan Citation[8]. Overall, these studies suggest that worms can be utilized as a model of frailty and functional deterioration.
Diseases and frailty
Atherosclerosis leads to a variety of diseases associated with frailty including congestive heart failure, peripheral vascular disease and cerebrovascular accidents. In males, erectile dysfunction has been shown to be a predictor of future cardiovascular disease Citation[9]. Atherosclerosis is associated with increased levels of cytokines, in themselves a factor involved in the pathogenesis of frailty. Fried et al. Citation[1] originally created their objective definition of frailty in persons with heart disease. Persons with congestive heart failure have a decline in cardiac output leading to a reduction in endurance capacity and, often, peripheral muscle wasting. This is particularly true in those persons who develop cardiac cachexia Citation[10]. While drugs that block angiotensin-converting enzyme activity appear to improve muscle strength Citation[11], many of the drugs used to treat heart failure may increase frailty. Recently, there has been an increased awareness that furosemide may lead to increased weakness, orthostasis, dehydration, worsening frailty and mortality.
Persons with chronic obstructive pulmonary disease (COPD) have a poor VO2max. They have elevated levels of cytokines. Hypoxia decreases the function of both muscles and the brain. Testosterone levels are often extremely low in persons with COPD Citation[12]. Testosterone replacement improves strength and function in patients with COPD Citation[13-15].
Anemia has been shown to be related to frailty, falls and functional decline in older persons Citation[16],Citation[17]. Even when the hemoglobin is between 10 g/dl to 14 g/dl there is an increased functional decline. Erythropoietin and its analogs can enhance function.
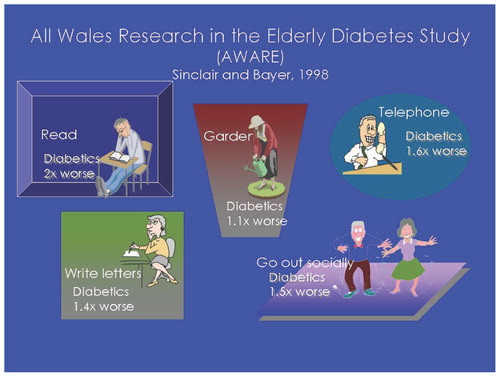
The disease par excellence that leads to frailty is diabetes mellitus. Diabetic patients have elevated cytokines, low testosterone levels and accelerated atherosclerosis Citation[18]. Depression further leads to problems in persons with diabetes mellitus Citation[19]. Numerous studies have shown that diabetics have excessive decline in function and an increased propensity to have injurious falls Citation[20-22]. The AWARE study demonstrated that older diabetics are much less capable of carrying out higher-level functions such as reading and gardening Citation[23] ().
Insulin resistance syndrome and frailty
Middle-aged men with visceral obesity are particularly prone to develop the insulin resistance syndrome Citation[5]. The hyperinsulinemia that occurs in conjunction with insulin resistance leads to hypertension, hyperglycemia, hypertriglyceridemia, low HDL cholesterol, increased uric acid, abnormalities in coagulation factors, non-alcoholic steatotic hepatitis (NASH), myosteatosis (fat infiltration in muscle) and cognitive dysfunction (). Persons with this syndrome also have leptin resistance related to the hypertriglyceridemia Citation[24]. The hypertriglyceridemia also appears to play a role in the development of the myosteatosis and the cognitive dysfunction. Insulin resistance plays a pathogenetic role in the “fat-frail” syndrome.
Sarcopenia, cytokines and hormones
Sarcopenia is the excessive loss of muscle mass. Sarcopenia occurs in 8.8% of young-old women, compared to 17.5% of old-old men Citation[25]. Persons with sarcopenia have an increase in functional decline. Obese persons with sarcopenia have the highest functional decline and mortality.
C-reactive protein, a nonspecific marker of cytokine excess, is related to loss of muscle mass and strength Citation[26]. Interleukin-6 has been termed the “geriatric cytokine”. Elevated levels of it and interleukin-2 are associated with loss of muscle mass, strength and function Citation[27]. Cytokines not only cause loss of muscle mass by inhibiting the NF-kappa-B system and activating the ubiquitin-proteasome system, but also modulate the immune system, decrease circulating albumin levels, produce anemia and anorexia, decrease cognition, enhance lipolysis and inhibit lipoprotein lipase, leading to hypertriglyceridemia and to sickness behavior. This has lead to the concept that there is a cytokine related aging process Citation[28].
Three hormones appear to play a major role in the pathogenesis of sarcopenia in males. These are testosterone, vitamin D and the muscle isoform of insulin growth factor-1. Testosterone levels decline with aging at the rate of 1% per year Citation[29],Citation[30]. Because of the increase in sex hormone binding globulin available to the tissue, hormone levels decline to an even greater extent. The decline in testosterone levels has been correlated with a decrease in muscle mass and strength Citation[31], and in one study with a decline in function Citation[32]. Testosterone administration to hypogonadal older males increased muscle strength Citation[33],Citation[34], and in persons who are borderline hypogonadal it increased muscle mass Citation[35]. Two small studies have suggested an improvement in function after testosterone administration Citation[36],Citation[37]. The effects of testosterone on muscle are listed in. The role of testosterone in the prevention and treatment of frailty requires large, multicenter trials to prove its utility.
Table II. Effects of testosterone on muscle
Vitamin D levels have been shown to decline with aging Citation[38]. Low vitamin D levels are associated with functional deterioration and falls Citation[39]. Vitamin D replacement enhances muscle strength and function in persons with low vitamin D levels, but not in those with normal levels Citation[40].
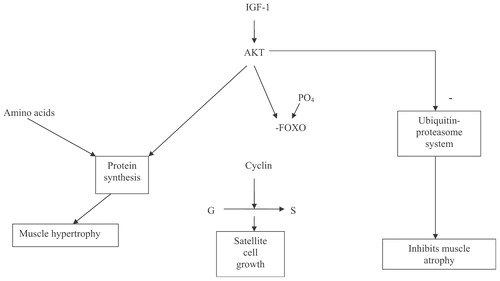
The muscle isoform of IGF-1 appears to be particularly important for the maintenance of aging muscle. The insertion of the transgene for the muscle isoform of IGF-1, into older rats, resulted in the reversal of muscle mass loss Citation[41]. The intracellular mechanisms by which IGF-1 controls muscle growth are shown in.
Myostatin D inhibits muscle growth. Inhibition of myostatin D leads to muscle hypertrophy Citation[42]. The role of myostatin D in sarcopenia is unknown. The other causes of sarcopenia include decreased neuronal stimulation of the muscles, decreased blood flow, decreased physical activity and a decrease in nutrient intake. There is some evidence that the intake of creatine may be particularly important for older muscles Citation[43-45].
Anorexia of aging and frailty
A physiological anorexia of aging occurs over the lifespan Citation[46]. Males develop a greater physiological anorexia than do females. This is due to the decline in testosterone levels in males leading to an increase in leptin. This physiological anorexia places the older male at an increased risk of developing malnutrition when they develop a disease process. Weight loss leads to institutionalization, hip fracture and death in older persons Citation[47].
A number of diseases are associated with a large increase in cytokine burden. This leads to marked loss of muscle and fat. This syndrome is called cachexia. Inadequate ingestion of calories and micronutrients can also lead to anemia and cognitive decline.
Pain
Pain is considered the fifth vital sign. Pain is very common among older persons and is often not treated Citation[48]. Pain can lead to disuse atrophy and contractures. Pain often limits the amount of physical activity an older person will undertake and limits involvement in exercise programs. Treatment of pain leads to enhanced response to resistance exercise Citation[49].
Central nervous system function and frailty
Dementia is not necessarily associated with frailty Citation[50]. Many demented males maintain strength and activities until near the end of the disease. The end stage of Alzheimer's disease is associated with some alterations in neuronal muscular function. Persons with Parkinson's disease and with vascular dementia often develop frailty early in the course of the disease.
Balance deteriorates along with the aging process. Poor balance is associated with an increase in falls and a fear of falling Citation[51]. Both of these can lead to frailty. Balance exercises can reverse the deterioration in balance and slow the development of frailty. In animals, enhancing the environment can improve balance and produce new neuronal growth Citation[52]. Alterations in the beta-adrenergic input of the cerebellum play a major role in the age related loss of balance Citation[53].
Persons with depression have a decreased motivation to undertake exercise and can develop a pseudodementia Citation[54]. They also have an increased propensity to develop myocardial infarction Citation[55] and have poor outcomes associated with rehabilitation Citation[56]. Depression increases corticotrophin releasing factor, which leads to anorexia and an increase in muscle wasting Citation[57]. All of these factors make depression a major factor in the pathogenesis of frailty.
Conclusion
Frailty is a complex condition representing pre-disability. It has multiple causes. Although it is less likely for males over the lifespan to become frail, when compared to females, early recognition of frailty and its aggressive treatment should slow the onset of functional deterioration. Resistance exercise is the cornerstone of the management of frailty. There is a need to intensively study the role of hormone replacement (testosterone and vitamin D) in the treatment of frailty. Stem cell research represents a potential promise for the treatment of frailty.
References
- Fried L P, Tangen C M, Walston J, Newman A B, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop W J, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci 2001; 56A: M146–M156
- Fried L P, Ferrucci L, Darer J, Williamson J D, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Med Sci 2004; 59A: 255–263
- Morley J E. The aging athlete. J Gerontol Med Sci 2000; 55A: M627–M629
- Morley J E, Perry H M, Miller D K. Something about frailty. J Gerontol Med Sci 2002; 57A: M698–M704
- Morley J E. The metabolic syndrome and aging. J Gerontol Med Sci 2004; 59A: 139–142
- Herndon L A, Schmeissner P J, Dudaronek J M, Brown P A, Listner K M, Sakano Y, Paupard M C, Hall D H, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C-elegans. Nature 2002; 419: 808–814
- Fisher A L. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc 2004; 52: 1185–1190
- Glenn C F, Chow D K, David L, Cooke C A, Gami M S, Iser W B, Hanselman K B, Goldberg I G, Wolkow C A. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol Biol Sci 2004; 59A: 1251–1260
- Morley J E, Korenman S G, Kaiser F E, Mooradian A D, Viosca S P. Relationship of penile brachial pressure index to myocardial infarction and cerebrovascular accidents in older men. Am J Med 1988; 84: 445–448
- Anker S D, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 2004; 90: 464–470
- Carter C S, Cesari M, Ambrosius W T, Hu N, Diz D, Oden S, Sonntag W E, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol Med Sci 2004; 59A: 416–423
- Kamischke A, Kemper De, Castel M A, Luthke M, Rolf C, Behre H M, Magnussen H, Nieschlag E. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J 1998; 11: 41–45
- Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis M I, Fournier M, Storer T W. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respirat Crit Care Med 2004; 170: 870–878
- Svartberg J, Aasebo U, Hjalmarsen A, Sundsfjord J, Jorde R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir Med 2004; 98: 906–913
- Ferreira I M, Verreschi I T, Nery L E, Godlstein R S, Zamel N, Brooks D, Jardim J R. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest 1998; 114: 19–28
- Cesari M, Penninx B W, Lauretani F, Russo C R, Carter C, Bandinelli S, Atkinson H, Onder G, Pahor M, Ferrucci L. Hemoglobin levels and skeletal muscle: results from the InCHIANTI study. J Gerontol Med Sci 2004; 59A: 249–254
- Thomas D R. Anemia and quality of life: unrecognized and undertreated. J Gerontol Med Sci 2004; 59A: 238–241
- Morley J E. Diabetes mellitus: a major disease of older persons. J Gerontol Med Sci 2000; 55A: M255–M256
- Rosenthal M J, Fajardo M, Gilmore S, Morley J E, Naliboff B D. Hospitalization and mortality of diabetes in older adults. A 3-year prospective study. Diabetes Care 1998; 21: 231–235
- Rodriguez-Saldana J, Morley J E, Reynoso M T, Medina C A, Salazar P, Cruz E, Torres A LN. Diabetes mellitus in a subgroup of older Mexicans: prevalence, association with cardiovascular risk factors, functional and cognitive impairment, and mortality. J Am Geriatr Soc 2002; 50: 111–116
- Miller D K, Lui L YL, Perry H M, Kaiser F E, Morley J E. Reported and measured physical functioning in older inner-city diabetic African Americans. J Gerontol Med Sci 1999; 54A: M230–M236
- Maty S C, Fried L P, Volpato S, Williamson J, Brancati F L, Blaum C S. Patterns of disability related to diabetes mellitus in older women. J Gerontol Med Sci 2004; 59A: 148–153
- Sinclair A J. Diabetes in the elderly–a perspective from the United Kingdom. Clin Geriatr Med 1999; 15: 225–237
- Banks W A, Coon A B, Robinson S M, Moinuddin A, Shultz J M, Nakaoke R, Morley J E. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 2004; 53: 1253–1260
- Morley J E, Baumgartner R N, Roubenoff R, Mayer J, Nair K S. Sarcopenia. J Lab Clin Med 2001; 137: 231–243
- Ceccarelli E, Donati C, Forconi S, Masotti L. C-reactive protein, physical disability, and prognosis in very old patients with ischemic stroke. J Gerontol Med Sci 2002; 57A: M520–M522
- Cesari M, Penninx B WJH, Pahor M, Lauretani F, Corsi A M, Williams G R, Guralnik J M, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol Med Sci 2004; 59A: 242–248
- Morley J E, Baumgartner R N. Cytokine-related aging process. J Gerontol Med Sci 2004; 59A: 924–929
- Morley J E, Kaiser F E, Perry H M, III, Patrick P, Morley P M, Stauber P M, Vellas B, Baumgartner R N, Garry P J. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exper 1997; 46: 410–413
- Matsumoto A M. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol Med Sci 2002; 57A: M76–M99
- Bhasin S. Testosterone supplementation for aging-associated sarcopenia. J Gerontol Med Sci 2003; 58A: 1002–1008
- Baumgartner R N, Waters D L, Gallagher D, Morley J E, Garry P J. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Devel 1999; 107: 123–136
- Morley J E, Perry H M, III, Kaiser F E, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry H M, Jr. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 1993; 41: 149–152
- Sih R, Morley J E, Kasier F E, Perry H M, Patrick P, Ross C. Testosterone replacement in older hypogonadal men – a 12-month randomized controlled trial. J Clin Endocrin Metab 1997; 82: 1661–1667
- Wittert G A, Chapman I M, Haren M T, Mackintosh S, Coates P, Morley J E. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol Med Sci 2003; 58A: 618–625
- Bakhshi V, Elliott M, Gentili A, Godschalk M, Mulligan T. Testosterone improves rehabilitation outcomes in ill older men. J Am Geriatr Soc 2000; 48: 550–553
- Amory J K, Chansky H A, Chansky K L, Camuso M R, Hoey C T, Anawalt B D, Matsumoto M, Bremner W J. Preoperative supraphysiological testosterone in older men undergoing knee replacement surgery. J Am Geriatr Soc 2002; 50: 1698–1701
- Perry H M, III, Horowitz M, Morley J E, Patrick P, Vellas B, Baumgartner R, Garry P J. Longitudinal changes in serum 25-hydroxyvitamin D in older people. Metab Clin Exper 1999; 48: 1028–1032
- Gallagher J C. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Molec Biol 2004; 89–90(1–5 Special Issue)497–501
- Bischoff-Ferrari H A, Dawson-Hughes B, Willett W C, Staehelin H B, Bazemore M G, Zee R Y, Wong J B. Effect of vitamin D on falls – a meta-analysis. JAMA 2004; 291: 1999–2006
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton E R, Sweeney H L, Rosenthal N. Localized IGF-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nature Genetics 2001; 27: 195–200
- Schuelke M, Wagner K R, Stolz L E, Hubner C, Riebel T, Komen W, Braun T, Tobin J F, Lee S J. Brief report –myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 2004; 350: 2682–2688
- Candow D G, Chilibeck P D, Chad K E, Chrusch M J, Davison K S, Burke D G. Effect of ceasing creatine supplementation while maintaining resistance training in older men. J Aging Physical Activity 2004; 12: 219–231
- Chrusch M J, Chilibeck P D, Chad K E, Davison K S, Burke D G. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exercise 2001; 33: 2111–2117
- Rawson E S, Wehnert M L, Clarkson P M. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol 1999; 80: 139–144
- Wilson M MG, Morley J E. Aging and energy balance. J Appl Physiol 2003; 95: 1728–1736
- Morley J E. Anorexia and weight loss in older persons. J Gerontol Med Sci 2003; 58A: 131–137
- Kamel H K, Phlavan M, Malekgoudarzi B, Gogel P, Morley J E. Utilizing pain assessment scales increases the frequency of diagnosing pain among elderly nursing home residents. J Pain Symptom Mgt 2001; 21: 450–455
- Baldwin A C, Stevenson S W, Dudley G A. Nonsteroidal anti-inflammatory therapy after eccentric exercise in healthy older individuals. J Gerontol Med Sci 2001; 56A: M510–M513
- Banks W A, Morley J E. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol Med Sci 2003; 58A: 314–321
- Morley J E. A fall is a major event in the life of an older person. J Gerontol Med Sci 2002; 57A: M492–M495
- Johansson B B. Functional and cellular effects of environmental enrichmentafterexperimentalbraininfarcts. Restorative Neurology & Neurosci 2004; 22: 163–174
- Rosenthal M J, Morley J E, Flood J F, Scarpace P J. Relationship between behavioral and motor responses of mature and old mice and cerebellar beta-adrenergic receptor density. Mech Ageing Devel 1988; 45: 231–237
- Doraiswamy P M, Krishnan K R, Oxman T, Kenkyn L R, Coffey D J, Burt T, Clary C M. Does antidepressant therapy improve cognition in elderly depressed patients?. J Gerontol Med Sci 2003; 58: M1137–M1144
- Blazer D G. Depression in late life: review and commentary. J Gerontol Med Sci 2003; 58A: 249–265
- Tur B S, Gursel Y K, Yavuzer G, Kucukdeveci A, Arasil T. Rehabilitation outcome of Turkish stroke patients: in a team approach setting. Int J Rehab Res 2003; 26: 271–277
- Fitten L J, Morley J E, Gross P L, Petry S D, Cole K D. Depression. J Am Geriatr Soc 1989; 37: 459–472