Abstract
Accurate measurement of serum testosterone (T) is essential for proper diagnosis of androgen deficiency. There are now several modern assay technologies, including automated ones, for measurement of T. In this study, we compared analytical performance of five modern immunoassay technologies commonly used for measurement of total T: Vitros ECi (Ortho-Clinical Diagnostics; normal range (n.r.) 4.6–34 nmol/L); Architect (Abbott Laboratories; n.r. 9.7–34 nmol/L); Access (Beckman Coulter; n.r. 5.3–23 nmol/L); Delfia (Perkin-Elmer; n.r. 9.3–34 nmol/L); and manual EIA DRG kits (n.r. 8.3–42 nmol/L), with the classical RIA (3H–T), after extraction (n.r. 11–33 nmol/L), as a reference method. Total T was measured using all above-mentioned methods in serum samples from 100 male patients, aged 16–65 years. Mean T concentrations in these 100 serum samples assayed by all non-isotopic methods were statistically significantly higher than those obtained by RIA. Delfia showed the highest T levels (19.3 nmol/L versus 12.1 nmol/L by RIA) with a positive bias 60–100%. Almost similar results were obtained using Architect, with a positive bias 40–70%. The closest correlation in results was found between Vitros ECi and RIA (12.7 nmol/L versus 12.1 nmol/L). In the studied samples, the median of differences ranged from minimal (−0.4 nmol/L for Vitros ECi) to maximal (−7.25 nmol/L for Delfia). For all non-isotopic methods, with the exception of Vitros ECi, differences in subjects with low T level (<10 nmol/L) were statistically significantly larger than in the subjects with high T (T > 10 nmol/L). All other methods showed different degrees of dissimilarities with the RIA, especially in the range of low testosterone concentrations, which is of importance in the clinical assessment of women and pubertal boys.
| Abbreviations | ||
| AE | = | acridinium ester |
| AP | = | alkaline phosphatase |
| DCL | = | direct chemiluminescence |
| ICL | = | indirect chemiluminescence |
| M-at | = | monoclonal anti-testosterone antibody |
| P-at IgG | = | polyclonal anti-testosterone antibody |
| SA | = | streptavidin |
Introduction
Measurement of serum testosterone plays an important role in the clinical evaluation of a number of very common endocrine disorders. In males, testosterone assays are used primarily to confirm/reject the diagnosis of hypogonadism or PADAM, to evaluate boys with delayed or precocious puberty and to monitor the adequacy of replacement therapy.
Determination of the circulating testosterone concentration, along with luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels, provides information about the etiology of testicular dysfunction. It is very important to measure testosterone when monitoring those patients with prostate cancer treated with releasing hormone (LH–RH) analogs.
In women, it is necessary to determine the level of testosterone in different pathologic states, such as hirsutism, alopecia, acne, suspected androgen-secreting tumors, and ovarian and adrenal tumors. Testosterone is an important diagnostic marker in the follow-up of patients with congenital adrenal hyperplasia (CAH) receiving treatment with glucocorticoids. All these conditions and diseases require highly sensitive and reproducible methods of testosterone determination in biological fluids.
Total testosterone was previously measured using the classical radioimmunoassay (RIA) procedure after extraction, with use of highly specific antisera, with procedures carried out by qualified personnel. The results were accurate, reliable, and reproducible measurements of total testosterone. But as time passed, new non-isotopic, non-extraction methods rapidly emerged, such as EIA, ELISA, and third generation methods, based on the principles of enhanced chemiluminescence, time-resolved fluorescence, and some others.
The vigorous increase in the use of these methods gave rise to some problems. The majority of modern commercial assay kits are based on the above-mentioned technological principles, and total testosterone measured with these kits is often either too high or too low when compared to results obtained using more standardized techniques. Sometimes this difference is so pronounced that it leads to misdiagnosis of PADAM and other forms of hypogonadism.
Since 1981, the Laboratory of Biochemical Endocrinology and Hormonal Analysis has been using the classical RIA method, with extraction, for the measurement of total testosterone, employing highly specific monoclonal antibodies. The same method was used in the WHO Human Reproduction Program and calibrated against the reference GC–MS method. With the RIA method, the range of normal concentrations for total testosterone is 12–35 nmol/L in healthy men of reproductive age.
The WHO External Quality Assessment (EQA) Scheme aims to monitor the performance of centers collaborating with WHO's Special Program of Research in multicenter clinical evaluation of fertility regulating agents. The objectives of the scheme are to detect and quantify bias of a specific laboratory using the standard method, with the intention of improving between-laboratory outcomes of measurements of testosterone, and to identify and assist laboratories that are experiencing difficulties in this regard. The standard in this between-laboratory comparison is the same as our classical RIA method, which we took as the ‘golden standard’ in this research in which we evaluated the analytical performance of a few modern immunoassay technologies widely used for total testosterone measurement in men of different age.
Materials and methods
Serum samples from 100 male patients were provided by National Center of Endocrinology, Moscow, Russia and the outpatient clinic for routine diagnostics, to determine the possible presence of androgenic disorders. All measurements of total testosterone were made in our laboratory using all above-mentioned 5 non-isotopic methods, with RIA as the reference method.
Patient age ranged from 16 to 65 years. Blood samples were taken from fasted subjects in the morning (9:00–10:00). Serum of each patient was aliquoted by 0.5 ml into 7 vials (one aliquot for each assay method), and kept at −20°C until analysis. No sample underwent additional thawing and refreezing.
Total testosterone measurement was performed using the following technologies:
Vitros ECi (Ortho-Clinical Diagnostics), automated multianalyte analyzer
Architect (Abbott Laboratories), automated multianalyte analyzer
Access (Beckman Coulter), automated multianalyte analyzer
Delfia (Perkin-Elmer), multianalyte analyzer
DRG, enzymeimmunoassay kits
RIA (3H–T), after extraction, WHO matched reagents.
Table I. Main analytical characteristics, as defined by manufacturers of five non-isotopic and one isotopic testosterone immunoassays.
Statistical analysis
The results of serum T measurements obtained by non-isotopic methods were compared to the results of the RIA measurement, serving as our standard, to determine the degree of agreement between methods with use of Statistica program (StatSoft Inc, USA, 1999).
As serum T concentrations were not normally distributed, we estimated the median and geometric mean of the values obtained using the different methods. The T concentrations measured by each assay and RIA were then compared using of the nonparametric Wilcoxon matched-pairs test. The correlations between RIA (independent variable) and other tested methods (dependent variables) were examined by simple linear regression models. The ratio of the result from each assay and the RIA method was calculated to assess the distribution of the values obtained by each assay compared with RIA and the magnitude of the discrepancy.
Results
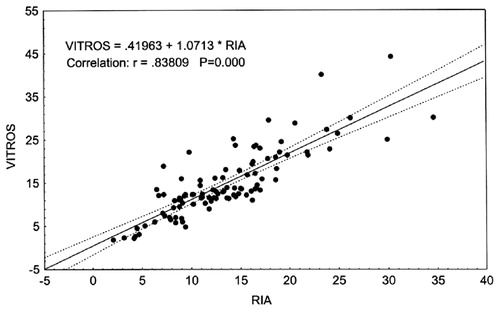
Mean serum testosterone concentrations in samples from 100 men assayed by all non-isotopic methods were statistically significantly higher than the concentrations measured by RIA (see ). The highest levels of testosterone were measured by the Delfia method (19.3 nmol/L versus 12.1 nmol/L in RIA). The Vitros ECi testosterone measurement results were very similar to those obtained by RIA (12.7 nmol/L versus 12.1 nmol/L).
Table II. Testosterone concentration in serum samples from 100 men obtained by RIA and five non-isotopic methods.
The results of testosterone measurement were highly variable between the different immunoassays. All methods showed significantly different geometric means and median testosterone values when compared with RIA. In addition, geometric means and median testosterone values were significantly different, not only when compared with RIA, but also when compared with the various immunoassay methods, the exception being Vitros-Access (p = 0.105).
In the samples analyzed, the median for differences ranged from minimal (−0.4 nmol/L for Vitros ECi assay) to maximal (−7.25 nmol/L for the Delfia assay) (see ).
Table III. Mean differences in T concentrations between RIA and tested immunoassays.
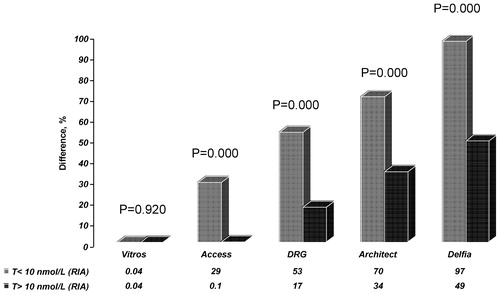
The mean difference in T concentrations obtained by RIA and Vitros ECi was 0.03%, whereas the interval of individual differences ranged from −49% to + 163%. The greatest differences were found to exist between RIA and the Delfia assay (65%) with individual differences ranging from −61% to + 951% (see ). For all non-isotopic methods, with exception of the Vitros ECi assay, differences were more pronounced in those individuals with low testosterone levels (<10 nmol/L, as determined by RIA) and statistically significantly larger than in those individuals with higher testosterone (T > 10 nmol/L, as determined by RIA; see ).
Table IV. Differences (%) in testosterone concentrations obtained by 5 non-isotopic methods and by RIA.
Figure 1. Percentage differences in testosterone concentrations measured by RIA and non-isotopic methods in two male subgroups, with RIA T < 10 nmol/L and RIA T > 10 nmol/L.
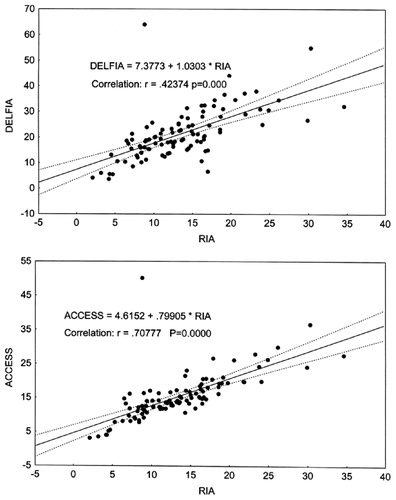
Regression analysis of the results showed that testosterone concentrations determined by RIA correlated reliably with those obtained with other non-isotopic immunoassay methods. The strongest correlation was found to exist between RIA and Vitros ECi, and the weakest correlations were seen to be between RIA and Delfia, and RIA and Architect (see ).
Figure 3. Correlation between total T concentrations (nmol/L)measured by RIA, Architect (upper plot) and DRG (lower plot).
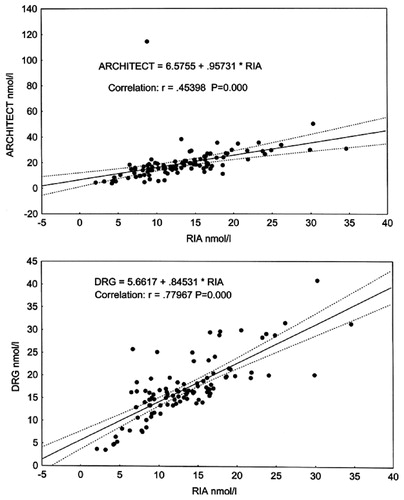
Figure 4. Correlation between total T concentrations (nmol/L)measured by RIA, Delfia (upper plot) and Access (lower plot).
In the subjects with low testosterone levels (<10 nmol/L, determined by RIA), correlation between RIA and Access, Delfia, DRG, and Vitros ECi was rather weak, and correlation between RIA and Architect was almost negligible (). In contrast to this, those subjects with high testosterone level (>10 nmol/L, determined by RIA) showed stronger correlation between RIA and Access, Delfia, DRG, and Vitros ECi (). The results for samples analyzed by ratio method are shown in .
Table V. Comparison between testosterone results obtained using RIA and 5 non-isotopic immunoassays for samples in a subgroup with low testosterone concentrations (RIA T results < 10 nmol/l; n = 32) using regression analysis.
Table VI. Comparison between testosterone results obtained using RIA and 5 non-isotopic immunoassays for samples in a subgroup with high testosterone concentrations (RIA t results > 10 nmol/l; n = 68) using regression analysis.
Figure 5. Ratio of testosterone concentrations measured by Access, Vitros and RIA in two subgroups of men, with RIA measuring T < 10 nmol/L and T > 10 nmol/L.
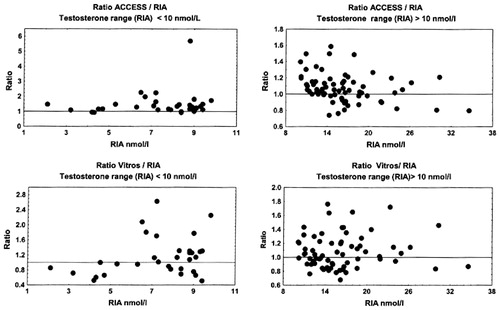
Figure 6. Ratio of testosterone concentrations measured by Architect, Delfia and RIA in two male subgroups, with RIA measuring T < 10 nmol/L and T > 10 nmol/L.
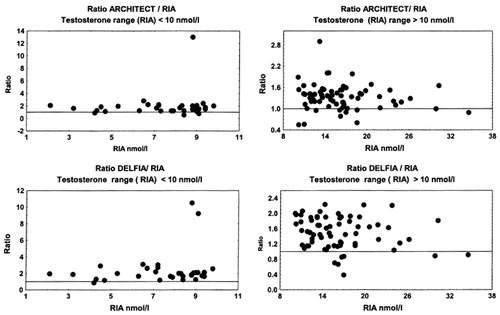
Figure 7. Ratio of testosterone concentrations measured by DRG and RIA in two male subgroups, with RIA measuring T < 10 nmol/L and T > 10 nmol/L.
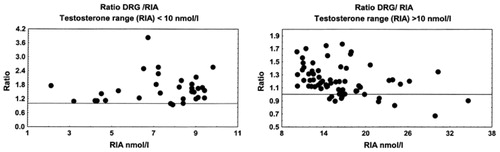
All non-isotopic immunoassay methods, with exception of Vitros ECi, showed a systematic positive bias in comparison with the results obtained by RIA. This was even more obvious in subjects with low testosterone concentrations (RIA T results < 10 nmol/L).
We investigated the capacity of the tested non-isotopic methods to adequately discriminate between normogonadal and men with partial androgen deficiency. In 38 out of 100 patients, low testosterone concentrations (2.1–9.8 nmol/L) were measured by RIA, all being under the low limit of normal range for testosterone, which is 12 nmol/L in our laboratory.
Comparing the results of testosterone measurement by non-isotopic methods in the same 38 patients, we found that each assay method used would establish the laboratory diagnosis of partial androgen deficiency in only a limited number of cases when compared to the RIA, if the above criteria for normal range are used:
for Vitros ECi – 22 patients out of 38 (58% of subjects)
for Access – 17 patients out of 38 (45% of subjects)
for Architect – 16 patients out of 38 (42% of subjects)
for DRG – 14 patients out of 38 (37% of subjects)
for Delfia – 9 patients out of 38 (24% of subjects).
Discussion
It is known that the modern non-isotopic immunoassay methods that are widely used to measure testosterone in human samples without extraction produce a high interassay variability Citation[1]. Usually, commercially available assays, measuring testosterone directly, generally overestimate concentrations of the different steroids Citation[2]. This becomes a source of serious concern. The classical RIA using iodinated testosterone assay also produced non-uniform results Citation[1]. In contrast, RIA procedures that use tritiated testosterone combined with extraction, which eliminates binding globulin and structurally related molecules and some lipids, measure values of testosterone concentrations corresponding to those measured by GC–MS Citation[2]. But also with this method, the degree of agreement between the RIA with the measurement by GC–MS varied depending on testosterone concentration. Nevertheless, it is generally accepted that GC–MS and tritiated testosterone RIA are the basic methods used in reference laboratories Citation[3],Citation[4].
Procedures based on non-isotopically labeled analytes (so-called third generation technology), including those based on enhanced chemiluminescence principles and adapted for use in automated analyzers, are now available and widely used in routine clinical laboratories Citation[5],Citation[6].
However, questions concerning the extent of agreement between several available analytical tests remain open. These methods provide concordant results primarily with samples from men with concentrations of testosterone in the male range, but not from women in whom testosterone concentrations, even if they are elevated, are considerably lower than those found in men. So, the poor performance of the newer assays is particularly obvious in the low range of serum testosterone values Citation[7]. The usual outcome of non-isotopic direct methods is an overestimation of testosterone concentrations in sera of men, but more so in women. Our findings are very similar to those reported by Wang et al. Citation[8] and by others Citation[9],Citation[10].
Our data obtained during this research clearly demonstrate that practically all tested non-isotopic methods show a poor agreement when using RIA as a reference method. The greatest differences were found with the Delfia kit utilizing polyclonal antibodies. In this case the positive bias exceeded 60%, and in the range of low testosterone concentrations (<10 nmol/L) the bias went up to 100% (). Regression analysis showed that the correlation between Delfia and RIA was weak, and that correlation was even poorer in the range of low testosterone concentrations.
The next automatic analytical method showing results close to those obtained by Delfia was Architect. Architect showed a positive bias of 40%, and it was 70% in the range of low testosterone concentrations (<10 nmol/L).
The strongest correlation was found between testosterone results determined by Vitros ECi and RIA. The difference between these methods was less than 0.04% over a full range of testosterone concentrations (), although individual differences between the two methods varied from −49% to + 169%. Testosterone results obtained using these two methods show a good correlation, although it is admittedly weaker in the range of low testosterone concentrations (<10 nmol/L). Testosterone concentrations measured by Vitros ECi could demonstrate androgen deficiency in 58% of subjects. If the normal range limits recommended in the Vitros ECi manual (4.6–28.2 nmol/L) are applied, the percentage of subjects with suspected androgen deficiency decreases to 19%. The same tendency was found for Access analyzer: if normal range limits recommended in the Access user manual are used, the percentage of subjects with suspected androgen deficiency drops markedly from 45% to 16%.
All these data emphasize the importance of proper and thorough selection of subjects for control groups in order to determine adequate normal ranges of testosterone concentrations.
There are several possible reasons for the lack of agreement between the results obtained with tested methods compared with RIA extraction assay. The first is the so-called matrix effect. The RIA procedure minimizes this effect, but it is clearly observed in non-isotopic assays. The second suggested reason for the lack of agreement between the results obtained with immunoassays tested and RIA include the limit of detection and functional sensitivity, which are particularly important for the determination of low testosterone concentrations. The third possible reason for the lack of agreement is the cross-reactivity of the polyclonal antibody (e.g., Delfia) when compared to monoclonal antibodies (all others, including our RIA). A final, important, source of possible disagreement between methods is the preparation of standards for testosterone.
According to our results, the most reliable immunoassay for testosterone measurement is Vitros ECi. Our results do not permit us to define a specific strategy or algorithm for each of the assays, to determine how automated non-isotopic testosterone immunoassay methods can and should be used to measure the androgenic status of male individuals in clinical practice. Neither of studied methods (except RIA) could provide accurate quantification of testosterone levels in the low range of concentrations for men of different age groups. This is particularly relevant for testosterone measurements of prepubertal subjects and women.
Acknowledgements
The authors would like to thank Schering AG, Germany for providing support for this study.
References
- Boots L R, Potter D, Azziz R. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril 1998; 69: 286–292
- Wudy S A, Wachter U A, Homoki J, Teller W M. 17α-hydroxyprogesterone, Δ4-androstenedione and testosterone profiled by routine stable isotope dilution/gas chromatography-mass spectrometry in plasma of children. Pediatr Res 1995; 38: 76–80
- Siekmann L. Determination of steroid hormones by the use of isotope dilution-mass spectrometry: a definite method in clinical chemistry. J Steroid Biochem 1979; 11: 117–123
- Lawson A M, Gaskell S J, Hjelm M. International Federation of Clinical Chemistry (IFCC), Office for Reference Methods and Materials (ORMM). Methodological aspects on quantitative mass spectrometry used for accuracy control in clinical chemistry. J Clin Chem Clin Biochem 1985; 23: 433–441
- Gosling J P. A decade of development in immunoassay methodology. Clin Chem 1990; 36: 1408–1427
- Wheeler M J. Automated immunoassay analysers. Ann Clin Chem 2001; 38: 217–229
- Jockenhövel F, Haase S, Hoermann R, Mann K. New automated direct chemiluminiscent immunoassay for the determination of serum testosterone. J Clin Ligand Assay 1996; 19: 138–144
- Wang C, Catlin D H, Demers L M, Starcevich B, Swerdloff R S. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocr Metab 2004; 89(2)534–543
- Taieb J, Mathian B, Millot F, Patricot M -C, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem 2003; 49(8)1381–1395
- Miller K K, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocr Metab 2004; 89(2)626–633