Abstract
The accurate measurement of testosterone remains a challenge. The determination of the blood testosterone concentrations in serum by conventional immunoassays is inaccurate in men and even more so in females and children. A new luminescence enzyme immunoassay (LIA) has been developed and validated. The high analytical (8.7 pmol/L) and functional (17.3 pmol/L) sensitivity allows the quantification of the very low concentration in saliva, as well as in serum, after 1/40 dilution. This study measured salivary testosterone levels and compared the results with the free levels calculated from total testosterone and sex hormone-binding globulin in eugonadal and hypogonadal men. Salivary testosterone concentrations in healthy men in morning hours were 369 pmol/L (mean), range 263–544 pmol/L, which was statistically significantly higher than that in men with androgen deficiency, 215 pmol/L (mean), range 51–249 pmol/L.
Repetitive determination of free testosterone concentrations in saliva (once a week for 5 weeks) showed high stability of results over time, with coefficient of variation 9% (range 5–23%).
In this study we showed that free salivary testosterone levels in morning samples correlated well with calculated free testosterone in blood, both in healthy men (R = 0.754, P = 0.001), and in patients with androgen deficiency (R = 0.889, P = 0.0001), though in cases with very low testosterone, salivary concentrations were systematically higher than calculated free testosterone levels in blood.
Keywords:
Introduction
At present, from clinical and research points of view, there is an unfortunate lack of consistency in use of the phrase ‘assessment of testosterone status’. This phrase is often used as a reference to a measure of biologic androgen activity, rather than to the specific measurement of the concentration of the testosterone as a biological active chemical molecule. The measurement of total testosterone concentration in sera is subject to considerable methodological different outcomes when using modern platform immunoassay technologies Citation[1],Citation[2]. In comparison with gas chromatography-mass spectrometry (GC-MS), all tested methods produced serious deviations, especially for the measurement of low level of testosterone, as in women and children. Our own data, published recently, confirm these observations Citation[3]. The variations in results do not permit to define a specific strategy or algorithm, for each of five tested platform assays, to determine how automated non-isotopic testosterone immunoassay methods can and should be used to measure the androgenic status of male individuals in clinical practice. None of the studied methods (except radioimmunoassay (RIA) with preanalytical extraction) provided accurate quantification of testosterone levels in the low range of concentrations for men of different age groups. This is particularly relevant for total testosterone measurements of prepubertal subjects, women, and late-onset hypogonadism in the aging male.
In addition, it remains an unresolved question which parameter (total testosterone, bioavailable testosterone or free testosterone) is best suited for assessment of androgen activity. When measurement techniques are compared, the results correlate with each other, but the outcome is certainly not unambiguous. There is a need for consensus as to which parameter of hormones should be measured to best assess androgen status, and there is a need to standardize the procedures used to measure hormones in different biological fluids.
Accurate measurement of serum free testosterone levels is necessary to diagnose androgen deficiency in women. Recently published data by Miller et al. demonstrated that a calculated value for free testosterone, using the mass action equation, is an acceptable substitute for measurement by equilibrium dialysis in normal women and women with androgen deficiency Citation[4]. However, accuracy of both methods depended strongly on the validity of both total testosterone measurement, as well as the sex hormone-binding glomerular (SHBG) assay and assumptions of SHBG binding conditions, which are variable among populations of women. The second method has some disadvantages too: it is rather expensive as it requires the measurement of the total concentration of testosterone and SHBG.
Saliva attracted the attention of researchers many years ago, as salivary glands possess a very particular feature to pass (by passive filtration through the membrane barrier into the salivary ducts) some substances which circulate in blood, including free steroids, not bound to SHBG and albumin. Lipid-soluble unconjugated steroids, such as testosterone, enter saliva predominantly via the intercellular route, produce salivary concentrations, which are not dependent upon saliva flow rate, and closely approximate their unbound sera level Citation[5]. Free testosterone, which is the biologically active form in target tissues, being measured by adequate technology, might become a good marker for the assessment of androgenic status both in men and in women. The first efforts to measure salivary steroids employing physicochemical methods were made about 50 years ago, but rather low sensitivity of these methods prevented their use in clinical diagnostics. Invention of RIA-methods in 1960 gave a strong impetus to use saliva, a biological material in which a wide spectrum of hormonal steroids could possibly be determined: androgens (testosterone, androstenedione, dehydroepiandrosterone (DHEA) and its sulfates), estrogens (estriol in pregnant women, estradiol and estrone), gestagens (progesterone and some others) and other steroid hormones (cortisol and 17-hydroxyprogesterone). Saliva collection is simple, non-invasive, and has great advantages over the traditional determination of steroids in venous blood, requiring qualified personnel. Besides this, blood withdrawal is an additional stress factor, capable of interfering with the results of hormonal measurements, particularly for testosterone.
A complete overview on the determination of salivary steroids by RIA has been presented in a book entitled ‘Immunoassays of steroids in saliva’ Citation[5-10]. Almost all researchers arrived at the conclusion that saliva offered great advantages for the determination of biologically available free steroids. Despite all the advantages of RIA methods, these methods were unable to provide sufficient sensitivity for the measurement of free steroids in saliva. The testosterone concentration in saliva is only about 1–2% of its concentration in blood. Therefore, large volumes of saliva, up to 3 ml, were required, in addition to an unpleasant procedure of extraction with diethyl-ether, which is a flammable and explosive solvent. As a result, this rather complicated method could not be adapted for routine use in biochemical laboratories for clinical diagnostics.
However, the invention of new immunoassay technologies, including those employing enhanced chemiluminescence Citation[11], combining ultra-sensitivity and high specificity, made it possible to determine free non-bound steroid hormones in very small volumes of saliva using direct methods. In the past, practically all methods of free steroid determination in blood, including dialysis and mathematical calculations, were not direct. The second method has one disadvantage; it is rather expensive, as it requires the measurement of the total concentration of steroid and the concentration of a binding protein. Dialysis as a golden standard is an excellent method, but it is problematic for use in routine diagnostics. Diagnostic kits for the direct measurement of free steroids, in particular testosterone in blood, have so far been yielding unreliable results Citation[12],Citation[13].
The correct estimation of free testosterone concentration in blood is based on measurement of total testosterone concentration. As indicated above, this is not an easy problem, as modern automated technologies of testosterone measurement produce quite diverse results Citation[1],Citation[14]. This study describes the measurement of salivary concentrations of testosterone in a number of subjects with a variety of clinical conditions.
Subjects and methods
Subjects
We selected the following groups of male volunteers:
Healthy male volunteers (n = 16), age 21–50 years (mean = 30 years). In this group, 12 men provided nine saliva samples (three in the morning, three in late afternoon, and three in the evening) following an approved sampling protocol as described below, in order to study the diurnal rhythm of free testosterone in saliva. The remaining four men provided only three morning saliva samples. All subjects from this group were healthy, as indicated by clinical examination, and blood and urine screening tests.
Healthy male volunteers (n = 15), age 38–55 years (mean = 48 years). Saliva was collected once a week in morning hours for 5 weeks. This group consisted of standardized subjects who were of the same profession, physical and emotional status, and daily regimen (wake-up time, dietary pattern).
Male patients (n = 14) with various etiologies of androgen deficiency, age 22–68 years (mean = 29.5 years). Ten men provided nine saliva samples each (similar to the men above), and four men provided only three morning saliva samples.
In one female-to-male transsexual receiving testosterone treatment, salivary testosterone levels were measured over the day.
All subjects included in this study gave their informed consent. The study was approved by the institute's review board on investigations in humans.
Saliva sampling
To investigate the short and long fluctuations of testosterone concentration, three saliva samples were collected during one hour, three times a day. Therefore, the patients were instructed to collect nine samples each:
Our experience showed that correct method of saliva sampling is the key determinant of achieving accurate and reproducible results of free testosterone measurement. The detailed procedure of saliva sampling was as follows.Saliva sampling was done using only special sampling devices: SaliCap equipped with a special straw. Sample was performed more than 30 minutes after eating, drinking, chewing gum, and teeth brushing. It took usually up to 2 minutes to collect the required amount of saliva, 0.6–0.8 ml.
Samples with even minimal coloration due to contamination with blood were discarded. All saliva sampling vials were properly labeled (patient name, date, time) using a permanent marker. Saliva samples can be stored in capped vials for up to 5 days at 20°C, 10 days at 2–8°C, and 6 months at −20°C until assays. Free testosterone was measured in 50 μL of saliva in duplicates.
Saliva collection was governed by the following rules:
Collection of saliva must not be interrupted and the straw should not be removed from the mouth or from the sampling vial.
Collection of saliva is easier if the upper end of a straw is squeezed.
Collection of saliva is best done in front of a mirror in order to control the process of vial infilling.
Methods
Salivary testosterone
The luminescent immunoassay (LIA) (IBL, Hamburg, Germany) used is based on the competition principle. An unknown amount of antigen present in the sample and a fixed amount of alkaline phosphatase conjugated 3-carboxymethyl oxime-testosterone derivative compete for the binding sites of the monoclonal antibody. After addition of the acridan-based luminescence substrate, the intensity of the glow luminescence measured is inversely proportional to the amount of the antigen in the sample. The assay was validated and got the 510 k submission of the FDA. The luminescence was measured on a Berthold MPL2 Luminometer, and a four parameter logistic was used for curve fitting. The calibration was established by use of testosterone ranging from 0 to 2635 pmol/L. The linear portion of the curve was between 34.7 and 693 pmol/L. The equivalent dose 50% of the curve was in the range 140–210 pmol/L. The slope of the curve at the turning point was between −0.9 and −1.1.
The detection range of the LIA encompasses the physiological range of testosterone concentrations in saliva and in 1:40 diluted serum samples. Cross reactivities (Abraham method) of the antiserum were measured against various compounds: 11β-hydroxytestosterone – 8.7%; 11α-hydroxytestosterone – 3.2%; 5α-dihydrotestosterone – 1.9%; androstendione – 0.8%; methyltestosterone – 0.44%; DHEAS – 0.07%; and other steroids <0.01%. We also tested the effect of different matrices on cross reactivities ().
Table I. Analytical specificity and cross reactivity in different matrices
Analytical sensitivity was calculated from the mean of the relative luminescence units (RLU) of the zero calibrator minus 2 standard deviations of 20 replicate analyses. The lowest detectable level that could be distinguished from the zero standard is 8.7 pmol/L. Functional sensitivity was determined from the inter-assay variation coefficient of very low concentrations in saliva samples. The lowest testosterone concentration that could be measured with a coefficient of variation below 20% is 17.3 pmol/L. The intra-assay variation, the inter-assay variation and the between-lot variation were determined by repeated measurements of control samples. The mean precision of five saliva samples in the range 69–1872 pmol/L was found to be 3.5%, 4.7% and 7.6% for intra-assay, inter-assay and between-lot variation.
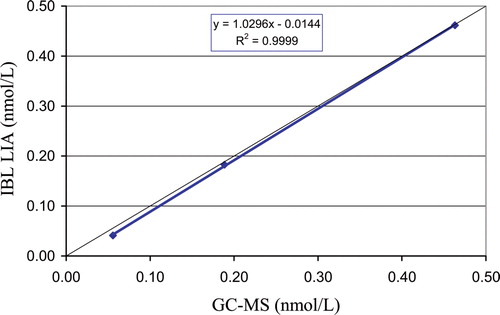
shows the result of direct comparison between GC-MS and testosterone LIA assay.
Figure 1. Correlation of chemiluminescence immunoassay (LIA) salivary testosterone with GC-MS performed in Professor Siekmann's Reference Laboratory, Bonn.
We estimated the analytical recovery of testosterone at five different concentrations. Increasing amounts of testosterone (22–1317 pmol/L) were added to the saliva samples. The mean recovery was 97.1% ± 11% (range 80–119%). The relation between expected and measured concentrations of saliva did not significantly deviate over the concentration range studied.
Five saliva samples having different testosterone levels (215–1816 pmol/L) were serially diluted with zero standard, and the testosterone concentrations in the diluted samples were assayed by the LIA. Five dilutions were performed for each sample. Each dilution was measured in duplicates in one assay run. No relevant deviation of the expected linearity was observed. The mean linearity CV for saliva was 108% ± 9% (range 90–129%).
Interferences of blood contamination in saliva were tested. Quality control samples with known concentration were enriched with specified amount of blood of the same person. Up to 0.2% blood content in saliva had no influence of testosterone concentration. Saliva samples with blood contamination of at least 0.2% are clearly red colored and must be excluded. The registration of chemiluminescence signal was done using Victor2 multilabel counter (Wallac).
Total serum testosterone (TT)
Serum T was measured in morning serum samples using the enhanced chemiluminescence immunoassay technology (Ortho-Clinical Diagnostics, J&J) with an automated analyzer Vitros ECi. The intra-assay coefficients of variation for 52.7 nmol/L and 2 nmol/L were 2.5% and 3.1%, respectively. The inter-assay coefficients of variation for 52.7 nmol/L and 2 nmol/L were 4.9% and 7.1%, respectively. The sensitivity of this assay was 0.03 nmol/L. Vitros ECi technology was chosen for this study as the one giving the closest results to those obtained with RIA after extraction Citation[3].
SHBG
Serum SHBG was assayed using time-resolved fluoroimmunoassay (Delfia) with an analyzer Autodelfia. The intra-assay coefficients of variation for 57 nmol/L and 19.7 nmol/L were 1.6% and 2.4%, respectively. The inter-assay coefficients of variation for 57 nmol/L and 19.7 nmol/L were 4.8% and 5.9%, respectively. The sensitivity of this assay was 0.5 nmol/L.
Calculation of free testosterone in serum
Free testosterone concentration in blood (fT) was estimated using the mathematical formula described by A. Vermeulen et al. Citation[13] and is available on-line at ISSAM site: http://www.issam.ch/freetesto.htm In this formula [T] is the concentration of total testosterone in blood serum.
| N: | = | Ka Ca = 1 |
| Ka: | = | association constant of albumin for T, 3.6 × 104 L/mol |
| Ca: | = | albumin concentration, 43 g/L |
| Kt: | = | association constant of SHBG for T, 109 L/mol |
Statistical analysis
Data analysis was performed using software program Statistica for Windows, StatSoft, Inc. (1999). Results are expressed as medians, standard deviations, 25th–75th and 10th–90th percentiles. The Mann-Whitney U test was used to determine the significance of differences between groups. Wilcoxon matched pairs test was used to determine the significance of the differences between correlated parameters. The relationships between different variables were calculated by Spearmen rank test and simple linear regression analysis. P < 0.05 was considered significant.
Results
Group of healthy men
Mean values for age, total testosterone in blood (TT), SHBG and calculated free testosterone (cfT) in blood are presented in .
Table II. Characteristics of the study groups (median and 10–90th percentiles)
Age-matched healthy men and androgen-deficient men had similar levels of SHBG. Mean levels of free salivary testosterone in morning, afternoon, and evening samples are presented in .
Table III. Diurnal patterns of free salivary testosterone (fT) in healthy and androgen-deficient men (median and 10–90th percentiles) (testosterone levels in pmol/L)
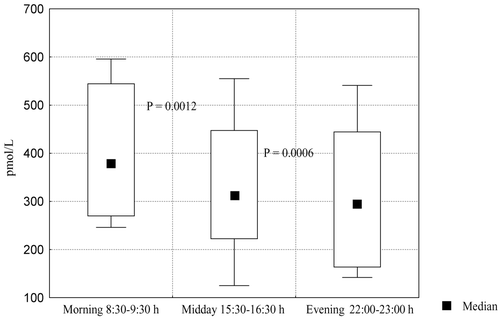
Mean concentration of testosterone in saliva of 12 healthy men (three morning samples from each, 8:30–9:30, 36 samples in total) was 380 pmol/L (270–544) pmol/L. Mean afternoon and mean evening concentrations of free testosterone in saliva (n = 36) were correspondingly 80 ± 15% and 71 ± 21% of morning concentrations. Diurnal patterns of salivary testosterone in healthy men are presented in .
shows individual data on free salivary testosterone and calculated blood free testosterone in healthy men. Levels of free testosterone in saliva may be slightly higher, lower, or equal to those of calculated free testosterone in blood.
Figure 3. Comparison of individual values of mean morning saliva testosterone (T) and blood calculated free testosterone (cfT) in healthy men.
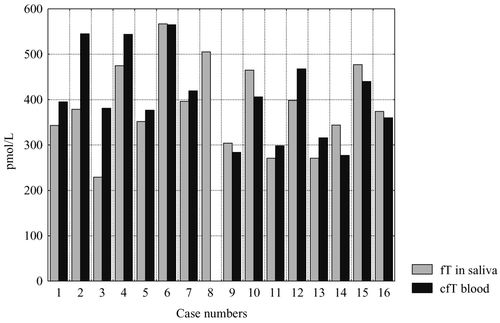
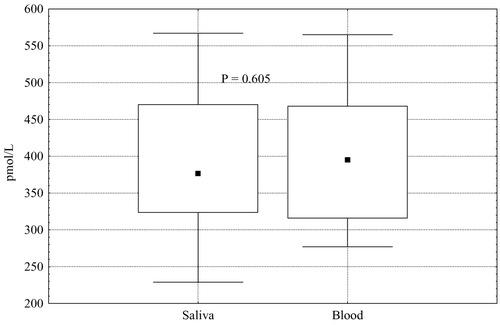
Mean concentrations of salivary free testosterone in the morning and calculated free testosterone in blood in healthy men did not differ significantly (P = 0.111) ().
Figure 4. Mean levels of morning salivary testosterone (T) and blood calculated free testosterone (cfT) in healthy men.
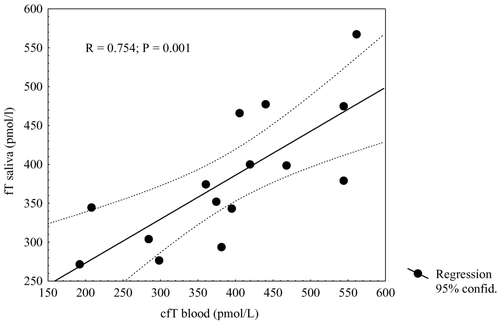
Results of regression analysis are given in (R = 0.769, P = 0.00080).
Figure 5. Relationship between calculated morning free testosterone (fT) in blood and average morning salivary testosterone (T) in healthy men.
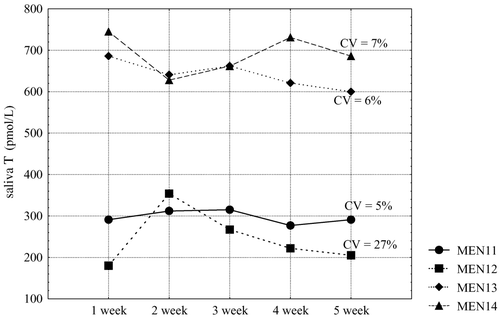
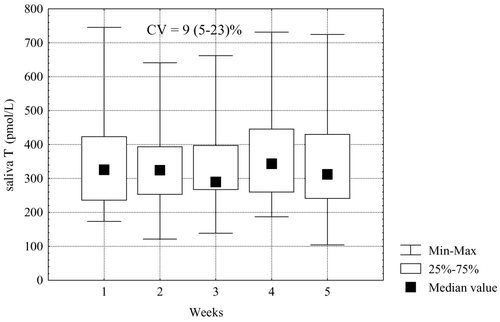
To study the reproducibility of free testosterone results in time we collected saliva samples in a group of healthy men during a period of 5 weeks (one sample a week). At the beginning of this study (week 1) free salivary testosterone concentrations in these subjects in morning hours ranged from 180 pmol/L to 745 pmol/L. Subsequent measurements made up to week 5 showed rather high reproducibility of results, with coefficient of variation 9% (5–23%) ().
Figure 6. Repeatability of salivary testosterone (T) measurement over five consecutive weeks (one sample a week) in healthy men.
To further illustrate this observation, we present the examples of individual dynamics in four men: two men with free salivary testosterone below the median (374 pmol/L), and two men above the median ().
Group of men with different forms of androgen deficiency
Characteristics of study men with androgen deficiency are shown in . Their free salivary testosterone is presented in .
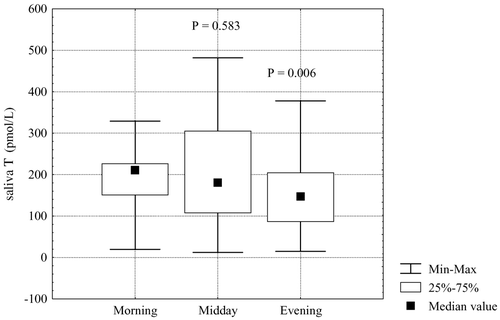
Unlike in healthy men, diurnal rhythm of free salivary testosterone in patients with androgen deficiency was less pronounced but still demonstrable ().
Concentrations of free salivary testosterone in morning hours and in the afternoon did not differ significantly. Statistically significant decreases of free salivary testosterone concentrations were observed only in the evening hours, being 68 ± 29% morning values.
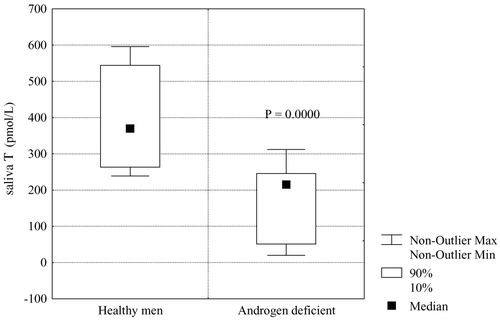
The median of morning salivary T concentration in patients with androgen deficiency was 215 pmol/L (range 51–249 pmol/L), which is considerably lower than those of healthy men ().
Figure 9. Comparison of mean levels of morning salivary free testosterone (fT) in healthy men and androgen-deficient men.
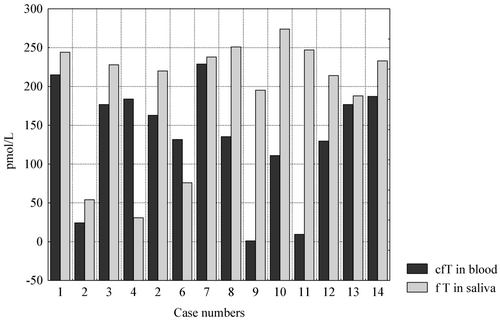
In this group, free testosterone concentrations in saliva often differed from those of calculated free testosterone in blood, with usually higher levels in saliva ().
Figure 10. Comparison of individual values of mean morning saliva free T (fT) and blood calculated fT in blood.
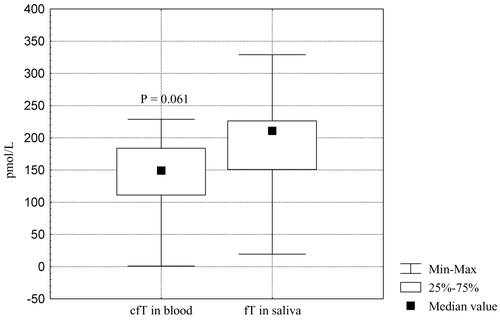
Median of free salivary testosterone in three morning samples was 215 pmol/L (range 55–249 pmol/L), being higher than the calculated free testosterone concentration in blood – 149 pmol/L (range 10–215 pmol/L) (P = 0.009) ().
Figure 11. Mean levels of morning salivary testosterone (T) and blood calculated free testosterone (cfT) in severely androgen-deficient men.
The biggest discrepancies were found in patientswith very low concentrations of total testosterone in blood and, to illustrate this, data of some individual patients are presented:
Patient 1 had prostate cancer and had undergone bilateral gonadectomy. Total testosterone concentration in blood was 0.2 nmol/L, and of SHBG level – 162 nmol/L. With this high SHBG level, the percentage of calculated fT in blood was 0.5% of total T only, whereas measured free salivary testosterone, 30 pmol/L, constituted more than 15% of total T in blood.
Patient 2 with primary hypogonadism. Total T concentration in blood – 1.2 nmol/L, and SHBG level – 104 nmol/L. Calculated fT in blood was 0.8% of total T, whereas the concentration of free salivary testosterone, 76 pmol/L, was more than 8% of total T in blood.
Patient 3 with Klinefelter's syndrome. Total T concentration in blood was 6.9 nmol/L, of SHBG – 34 nmol/L, and calculated fT in blood was 2% of total T. Free salivary testosterone concentration, 276 pmol/L, was about 4% of total T in blood.
Patient 4 (not diagnostically classified). Total T level in blood was 5.2 nmol/L, and SHBG – 26.5 nmol/L. Calculated fT in blood was 2.1% of total T. Free salivary testosterone level, 208 pmol/L, constituted 4% of total T in blood.
Patient 5 with hypergonadotropic hypogonadism. Total T level in blood was 1.4 nmol/L, and SHBG – 35 nmol/L. Calculated fT in blood was 1.7% of total T. Free salivary testosterone concentration (56 pmol/L) was 4% of total testosterone in blood.
Patient 6 with obesity and disturbed liver function. Total T was 6.1 nmol/L, with a very low level of SHBG – 4.2 nmol/L (normal range 12.9–61 nmol/L). Free salivary testosterone was 274 pmol/L (4.6% of total T), calculated fT in blood was 229 pmol/L.
Another important application of salivary testosterone is monitoring testosterone replacement therapy by measuring free salivary testosterone. An example of this is presented in .
Figure 12. Diurnal patterns of salivary testosterone (T) in a transsexual woman before and one week after Sustanon administration.
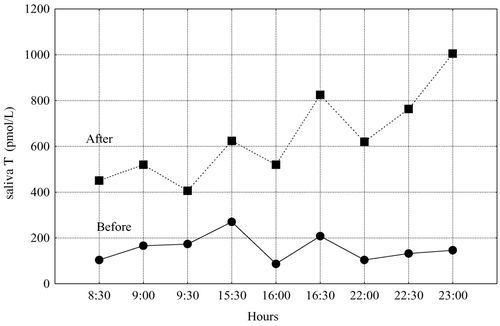
We monitored the treatment of transsexual woman (F→M) with Sustanon. Before treatment, the levels of total and free testosterone in blood were low (3.9 nmol/L and 42.3 pmol/L). One week after Sustanon injection, total testosterone levels had increased by 23%, while the free testosterone concentration in blood rose by 140%. But the most informative data about androgen overload in this woman came from the examination of free salivary testosterone diurnal dynamics: in morning saliva samples the testosterone rise was found to be 275%; in the evening saliva the highest testosterone rise was by 527%.
Androgen replacement therapy can be monitored more adequately by free testosterone in saliva measurement with LIA technology. It allows study of the pharmacokinetics more easily in individual cases, when it is important to adjust the dosage and mode of administration for individual patients.
Discussion
This study tested whether salivary testosterone measurement provides a reliable reflection of the unbound testosterone fraction in serum. Since free fraction is thought to determine exposure of the tissues to androgens, salivary testosterone concentration should provide a better indication of biologically active steroid than total serum levels, especially in conditions of altered SHBG binding. The technique of sampling is simple and non-invasive, and allows the collection of multiple samples with little inconvenience to the patient. Frequent collection and mailing of samples is practicable for the patients Citation[2].
The normal range of free salivary testosterone concentrations in healthy men in morning hours established in this research are in good agreement with those reported by Lac et al. Citation[15]. Their procedure involved: RIA after extraction and chromatographic separation, employing highly specific antiserum, produced a normal range of testosterone concentrations in saliva of 260 ± 14 pmol/L. These results are very similar to our data obtained by using direct non-extraction immunoassay method based on enhanced chemiluminescence technology employing highly specific monoclonal antibodies. The authors of above-cited publication reviewed the results on salivary testosterone measurement by eight other research groups, and in all of them reference concentrations of testosterone fell within the range of 233–314 pmol/L. Similar levels of salivary testosterone were reported by Corradi and Szathmari Citation[16]. According to their data, free salivary testosterone in patients with erectile dysfunction ranged from 198 pmol/L to 240 pmol/L, and in healthy volunteers it was 200–1000 pmol/L. These authors concluded that free testosterone in saliva could be a good marker of androgenic status in men. Nieschlag and coworkers used the measurement of free testosterone in saliva not only for the diagnosis of hypogonadism but also for the monitoring of the pharmacokinetics of administration of testosterone buciclate Citation[17].
In this study, we were also interested in monitoring salivary testosterone in healthy men over time, namely the variation of testosterone concentrations over a period of 5 weeks. shows that this variation is relatively small, with a coefficient of variation of 9% (5–23%). This observation is supported by the data on individual variations of salivary testosterone concentrations during this time period in two men having salivary testosterone below 374 pmol/L (median), and in two men with salivary testosterone higher than 374 pmol/L. Their levels of free testosterone were reproducible and therefore measurement of saliva testosterone levels allows a fair assessment of the androgenic status of men. Of course, further studies are necessary to disclose the biological role of diverse concentrations of circulating testosterone.
Our data showed a diurnal rhythm of salivary testosterone levels, very clear in eugonadal men but less so in hypogonadal men.
In deeply hypogonadal men, salivary testosterone concentrations tended to be higher than free concentrations in blood, as calculated from levels of total testosterone and of SHBG. It is not possible to determine, from the results of this study, which methods provided the most accurate assessment. The salivary testosterone is a direct measurement, while the serum free testosterone is based on a calculation which makes a number of assumptions. So, the likelihood of errors in the latter is somewhat larger than in a direct measurement. Since the difference in outcome is systematical – higher in saliva than in blood – this can be taken into account when interpreting results.
In conclusion, LIA technology for salivary testosterone measurement provides an acceptable reflection of the unbound testosterone in plasma. Since free fraction determines exposure of the tissues to testosterone, salivary testosterone concentration presents a better indicator of a biologically active steroid than total plasma levels, especially in conditions where SHBG-binding characteristics vary.
With the introduction of very sensitive and specific LIA technology of analysis, salivary testosterone determination can be widely used for diagnosis of different forms of hypogonadism in men and evaluation of androgen status in women, and become the method of choice for measurement of testosterone in scientific research, in pharmacokinetic studies of new testosterone preparations, and in monitoring of treatment of gonadal dysfunction.
References
- Wang C, Catlin D H, Demers L M, Starcevic B, Swerdloff R S. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 2004; 89: 534–543
- Taieb J, Mathian B, Millot F, Patricot M C, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women and children. Clinical Chemistry 2003; 49: 1381–1395
- Goncharov N, Katsya G, Dobracheva A, Nizhnik A, Kolesnikova G, Todua T, Lunenfeld B. Serum testosterone measurement in men: evaluation of modern immunoassay technologies. The Aging Male 2005; 8(3/4)194–202
- Miller K K, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab 2004; 89(2)525–533
- Vining R F, McGinley R A. Transport of steroids from blood to saliva. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff 1982; 56–63
- James V, Baxendale P. Androgens in saliva. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff, Wales 1982; 193–201
- Read G, Walker R. Variation of salivary testosterone with age in men: discussion. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff, Wales 1982; 215–220
- Schürmeyer T, Nieschlag E. Salivary and serum testosterone under physiological and pharmacological conditions. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff, Wales 1982; 202–209
- Walker R F, Read G F, Riad-Fahmy D, Griffiths K. The assessment of ovarian function by the radioimmunoassay of oestradiol-17-beta in saliva. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff, Wales 1982; 155–162
- Landon J, Mahmod S. Distribution of drugs between blood and saliva. Immunoassays of steroids in saliva, G F Read, D Riad-Fahmy, R F Walker, K Griffiths. Alpha Omega, Cardiff, Wales 1982; 47–55
- Albrecht S, Zimmermann T, Brandl H, Saeger H D, Distler W. Chemiluminescence at the turn of the millennium – from annual fair curiosity to indispensable tool of laboratory diagnostics. J Lab Med 1997; 21: 191–204
- Rosner W. Measurement of free testosterone in serum by DPC or DSL RIA – an extraordinarily inaccurate assay for free testosterone is still with us. J Clin Endocrinol Metab 2001; 86: 2903
- Vermeulen A, Verdonck L, Kaufmann J M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666–3672
- Matsumoto A M, Bremner W J. Serum testosterone assays – accuracy matters. J Clin Endocrinol Metab 2004; 89: 520–524
- Lac G, Lac N, Robert A. Steroid assays in saliva: a method to detect plasmatic contaminations. Arch Int Physiol Biochem Biophys 1993; 101(5)257–262
- Corradi G, Szathmari M. Serum and salivary testosterone levels in erectile dysfunction. Orv Hetil 1998; 139(34)2021–2024
- Tschop M, Behre H M, Nieschlag E, Dressendorfer R A, Strasburger C J. A time-resolved fluorescence immunoassay for the measurement of testosterone in saliva: monitoring of testosterone replacement therapy with testosterone buciclate. Clin Chem Lab Med 1998; 36(4)223–230