Abstract
Most persons with diabetes mellitus are over the age of 60 years. Males develop diabetes more commonly than females. Older diabetics tend to have both impaired insulin release as well as insulin resistance. In older persons diabetes mellitus is associated with decreased functional status and cognitive dysfunction. In general, older persons with diabetes are inclined to be underdiagnosed and undertreated. Managing diabetes in older persons requires special considerations because of their differences in pathophysiology of diabetes and strong association with functional, cognitive impairments and comorbidities. The use of strict therapeutic diets is not recommended in older persons. Treatment of hypertension and hyperglycemia can improve outcomes in older persons. The interdisciplinary team approach is important for care of older diabetic persons.
Introduction
I was eating bad stuff. Lots of sugar and carbs, junk food all the time. It makes you very irritated.
(Singer Avril Lavigne)
In 600 BC, Susruta, the Aryuvedic physician noticed that certain individuals had honey urine (‘madhumeha’) that was strongly attracting to ants. He also noted that there were two types of persons with honey urine, one being younger, thin people and the other older, fatter people. Thus, the concept that older persons had a different form of diabetes mellitus compared to younger persons has existed since the beginning of recorded medicine.
The prevalence of diabetes mellitus is rapidly increasing. In 2000, 2.8% of the world-wide population had diabetes, with an expected increase to 4.4% (or 366 million persons) by 2030. The majority of diabetics in developed countries are over 60 years of age. By the year 2030 there are expected to be almost twice as many older persons with diabetes in developing countries compared to the more developed ones Citation[1]. The largest number of diabetics live in India, China and the United States.
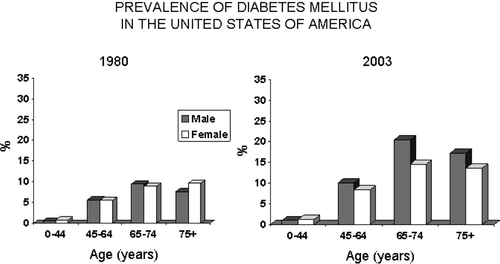
From 1980 to 2004 the prevalence of diabetes mellitus in the United States has doubled. Diabetes mellitus is more common in men than women (). At 65–74 years of age, 20.5% of men living in the United States are diabetic, and in the 75+ age group 17.3% are diabetic. Diabetes is undiagnosed in large numbers of the population and this occurs more often in men than women. In the 60–74 year age group 41.5% of men with diabetes were undiagnosed and in the 75+ age group 34.5% were not diagnosed Citation[2]. In nursing homes the prevalence of diabetes is approximately 30%, with the diagnosis being made in approximately one-third of those with diabetes. Although there is evidence that optimal glycemic control can reduce the rate of occurrence of the secondary complications of diabetes, as in younger persons, the majority of older persons with diabetes did not achieve American Diabetes Association (ADA) recommendations for glycemic control.
There are many similarities between diabetes mellitus and the aging process. Diabetes appears to accelerate aging by about a decade. Thus, both diabetes and aging at the biochemical level lead to a decline in DNA unwinding rate, increased collagen cross-linking, increased capillary basement membrane thickening and a decline in the Na+K+ATPase activity Citation[3]. Clinically, persons with diabetes have twice the prevalence of cataracts of the general population, accelerated atherosclerosis, cognitive decline and a decreased functional status.
Pathogenesis of diabetes mellitus in older persons
Older persons have their own characteristics for the pathogenesis of diabetes. Older diabetics tend to have both impaired insulin release as well as insulin resistance. Older persons with diabetes have a decrease in insulin-mediated glucose disposal, as well as a decrease in non-insulin mediated glucose uptake into organs such as the brain Citation[4]. However, they tend to have little change in fasting hepatic glucose output, unlike classic type II diabetics. Older persons with diabetes often tend to be less obese than younger or middle-aged diabetics Citation[5],Citation[6]. Older persons also tend to have more evidence of pancreatic islet cell dysfunction to produce insulin, compared to middle-aged Type II diabetics. Clinically, this results in older diabetics often presenting with a mixed hyperosmolar/ketoacidotic coma. However, the mechanism for the decline in islet cell function with aging is still uncertain. Because of these differences in the pathophysiology of diabetes in the older person, we have suggested that the classic diabetes in an older person should be considered a type 1 ½ () Citation[7]. In the New Mexico Aging Process study, we showed that persons who reach 70 years of age, with no evidence of glucose intolerance are very unlikely to develop diabetes mellitus over the next 14 years Citation[8].
Table I. Differences in the pathophysiology of diabetes mellitus in the young, middle-aged and old.
The reason for insulin resistance occurring with aging appears to be due to accumulation of lipid within muscle Citation[9]. This can either be due to a mitochondrial defect resulting in decreased utilization of intramuscular lipid or hyperlipidemia leading to myosteatosis. Increased intracellular lipid leads to altered phosphorylation of the insulin receptor substrate resulting in decreased activity of the glucose transporters.
Adiponectin is a fat cell hormone belonging to the tumor necrosis factor superfamily Citation[10]. It enhances insulin sensitivity and decreases triglycerides. In animal models it reverses insulin resistance. In the elderly, levels of adiponectin are higher in lean compared to obese subjects and in women Citation[11]. Insulin resistance was negatively correlated with adiponectin levels and positively correlated with leptin levels. Its potential role in diabetes of older men has yet to be determined.
Cytokines, such as tumor necrosis alpha, have been implicated in the production of insulin resistance. Aging is often associated with elevated cytokines due to visceral obesity and a low level inflammatory state. The role of cytokines in the modulation of insulin resistance in older men requires further exploration.
The metabolic syndrome (insulin resistance syndrome) which was originally described by Camus in 1966 Citation[12], is associated with an increased risk of diabetes and is extremely common in older persons. Its prevalence is almost one-fourth of adult Americans Citation[13]. The metabolic syndrome consists of hyperinsulinemia, hypertension, glucose intolerance, hyperuricemia, altered clotting abnormalities (such as an elevated plasminogen activating inhibitor 1), lipid abnormalities (including increased triglycerides, decreased high density lipoprotein lipase and increased small dense low density lipoprotein), myosteatosis, nonalcoholic steatotic hepatitis and possibly cognitive dysfunction. It is caused by an interaction of decreased exercise and overeating with a genetic milieu that promotes its development Citation[14]. It is not surprising that the metabolic syndrome predicts future diabetics, since glucose intolerance is a component of the metabolic syndrome. To date, the most accepted pathophysiology of the metabolic syndrome is insulin resistance. It is unclear, however, whether in older persons with metabolic syndrome the insulin resistance is primary cause or secondary result of coexisting illness.
Reasons for maintenance of euglycemia in older diabetics
The major reasons for maintenance of euglycemia in older persons with diabetes are listed in. There is evidence that age interacts with diabetes to accelerate the rate at which secondary complications, such as retinopathy, neuropathy and nephropathy, occur Citation[15]. Progression rate of retinopathy in older persons has been shown to be directly related to their HbA1C level Citation[16]. Persons with diabetes are more likely to develop infection, including recurrence of tuberculosis Citation[17]. Osmotic diuresis from elevated glucose levels leads to nocturia with interference of sleep, increased incontinence and dehydration. Dehydration is particularly likely to occur in older persons because of their impaired thirst drive Citation[18].
Table II. Reasons for the maintenance of euglycemia in older persons.
Diabetics are more likely to complain of pain than other persons with chronic diseases Citation[19]. Infusion of glucose into healthy young men decreased both the pain threshold and the maximal pain tolerated Citation[20]. Older male diabetics could tolerate less pain than age-matched non-diabetics. Glucose appears to decrease pain-tolerance by inhibiting the ability of beta-endorphin to down-regulate perceived pain.
Diabetes, besides being associated with an increased incidence of vascular ulcers, is also associated with a higher incidence of pressure ulcers Citation[20]. In diabetics, these ulcers tend to heal slower.
Postprandial hypotension is associated with an increase in falls, syncope, stroke, myocardial infarction and death Citation[21]. It is stimulated by carbohydrate which releases the vasodilatory peptide, calcitonin gene related peptide Citation[22]. Postprandial hypotension is more common in persons with diabetes mellitus.
Aretaeus, the Cappadocian (∼AD80) stated that diabetes is a disease of the stomach. Diabetes, especially when poorly controlled, delays stomach emptying Citation[23]. This can interact with the delayed stomach emptying already seen in older persons Citation[24]. Poor glycemic control compared to good control is associated with a marked increase in gastrointestinal symptoms including dysphagia, heartburn, nausea, postprandial fullness, diarrhea, constipation and fecal incontinence.
Zinc deficiency occurs in approximately 10% of older diabetics Citation[25],Citation[26]. Diabetes is associated with impaired zinc absorption. In addition, glucose infusion results in hyperzincuria. Zinc deficiency has been associated with decreased phytohemoglutinin T cell proliferation, increased lipid peroxidation, decreased zinc serum thymic factor, decreased antioxidant activity, decreased wound healing and decreased testosterone levels Citation[27].
Impaired cognition, functional decline and diabetes
A systematic literature review found that diabetes, hypertension, stroke and arthritis were the conditions most likely to be associated with subsequent functional decline Citation[28]. The Manitoba Longitudinal Study on Aging showed in a 12-year follow-up study of 3,573 individuals 65 to 84 years of age that not having diabetes was one of the factors that predicted maintaining functional independence Citation[29]. Numerous other studies from around the world have shown that diabetes is a strong predictor of disability Citation[5],Citation[6],Citation[30-32]. In the third National Health and Nutrition Examination Survey (NHANES III), disability was found to be present in 39% of diabetic men and 63% of diabetic women Citation[30]. In men, but not women, stroke was an important predictor of disability.
The All Wales Research in Elderly Diabetes (AWARE) study compared 400 diabetics over the age of 65 years with an age-matched cohort of non-diabetics Citation[33]. This study found that diabetics had a greater disability on the Barthel Index and were more likely to use a walking aid. This study also found that diabetics were less likely to read, use the telephone, go out socially, write letters or garden than were non-diabetics.
Lower body disability and impaired mobility are particularly common in older person with diabetes Citation[34]. A community based study found that persons with diabetes mellitus were more likely to have injurious falls than non-diabetics Citation[6]. Maurer et al. Citation[35] found in a nursing home that the fall rate in persons with diabetes was 78% and only 30% in those without. Multiple factors besides lower body disability, such as visual impairment, orthostatic hypotension, postprandial hypotension and cognitive impairment appear to play a role in the increased fall risk seen in older diabetics. It should be recognized that autonomic abnormalities, such as orthostatic hypotension, are among the earliest manifestations of diabetes Citation[36].
Animal studies have demonstrated that hyperglycemia, as well as hypoglycemia, is associated with poor memory Citation[37]. Numerous community based studies have found that Type II diabetes mellitus is associated with cognitive impairment Citation[38-40]. In older women, Gregg et al. Citation[41] reported a 2-fold increase in cognitive impairment when followed for 6 years. Improvement of glucose control in older diabetics has been demonstrated to result in improvement in cognitive function Citation[42],Citation[43]. Type 2 diabetes is associated with an increased risk in developing vascular dementia Citation[44].
The possible reasons for diabetes and poor diabetic control being associated with cognitive impairment are listed in. Diabetics, besides having an increased likelihood of developing vascular dementia, also appear to be more likely to develop Alzheimer's disease Citation[45]. Depression is more common in persons with diabetes than in those without diabetes Citation[46]. Depression is a key factor in worsening diabetic control, decreased treatment compliance, hospitalization and subsequent death in persons with diabetes Citation[15],Citation[47].
Table III. Potential causes of poor cognition in older persons with diabetes mellitus.
Hyperinsulinemia has been associated with poor cognitive function and insulin administered directly into the hippocampus in mice produces poor memory [49 and unpublished observations). Hypertriglyceridemia has been associated with delirium. Elevated triglycerides in persons with Type II diabetes are associated with poor performance on the digit symbol substitution test, digit span backward test and reaction time Citation[48]. In a second study, elevated triglycerides were associated with poor retrieval from semantic memory in Type II diabetics Citation[49]. Finally, reducing hypertrigylceridemia with gemfibrozil improved cerebral blood flow and function on the cognitive capacity screening examination Citation[50]. Our unpublished studies in mice have shown that triglycerides injected into the brain can impair memory and in vitro triglycerides impair long term potentiation. Lowering hypertriglyceridemia with gemfibrozil in mice improves memory and reduces oxidative damage.
Leptin enhances long term potentiation and improves memory Citation[51]. Hypertriglyceridemia impairs the ability of leptin to cross the blood brain barrier and enter the brain Citation[52]. Thus, impaired ability of leptin to enter the brain may provide another mechanism by which hypertriglyceridemia impairs memory.
Overall, the recognition of the role of hyperglycemia and hypertriglyceridemia in impaired cognition and functional impairment represents a key reason for maintenance of good diabetic control.
Diabetes and hypertension
The United Kingdom Prospective Diabetes Study (UKDPS) demonstrated that lowering blood pressure to below 150/85 mm Hg found a decrease in microvascular disease, heart failure, and mortality in the well controlled group Citation[53]. Treatment of hypertension appeared to be more effective than treating glucose in improving outcomes. There was no difference between those subjects receiving atenolol compared to those receiving captopril. Because of the entry age cut off of 65 years, this study cannot be applied to persons over 75 years of age.
The ALLHAT/The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) followed 33,357 patients with a mean age of 67 years Citation[54]. Type 2 diabetes was present in 36%. Chlorthalidone reduced heart failure to a greater degree than did amlodipine and was more effective than lisinopril in reducing blood pressure and preventing cardiovascular events. Persons with diabetes had similar results as those without diabetes.
Numerous studies have investigated the effects of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in older persons with diabetes and hypertension Citation[55-57]. They appear effective at reducing cardiovascular endpoints and microalbuminuria. The available data does not support that they are more effective than other antihypertensive medications. Both ramipril and losartan appear to lower the risk of diabetes occurring in persons over 55 years of age Citation[56],Citation[58]. The reason for this is not apparent, though there is some evidence that these drugs may enhance skeletal muscle function and thus lower insulin resistance.
Diabetes and hypogonadism
It is now well recognized that late onset hypogonadism is a very common condition Citation[59]. Diabetes mellitus is associated with an increased prevalence of hypogonadotrophic hypogonadism Citation[60],Citation[61]. The reasons for this are uncertain, but the above mentioned zinc deficiency may be one of the causes. In addition, adipokines, such as leptin and tumor necrosis factor alpha, may play a role. Increased insulin levels decrease sex hormone binding globulin levels, leading to a decrease in total testosterone. Testosterone replacement therapy may have beneficial effects on insulin sensitivity in older diabetic men with late onset hypogonadism. A reduction in fat mass in response to testosterone replacement therapy leads to decreased circulating free fatty acids, possibly resulting in an improvement of insulin sensitivity. Until recently, however, the available data on testosterone replacement therapy for enhancing insulin sensitivity in older men with diabetes does not yet exist.
Diabetes and Nutrition
We have to lament that our mode of cure is so contrary to the inclinations of the efficacy of the [diet] regimen, and the impropriety of deviation, yet they commonly trespass, concealing what they feel as a transgression on themselves. They express a regret that a medicine could not be discovered however nauseous or distasteful, which would suppress the necessity of any restriction of diet.
(John Rolo, 1798)
Two studies have shown that at least in older persons in nursing homes, a regular diet and even ingestion of concentrated sweets makes little difference to glycemic control Citation[62],Citation[63]. This has led to both the American Diabetic Association and the American Dietetic Association to no longer recommend the use of therapeutic diabetic diets in nursing homes Citation[64],Citation[65]. The major reason to avoid diabetic diets in older persons is that they can interact with the anorexia of aging and lead to inappropriate weight loss and malnutrition.
Recent studies have strongly suggested that the hyperglycemia and hyperlipidemia following ingestion of a meal are more strongly related to future atherosclerotic cardiac disease than are fasting glucose levels Citation[66]. The mechanism(s) of this effect include oxidative stress, tissue glycation, endothelial dysfunction, activation of coagulation and toxic effects of triglycerides and free fatty acids. The area of the hyperglycemic excursion following a meal can be limited by adding high fiber foods or polyunsaturated fatty acids to the meal.
Diabetics have an increase in gut wall permeability Citation[67] which can lead to increased translocation of bacteria from the gut into the portal vein and thoracic duct. This leads to an increase in circulating endotoxins, such as lipopolysacarides, with activation of monocytes. Monocyte activation results in increased circulating cytokines, e.g. tumor necrosis factor α. Elevated cytokines can result in anorexia, muscle loss (sarcopenia), functional decline and immune dysfunction. The use of prebiotics, e.g. oligofructosaccharides, may result in a decrease in cytokine production by altering the intestinal bacterial milieu.
Homocysteine levels are often elevated in older diabetics and are associated with accelerated atherosclerosis, osteopenia and Alzheimer's disease Citation[68]. One cause of high homocysteine levels is vitamin B12 deficiency, which occurs more commonly in diabetics. The diagnosis can be made by the combination of a low or borderline low vitamin B12 level and an elevated methylmalonic acid level. High dose folate will lower the levels in some other patients.
A number of other nutritional issues need to be considered in the older diabetic. These include (i) zinc replacement in older persons with vascular or pressure ulcers, (ii) high doses of vitamin C and E can interfere with the glucose oxidation reaction used to measure blood glucose levels, (iii) low magnesium levels, that occur with osmotic diuresis, may lead to increased levels of systolic hypertension, (iv) hypovitaminosis D is common in medical patients with diabetes Citation[69] and there is an increased incidence of hip fracture in older diabetics Citation[70], (v) high copper levels may accelerate atherosclerosis and (vi) the role of chromium together with nicotinic acid (glucose tolerance factor) in diabetics is uncertain, but appears minor Citation[71].
Lifestyle interventions and diabetes
A number of studies have demonstrated that in middle-aged persons lifestyle modifications (diet and exercise) can reduce the progression of impaired glucose tolerance to Type II diabetes by approximately 50% Citation[72-74]. This is in keeping with a large epidemiological study of 42,000 males aged 40 to 75 years where it was demonstrated that a ‘western’ style pattern (refined grains, red meat, French fries, high-fat dairy products and sweets/desserts) and low physical activity were associated with an increase in risk for Type 2 diabetes Citation[75]. Moderate alcohol consumption, at least 5 drinks/week was protective against developing Type II diabetes. In the intervention studies lifestyle modification was more protective than metformin or acarbose Citation[76]. In persons over 60 years of age metformin was no better than placebo.
Exercise is recommended for all older persons with diabetes. An exercise program should consist of endurance, resistance, balance, postural and flexibility exercises.
Medications and diabetes
In general, the same drugs as are used for the management of older as well as younger diabetics with some caveats (). A particularly important point is to recognize that in older persons loss of muscle mass can lead to severe renal failure occurring in the presence of a normal creatinine – the so-called ‘concealed renal failure’ Citation[77]. This can lead to increased adverse drug reactions to water soluble drugs such as oral hypoglycemic agents, digoxin and angiotensin converting enzyme inhibitors.
Table IV. Medications used for the treatment of diabetes mellitus in older men.
The United Kingdom Prospective Diabetes Study demonstrated that it is very difficult to maintain intensive control of diabetes, as demonstrated by an increase in HbA1C from approximately 6 to 8 over twelve years Citation[78]. However, it should be pointed out that increases in the conventional care group were even higher. In middle-aged diabetics who were obese, metformin proved to be the most effective drug at improving outcomes. However, metformin tends to become less effective in older diabetics and the development of renal dysfunction with aging and disease such as congestive heart failure increase the risk of lactic acidosis occurring. In the United States metformin is not recommended for persons over 80 years of age. Metformin also can cause anorexia and weight loss Citation[79] which can accelerate the development of protein energy undernutrition, a common problem in older persons Citation[80].
Sulfonylurea drugs are still commonly used in older persons. Chlorpropramide should not be used over the age of 65 years as it has a prolonged half-life and is associated with a high incidence of hyponatremia. Similarly glybenclamide is not recommended in persons over 70 years of age. Glyburide, glipizide, and glimeperide all can be used safely in older persons. There does not appear to be a major difference between the drugs, with an equivalent prevalence of hypoglycemia occurring when they obtain the same degree of hypoglycemia. In some very old individuals, tolbutamide once a day may be sufficient to maintain euglycemia. Repaglinide, a meglitinide, and nateglinide, a D-phenylalanine derivative, have very short half-lives and therefore, need to be given multiple times a day making them less useful in older persons. There is no difference in hypoglycemic episodes between these agents and sulfonylureas.
Thiazolidinediones work by activating the PPARγ (peroxisome proliferator-activated receptor). These drugs enhance peripheral tissue sensitivity to insulin. Troglitizone was withdrawn because of a high level of liver failure associated with its use. At present pioglitasone and rosiglitasone are available. They are excellent monotherapy drugs in the old-old (>80 years of age). These drugs are associated with occasional liver dysfunction, water retention and occasionally overt heart failure.
The alpha-1 glucosidase inhibitors, acarbose and miglitol, are drugs which tend to smooth out the glycemic excursion following a meal. This appears to be due to both delayed carbohydrate digestion and absorption as well as due to an increase in glucagon-like peptide-1 Citation[81]. These drugs are about half as effective as sulfonylureas at lowering the HbA1C Citation[82]. They are associated with abdominal pain, flatulence and diarrhoea. Acarbose has been demonstrated to decrease cardiovascular events in people with impaired glucose tolerance Citation[83].
Glucagon-like peptide-1 (GLP-1) is a peptide that is produced by the duodenum and stimulates insulin production, inhibits hepatic glucose production, delays gastric emptying and produces anorexia. Recently, a frog skin homolog, GLP-1 receptor agonist, exendin-4, has been approved in the United States for treatment of Type 2 diabetes. It lowers Hb A1C by approximately 1 in persons who are not controlled on sulfonylureas and/or metformin Citation[84]. It is uncertain whether it will have a place in the management of the older diabetic.
Because of the problems with polypharmacy in older persons, it makes sense to move to insulin earlier, rather than later, when glycemic control cannot be easily obtained with oral agents. Of course, this approach depends on the ability of the older person or the caretaker to monitor blood glucose and administer insulin. Intermediate acting (NPH) and short-acting regular insulin remain the mainstay of the management of the older diabetic. Long acting drugs such as glargine insulin increase the risk for hypoglycemia when the older person doesn't eat or food is delayed. Rapid acting insulins require administration 3 times a day and in the nursing home they are often given at a routine time and lead to hypoglycemia when the meal is delivered late.
Of the drugs under development, dipeptidyl peptidase (DPP) IV inhibitors appear to have the most promise for older diabetics. This enzyme is a member of a family of serine peptidases Citation[85]. DPP inhibitors increase the release of insulin by stabilizing circulating concentrations of the gut incretin hormones via GLP-1 and glucose-dependent insulinotropic peptide (GIP). Efficacy of this drug-type has been demonstrated in humans Citation[86].
The interdisciplinary team
The goals of diabetic management programs for the older people should be to optimize glycemic control for minimizing metabolic decompensation and long term complications and improve quality of life. Accordingly, managing diabetes in older persons requires an interdisciplinary team. Use of a team of health professionals has been shown to improve diabetic care over a single provider Citation[87]. For the team to be optimally effective, physician extenders need to have the ability to make changes in medication dosage.
A number of studies have demonstrated that short-term educational programs can not only improve knowledge, but produce modest improvements in glycated hemoglobin, blood pressure and perceived quality of life Citation[88-93]. Frequent follow-up and self-monitoring of blood glucose are key components to success. Older persons were shown to have low participation in self-monitoring of blood glucose in two studies Citation[87],Citation[94].
Conclusion
In older persons the diagnosis of diabetes mellitus requires a fasting glucose of greater than 126 mg/dl (7 mmol/L) or a postprandial glucose greater than 200 mg/dL (11.1 mmol/L). Older diabetics have multiple problems due to the interaction of the aging process with the disease. This means that they are best treated within an interdisciplinary team. Both blood pressure and glucose should be carefully controlled, while minimizing hypoglycemia and orthostatic hypotension.
References
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053
- Harris M I, Flegal K M, Cowie C C, Eberhardt M S, Goldstein D E, Little R R, Wiedmeyer H M, Byrd-Holt D D. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey. 1988–1994. Diabetes Care 1998; 21: 518–524
- Morley J E. The elderly Type 2 diabetic patient: special considerations. Diabetic Medicine 1998; 15(Suppl 4)S41–S46
- Meneilly G S, Tessier D. Diabetes in elderly adults. J Gerontol Med Sci 2001; 56: M736–M737
- Rodriguez-Saldana J, Morley J E, Reynoso M T, Medina C A, Salazar P, Cruz E, Torres A L. Diabetes mellitus in a subgroup of older Mexicans: prevalence, association with cardiovascular risk factors, functional and cognitive impairment, and mortality. J Am Geriatr Soc 2002; 50: 111–116
- Miller D K, Lui L Y, Perry H M, 3rd, Kaiser F E, Morley J E. Reported and measured physical functioning in older inner-city diabetic African Americans. J Gerontol Med Sci 1999; 54: M230–M236
- Morley J E. Diabetes mellitus: a major disease of older persons. J Gerontol Med Sci 2000; 55: M255–M256
- Lindeman R D, Yau C L, Baumgartner R N, Morley J E, Garry P J, New Mexico Aging Process Study. Longitudinal study of fasting serum glucose concentrations in healthy elderly. The New Mexico Aging Process Study. J Nutr Hlth Aging 2003; 7: 172–177
- Lowell B B, Shulman G I. Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307: 384–387
- Haluzik M. Adiponectin and its potential in the treatment of obesity, diabetes and insulin resistance. Curr Opin Ivestig Drugs 2005; 6: 988–993
- Matsuda Y, Tanioka T, Yoshioka T, Nagano T, Hiroi T, Yoshikawa K, Okabe K, Nagamine I, Takasaka Y. Gender differences in association of plasma adiponectin with obesity reflect resultant insulin resistance in non-diabetic Japanese patients with schizophrenia. Psychiat Clin Neurosci 2005; 59: 266–273
- Camus J P. Gout, diabetes, hyperlipidemia: a metabolic trisyndrome. Rev Rheum Mal Osteoarth 1966; 33: 10–14
- Zhu S, St-Onge M P, Heshka S, Heymsfield S B. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism 2004; 53: 1503–1511
- Banks W A, Altmann J, Sapolsky R M, Philips-Conroy J E, Morley J E. Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab 2003; 88: 1234–1240
- Rosenthal M J, Fajardo M, Gilmore S, Morley J E, Naliboff B D. Hospitalization and mortality of diabetes in older adults. A 3-year prospective study. Diabetes Care 1998; 21(2)231–235
- Nathan D M, Singer D E, Godine J E, Harrington C H, Perlmuter L C. Retinopathy in older type II diabetics. Association with glucose control. Diabetes 1986; 35: 797–801
- Banerjee S, Banerjee M. Diabetes and tuberculosis interface. J Indian Med Assoc 2005; 103: 318, 320,322
- Silver A J, Morley J E. Role of the opioid system in the hypodipsia associated with aging. J Am Geriatr Soc 1991; 40: 556–560
- Morley G K, Mooradian A D, Levine A S, Morley J E. Mechanism of pain in diabetic peripheral neuropathy. Effect of glucose on pain perception in humans. Am J Med 1984; 77: 79–82
- Brandeis G H, Ooi W L, Hossain M, Morris J N, Lipsitz L A. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. J Am Geriatr Soc 1994; 42: 388–393
- Morley J E. Postprandial hypotension—the ultimate Big Mac attack. J Gerontol Med Sci 2001; 56A: M744–M748
- Edwards B J, Perry H M, 3rd, Kaiser F E, Morley J E, Kraenzle D, Stevenson R, Kreutter D. Relationship of age and calcitonin gene-related peptide to postprandial hypotension. Mech Ageing Dev 1996; 87(2)61–73
- Kong M F, Horowitz M. Gastric emptying in diabetes mellitus: relationship to blood-glucose control. Clin Geriatr Med 1999; 15: 321–338
- Clarkston W K, Pantano M M, Morley J E, Horowitz M, Littlefield J M, Murton F R. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol 1997; 272(1Pt 2)R243–R248
- Niewoehner C B, Allen J I, Boosalis M, Levine A S, Morley J E. Role of zinc supplementation in type II diabetes mellitus. Am J Med 1986; 81: 63–68
- Kinlaw W B, Levine A S, Morley J E, Silvis S E, McClain C J. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med 1983; 75: 273–277
- Mooradian A D, Morley J E. Micronutrient status in diabetes mellitus. Am J Clin Nutr 1987; 45(5)877–895
- Stuck A E, Walthert J M, Nikolaus T, Bula C J, Hohmann C, Beck J C. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med 1999; 48: 445–469
- Roos N P, Havens B. Predictors of successful aging: twelve-year study of Manitoba elderly. Am J Public Hlth 1991; 81(1)63–68
- Gregg E W, Beckles G L, Williamson D F, Leveille S G, Langlois J A, Engelgau M M, Narayan K M. Diabetes and physical disability among older U.S. adults. Diabetes Care 2000; 23(9)1272–1277
- Perkowski L C, Strou-Benham C A, Markides K S, Lichtenstein M J, Angel R J, Guralnik J M, Goodwin J S. Lower-extremity functioning in older Mexican Americans and its association with medical problems. J Am Geriatr Soc 1998; 46(4)411–418
- Wray L A, Ofstedal M B, Leung K M, Lawn L S. The effect of diabetes on disability in middle-aged and older adults. J Gerontol Med Sci 2005; 60A: 1190–1191
- Sinclair A J, Girling A J, Bayer A J. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50: 203–212
- Volpato S, Blaum C, Resnick H, Ferrucci L, Freid L P, Guralnik J M. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women's Health and Aging Study. Diabetes Care 2002; 25(4)678–683
- Maurer M S, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol Med Sci 2005; 60: 1157–1162
- Morley J E, Asvat M S, Klein C, Lowenthal M N. Autonomic neuropathy in Black diabetic patients. S Afr Med J 1977; 16:52: 115–116
- Flood J F, Mooradian A D, Morley J E. Characteristics of learning and memory in streptozocin-induced diabetic mice. Diabetes 1990; 39: 1391–1398
- Mooradian A D, Perryman K, Fitten J, Kavonian G D, Morley J E. Cortical function in elderly non-insulin dependent diabetic patients. Behavioral and electrophysiologic studies. Arch Int Med 1988; 148: 2369–2372
- Vanhanen M, Koivisto K, Kuusisto J, Mykkanen L, Helkala E L, Hanninen T, Reikkinen P, Sr, Soininen H, Laakso M. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care 1998; 21(3)398–402
- Strachan M W, Deary I J, Ewing F M, Frier B N. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care 1997; 20(3)438–445
- Gregg E W, Yaffe K, Cauley J A, Rolka D B, Blackwell T L, Narayan K M, Cummings S R. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med 2000; 160(2)174–180
- Gradman T J, Laws A, Thompson L W, Reaven G M. Verbal learning and/or memory improves with glycemic control in older subjects with non-insulin-dependent diabetes mellitus. J Am Geriatr Soc 1993; 41(12)1305–1312
- Meneilly G S, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol Med Sci 1993; 48(4)M117–M121
- Elias P K, Elias M F, D'Agostino R B, Cupples L A, Wilson P W, Silbershatz H, Wolf P A. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care 1997; 20(9)1399–1395
- Morley J E. The metabolic syndrome and aging. J Gerontol Med Sci 2004; 59A: 139–142
- Amato L, Paolisso G, Cacciatore F, Ferrara N, Canonico S, Rengo F, Varricchio M. Non-insulin-dependent diabetes mellitus is associated with a greater prevalence of depression in the elderly. The Observatorio Geriatrico of Campania Region Group. Diabetes Metab 1996; 22(5)314–318
- Lustman P J, Anderson R J, Freedland K E, de Groot M, Carney R M, Clouse R E. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000; 23(7)934–942
- Perlmuter L C, Nathan D M, Goldfinger S H, Russo P A, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. J Diabet Complications 1988; 2(4)210–213
- Helkala E L, Niskanen L, Viinamaki H, Partanen J, Uusitupa M. Short-term and long-term memory in elderly patients with NIDDM. Diabetes Care 1995; 18: 681–685
- Rogers R L, Meyer J S, McClintic K, Mortel K F. Reducing hypertriglyceridemia in elderly patients with cerebrovascular disease stabilizes or improves cognition and cerebral perfusion. Angiology 1989; 40(4 Pt 1)260–269
- Wayner M J, Armstrong D L, Phelix C F, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 2004; 25: 991–996
- Banks W A, Coon A B, Robinson S M, Moinuddin A, Shultz J M, Nakaoke R, Morley J E. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 2004; 53(5)1253–1260
- Mooradian A D, Chehade J. Implications of the UK prospective diabetes study: questions answered and issues remaining. Drugs Aging 2000; 16: 159–164
- ALLHAT Officers and Coordinators for the ALL HAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288(23)2981–2997
- Niklason A, Hedner T, Niskanen L, Lanke J, Captopril Prevention Project (CAPPP). Development of diabetes is retarded by ACE inhibition in hypertensive patients – a subanalysis of the Captopril Prevention Project (CAPPP). J Hypertens 2004; 22: 645–652
- Dahlof B, Devereux R B, Kjeldsen S E, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pederson O, Lindholm L H, Nieminen M S, Omvik P, Oparil S, Wedel H, LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet 2002; 359(9311)995–1003
- Morgensen C E, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts R W, Cooper M E. Randomised controlled trial of dual blockade of rennin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000; 321(7274)1440–1444
- Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel B H, Zinman B, HOPE Study Investigators. Ramipril and the development of diabetes. JAMA 2001; 286: 1882–1885
- Morley J E, Tariq S H. Sexuality and disease. Clin Geriatr Med 2003; 19: 563–573
- Distiller L A, Sagel J, Morley J E, Seftel H C. Pituitary responsiveness to luteinizing hormone-releasing hormone in insulin-dependent diabetes mellitus. Diabetes 1975; 24: 378–380
- Corona G, Mannucci E, Petrone L, Ricca V, Balercia G, Mansani R, Chiarini V, Giommi R, Forti G, Maggi M. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res Sep 1, 2005, E-Pub ahead of print]
- Tariq S H, Karcic E, Thomas D R, Thomson K, Philpot C, Chapel D L, Morley J E. The use of no-concentrated-sweets diet in the management of type 2 diabetes in nursing homes. J Am Diet Assoc 2001; 101: 1463–1466
- Coulston A M, Mandelbaum D, Reaven G M. Dietary management of nursing home residents with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1990; 51: 67–71
- Dorner B, Niedert K C, Welch P K, American Dietetic Association. Position of the American Dietetic Association: liberalized diets for older adults in long-term care. J Am Diet Assoc 2002; 102: 1316–1323
- Franz M J, Bantle J P, Beebe C A, Brunzell J D, Dhiasson J L, Garg A, Holzmeister L A, Hoogwerf B, Mayer-Davis E, Mooradian A D, Purnell J Q, Wheeler M, American Diabetes Association. Nutrition principles and recommendations in diabetes. Diabetes Care 2004; 27(Suppl 1)S36–S46
- Hanefeld M, Temelkova-Kurktschiev T. Control of post-prandial hyperglycemia—an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr Metab Cardiovasc Dis 2002; 12: 98–107
- Mooradian A D, Morley J E, Levine A S, Prigge W F, Gebhard R L. Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 1986; 29: 221–224
- Kuo H K, Sorond F A, Chen J H, Hashmi A, Melberg W P, Lipsitz L A. The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol Med Sci 2005; 60A: 1190–1201
- Perry H M, 3rd, Horowitz M, Morley J E, Patrick P, Vellas B, Baumgartner R, Garry P J. Metabolism 1999; 48: 1028–1032
- Taylor B C, Schreiner P J, Stone K L, Fink H A, Cummings S R, Nevitt M C, Bowman P J, Ensrud K E. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc 2004; 52: 1479–1486
- Mertz W. Chromium in human nutrition: a review. J Nutr 1993; 123: 626–633
- Hu F B, Manson J E, Stampfer M J, Colditz G, Liu S, Solomon C G, Willett W C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345: 790–797
- Pan X R, Li G W, Hu Y H, Wang J X, Yang W Y, An Z X, Hu Z X, Lin J, Xiao J Z, Cao H B, Liu P A, Jiang X G, Jiang Y Y, Wang J P, Zheng H, Zhang H, Bennet P H, Howard B V. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20(4)537–544
- van Dam R M, Willett W C, Rimm E B, Stampfer M J, Hu F B. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002; 25: 417–424
- Tuomilehto J, Lindstrom J, Eriksson J G, Valle T T, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350
- Knowler W C, Hamman R F, Edelstein S L, Barrett-Connor E, Ehrmann D A, Walker E A, Fowler Se, Nathan D M, Kahn S E, Diabetes Prevention Program Research Group. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 2005; 54: 1150–1156
- Corsonello A, Pelone C, Corica F. Concealed renal failure and adverse drug reactions in older patients with Type 2 diabetes mellitus. J Gerontol Med Sci 2005; 60A: 1147–1151
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131)837–853
- Lee A, Morley J E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obesity Res 1998; 6: 47–53
- Morley J E. Protein-energy malnutrition in older subjects. Proc Nutr Soc 1998; 57(4)587–592
- Lee A, Patrick P, Wishart J, Horowitz M, Morley J E. The effects of miglitol on glucagons-like peptide-1 secretion and appetite sensations in obese type 2 diabetics. Diabetes, Obesity & Metab 2002; 4: 329–335
- Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. J Diabetes Complications 1998; 12: 228–237
- Delorme S, Chiasson J L. Acarbose in the prevention of cardiovascular disease in subjects with impaired glucose tolerance and type 2 diabetes mellitus. Curr Opin Pharmacol 2005; 5: 184–189
- Taylor K, Kim D, Nielsen L L, Aisporna M, Baron A D, Fineman M S. Day-long subcutaneous infusion of exanatide lowers glycemia in patients with Type 2 diabetes. Horm Metab Res 2005; 37: 627–632
- Lankas G R, Leiting B, Roy B S. Dyseptidyl Peptidase IV inhibition for the treatment of Type 2 diabetes. Diabetes 2005; 54: 2988–2994
- Ahren B, Gorris R, Standl E, Mills D, Schweizer A. Twelve and 52 week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin treated diabetes with type 2 diabetes. Diabetes Care 2004; 27: 2874–2880
- Adams A S, Mah C, Soumerai S B, Zhang F, Barton M B, Ross-Degnan D. Barriers to self-monitoring of blood glucose among adults with diabetes in an HMO: a cross sectional study. BMC Health Serv Res 2003; 3(1)6
- Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and pschysocial interventions on management of diabetes mellitus. J Clin Epidemiol 1988; 41: 1007–1030
- Jaber L A, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmacetucial care model on diabetes management. Ann Pharmacother 1996; 30(3)238–243
- Gilden J L, Hendryx M, Casia C, Singh S P. The effectiveness of diabetes education programs for older patients and their spouses. J Am Geriatr Soc 1989; 37: 1023–1030
- Glasgow R E, Toobert D J, Hampson Se, Brown J E, Lewinsohn P M, Donnelly J. Improving self-care among older patients with type II diabetes: The ‘Sixty Something …’ Study. Patient Educ Couns 1992; 19(1)61–74
- Weinberger M, Kirkman M S, Samsa G P, Shortliffe E A, Landsman P B, Cowper P A, Simel D L, Feussner J E. A nurse-coordinated intervention for primary care patients with non-insulin-dependent diabetes mellitus: impact on glycemic control and health-related quality of life. J Gen Intern Med 1995; 10(2)59–66
- Agurs-Collins T D, Kumanyika S K, Ten Have T R, Adams-Campbell L L. A randomized controlled trial of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care 1997; 20: 1503–1511
- Bruce D G, Davis W A, Cull C A, Davis T M. Diabetes education and knowledge in patients with type 2 diabetes from the community: the Fremantle Diabetes Study. J Diabetes Complications 2003; 17(2)82–89