Abstract
Purpose. To determine the value of available questionnaires used for the diagnosis of testosterone deficiency syndromes (TDS) by correlating their ratings with a panel of hormonal determinations in a male population.
Materials and methods. Participants completed the ADAM questionnaire and underwent biochemical evaluation at the local site. Assessments determined entry into Group A (symptomatic) or Group B (non-symptomatic). After stratification, subjects provided a morning sample of blood, completed the Aging Male Survey (AMS) and the newly developed Canadian Society for the Study of the Aging Male (CSAM-Q) questionnaires. Serum aliquots were analysed at a central lab for 8 putative markers commonly associated with symptomatic testosterone deficiency associated with aging: total testosterone (T); bioavailable T (BT); dehydroepiandrosterone sulphate (DHEA-S); sex-hormone binding globulin (SHBG); luteinizing hormone (LH), prolactin (PRL); thyroid-stimulating hormone (TSH) and insulin-like growth factor-1 (IGF-1).
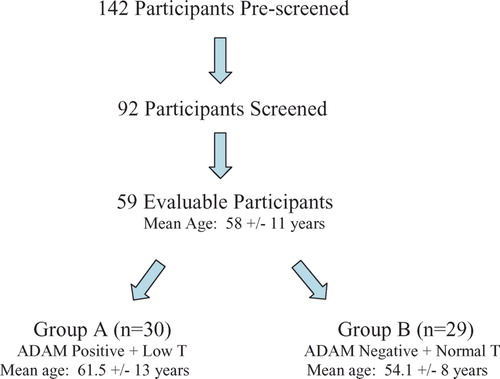
Results. 92 men were screened; of these 59 (mean age of 58 ± 11 years) completed the study, 30 (51%) scored positively (mean 61.5 years) to the ADAM while 29 (49%) did not (mean 54.1 years). For the AMS the weight of the three domains (psychological, somato-vegetative and sexual) was significantly greater in Group A (p < 0.001) than in Group B. Equally, for the CAS questionnaire, the scores for the variables energy, global performance, frequency of intercourse, mood and quality of sleep were lower in Group A than in their asymptomatic counterparts (p < 0.001). The domain of memory assessment within the CSSAM-Q was not discriminatory. ADAM and AMS are self-administered and completed within 10 minutes. CSSAM-Q is more time consuming, requires an investigator to administer, and memory domain is biased in favour of specific professional training.
No difference was found between the two groups in 6 of 8 biochemical tests. However, significant lower values (p < 0.001) were found for DHEA-S and IGF-1 in the symptomatic group as compared with the non-symptomatic cohort.
Conclusions. This study confirms that newer, more complex tools perform similarly to the simpler ADAM questionnaire. The lack of correlation between the clinical picture and the most commonly used biochemical confirmatory tests, again, clearly points to the paramount importance of the clinical evaluation. An emphasis and reliance on serum T alone hinders the clinician's ability to manage testosterone deficiency syndromes (TDS).
Introduction
The decline in testosterone (T) production in men in association with aging is incontrovertible and well documented. It is also beyond doubt that other hormones, including precursors of sex steroids such as dehydroepiandrosterone, also decline with age at similar or even higher rates Citation[1],Citation[2]. What remains controversial is the clinical significance of the well documented biochemical changes and the relationship between clinical manifestations and the degree of the hormonal alterations. An association between the signs and symptoms and the levels of T and other hormones has been sufficiently investigated but the results remain puzzling and contradictory.
Indeed, the clinical diagnosis of T deficiency in the adult and aging man can difficult because the manifestations are not specific. In an attempt to alleviate this obstacle, a number of questionnaires have been developed with the intention of facilitating the diagnosis. In most instances the clinical manifestations have been correlated to measurement of gonadal steroids, mostly T and its free and bioavailable fractions Citation[3],Citation[4]. To further complicate the situation, the clinical picture commonly associated with hypogonadism shares several of the features also found in men with hypothyroidism Citation[5] as well as with a decrease in the production of growth hormone Citation[6].
In this study, a screening questionnaire previously designed and validated for diagnosis of hypogonadism was correlated with a battery of putative serum markers usually associated with hypogonadism. In addition, we aimed to determine the reliability and internal consistency of a newly developed questionnaire and to identify its strengths and weaknesses in comparison to existing instruments and common biochemical markers.
Material and methods
Approval for the study was obtained from the Queen's University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. To participate in the study, men had to be over the age of 40 years and had no prior history of taking any hormonal preparations or chemotherapy. Men were excluded from the study for any of the following reasons: clinically significant hepatobiliary abnormalities, renal disease or anaemia or any chronic debilitating condition (i.e., cancer, cardiac disease or HIV infection). They also had to fit into either of two groups: Group A which exhibited signs and symptoms of hypogonadism, a positive score in the ADAM questionnaire Citation[2] and had serum levels of T below normal range (<10–28 ng/L). Group B did not exhibit signs or symptoms of hypogonadism and had serum levels of total testosterone within the normal range in the local laboratory. In addition they must have had a negative score in the ADAM questionnaire. On the basis of this initial assessment they were classified as clinically hypogonadal or eugonadal. Further evaluation included the Aging Male Survey (AMS) Citation[7] questionnaire and a newly developed diagnostic instrument, the Canadian Society for the Study of the Aging Male Questionnaire (CSSAM-Q). ADAM and AMS have been previously validated while the CSSAM-Q was used for the first time in an attempt to establish its viability as a screening/diagnostic tool. It is shown in its entirety in. The CSSAM-Q was developed independently by one of us (MS) with input of members of the CSSAM. It consists of 2 Sections: Section 1 contains 7 domains (A to G) and it is self-administered. Section 2 contains a single domain (H) and it is administered by a study coordinator or an investigator.
Subjects provided a morning (07:00 to 11:00 hrs.) sample of blood from which serum aliquots were prepared and submitted to a central biochemistry laboratory (Department of Medical Biology of the Hospital Centre of the University of Laval, Ste-Foy, PQ, Canada) for determination of the following eight hormonal assays: 1) total testosterone (T), 2) bioavailable testosterone measured by the ammonium (BT), 3) dehydroepiandrosterone sulphate DHEA-S; 4) sex hormone-binding globulin (SHBG), 5) luteinizing hormone (LH), 6) prolactin (PRL), 7) thyroid stimulating hormone (TSH) and 8) insulin-like growth factor-1 (IGF-1). Measurements of T, DHEA-S, SHBG, LH, PRL and TSH were performed by radioimmunoassay. BT was measured by the ammonium sulphate precipitation method Citation[8] while IGF-1 was measured by an enzyme immunoassay.
Statistical analyses were carried out using the Student t-test, Mann-Whitney Rank Sum test and the Spearman Rank Order Correlation as appropriate.
Results
One hundred and forty-two men completed the screening visit and agreed to participate but only 59 (mean age: 58 ± 11 years, SD) completed all the criteria and were evaluable. Reasons for screening failures included: current or previous use of hormones, inability to provide a blood sample, inability to complete any of the questionnaires or loss of the serum aliquot by the central laboratory or in transit.
Questionnaires
Thirty men (51%), with a mean age of 61.5 years, fitted the clinical picture of hypogonadism, including a positive score in the ADAM questionnaire (Group A), while 29 men (49%) were not deemed to be hypogonadal on purely clinical grounds (Group B). The mean age in this Group B was significantly lower (54.1 years).
The AMS questionnaire is rated 1 to 5 for each category; the higher the score the higher the severity of symptoms. Considering the weight of its three domains (psychological, somato-vegetative and sexual), as shown in, the weight of each domain was significantly greater in Group A (p < 0.001) than in Group B. The CSSAM-Q contains more domains (8) which are rated contrary to the AMS: the higher the score the better the performance in each particular domain. For CSSAM-Q, the scores for the variables of energy, global performance, frequency of intercourse and quality of sleep were significantly lower in the clinically hypogonadal men than in their eugonadal counterparts (). No differences existed between the two cohorts in the domains of energy, memory, sexual interest or masturbation.
Table I. Mean AMS scores.
Table II. Mean CAS scores.
The ADAM questionnaire is a self-administered tool completed by every subject in less than 2 minutes. The AMS is also self-administered and completed by every participant in less than 6 minutes. The CSSAM-Q is partially self-administered but contains a portion (memory domain) that requires the presence of an assessor to administer it. The self-administered portion is long, requiring over 25 minutes in most cases with an additional 5–10 minutes for the investigator-administered domain.
Biochemical evaluation
The 8 laboratory tests were correlated with the ADAM questionnaire since this was one of the criteria employed to assign the participants to Groups A or B. Surprisingly, only two biochemical parameters (IGF-1 and DHEA-S) were significantly lower in subjects in Group A (clinically hypogonadal) than Group B ().
Table III. ADAM ‘positive’ and hormone levels.
The results of the 8 assays were also correlated with both the AMS and CSSAM-Q instruments. Again, the correlation was poor and significant only between a few domains and hormonal assays ().
Table IV. AMS and CAS Questionnaire domain correlations with hormone levels.
Discussion
The finding that the AMS questionnaire correlates with the clinical evaluation supports several reports in the literature regarding its screening/diagnostic efficacy Citation[9],Citation[10]. Both the ADAM and AMS instruments are self-administered and easy to complete and score; average time is less than 10 minutes. Their sensitivity is high (>80%) but both lack adequate specificity. To our disappointment, the CSSAM-Q is a different story. On average it takes 15 minutes to complete and one of the portions requires an investigator. Average time for its administration and scoring is about 30 minutes. In addition the CSSAM-Q appears to be less sensitive than its counterparts and lacks both sensitivity and specificity in several domains (masturbation, sexual interest and physical performance). It was also noted that the memory domain exhibits a favourable bias for certain professions such as engineering and accounting, suggesting that such a domain should not be limited to numerical evaluation. Because of its inherent inconsistencies and limitations it cannot be recommended as an alternative to existing tools. However, a modified CSSAM-Q that retains the more discriminatory domains and introduces refinements in the less effective categories could, potentially make the CSSAM-Q a viable screening tool for hypogonadism.
Several relevant observations came out of this study. The finding of a poor correlation between symptoms compatible with the diagnosis of hypogonadism and a number of tests that are frequently used to support the clinical impression is a prominent one. In particular, among the sex steroids only DHEA offered some discriminatory value. Surprisingly, IGF-1, an indirect measure of GH production performed better than any other measurement. Since all patients in Group A had had a serum T level below the normal range in order to enter the study, it was surprising that only 16 out of 29 (55.2%) had the abnormal values confirmed on repeat testing. Among those with normal T values at presentation (Group B) 23/30 (77%) were confirmed to have normal T values when tested in the central facility. These discrepancies point to and emphasize that, when a predominant diagnostic weight is given to laboratory values, a single T measurement may be misleading. The option of sending serum specimens to a central laboratory is fraught with financial difficulties and issues of practicality. In addition, there are variations among different laboratories for assessing T. Notwithstanding these observations, one of us (R.R.T.) has recently shown that among a pre-selected population of men with symptoms of hypogonadism, 48% of the patients could meet the biochemical criteria of ISSAM in defining the entity, namely a TT lower than 10.0 nmol/L, an a BT lower than 3.8 nmol/L and a cFT lower than 255 pmol/L Citation[11]. Thus, the importance of repeating evaluation to confirm or deny the initial finding is paramount. It further underscores the need to allow sufficient weight and relevance for an appropriate clinical assessment, as a more reliable guidance to therapeutic decisions in male hypogonadism because the performance of hormonal assays is particularly vexing Citation[12].
Regarding the measurement of DHEA-S, its slight decrease in patients of group A might simply reflect differences in lipid profile, body mass index (BMI) or age in comparison with Group B. The role of DHEA in well-being and sexual function in men remains highly controversial. Studies using variable doses of DHEA have reported anywhere from a beneficial effect Citation[13],Citation[14] to serious doubts Citation[15] and to a complete lack of response Citation[16]. Reasons offered for these contradictory results range from inefficacious preparations (tablets devoid of active drug) or insufficient dose to the fact that DHEA truly has limited effect in physiological functions and its deficiency is of little or no clinical consequence. Accuracy in the methodology for measurement of DHEA and DHEA-S is not at issue. Since controlled trials have not clarified the controversy, the doubts and puzzling questions will remain until further functions of the hormone are established and more sophisticated ways to determine its effects at tissue level Citation[17] become available Citation[18].
Similarly to DHEA and T, GH also decreases with age. This decline in GH production has been implicated in many of the manifestations commonly associated with T deficiency. IGF-1 is easier to measure than GH and it is a reliable reflection of GH production Citation[19]. Due to cost and availability neither GH nor IGF-1 is commonly measured in men in whom hypogonadism is suspected. This study found the best correlation between clinical symptoms and biochemical assays to occur with IGF-1.
Measurement of thyroid function was not helpful either. Despite the rekindled interest on its effect on sexual parameters it does not appear to be a major player as a confounding factor in men suspected of having a decreased production of T.
Our results do not help in clarifying the controversy about the accuracy of the diagnosis of adult hypogonadism by questionnaires or biochemical tests. The study also has the major drawback of a limited number of subjects and the assessment of a single aliquot for determination of the various hormonal parameters. Despite these limitations, not uncommon in the literature, it offers some important clinical insights. It supports the value of both the ADAM and AMS instruments and recognizes the difficulties in developing a new tool. It emphasizes the usefulness of the ADAM and AMS questionnaires as screening tools not suitable, by themselves, as diagnostic instruments. Although it is unproven, we suspect that their use as outcome measures is also limited. Although some of the domains of the CSSAM-Q proved to be discriminatory, additional efforts for its further refinement do not appear a viable proposition when the cost, time investment and doubtful diagnostic accuracy are taken into account.
Without ignoring the important supportive value of the biochemical evaluation in the diagnosis of T deficiency syndromes, recently confirmed Citation[8], we believe that health professionals should place increasing value on the clinical assessment and see the blood tests only as playing an adjunctive and supportive role in diagnosis of adult male hypogonadism Citation[9]. This may prove to be a task fraught with difficulties in view of the long established reliance on and trust of biochemical evaluation ion medical diagnoses.
Conclusion
This study confirms that newer, more complex tools perform similarly to the simpler ADAM questionnaire for the initial screening of men suspected of hypogonadism resulting from the aging process. The lack of correlation between history and physical examination with the most commonly used confirmatory tests, again, clearly points to the importance of the clinical evaluation, the pitfalls of the single biochemical assessment and the need to consider both together in the comprehensive evaluation of men with symptoms compatible with hypogonadism. Focusing on a determination of serum T alone hinders the clinician's ability to manage men with an adult testosterone deficiency syndrome (TDS).
Acknowledgements
This study was sponsored by the Canadian Andropause Society (now Canadian Society for the Study of the Aging Male). We thank Joe Downey for statistical analysis of this study.
References
- Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann NY Acad Sci 1995; 774: 121–128
- Morley J E, Charlton E, Patrick P, Kaiser F E, Cadeu P, McCready D. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49: 1239–1242
- Moore C, Huebler D, Zimmermann T, Hineman L AJ, Saad F, Thai D M. The Aging Male Symptom Scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46: 80–87
- Carani C, Isidori A M, Granata A, Carosa E, Maggi M, Lenzi A, Jannini E A. Multicenter study of the prevalence of sexual symptoms in male hypo and hyperthyroid patients. J Clin Endocrinol Metab 2005; 90: 90–94
- Vance M L. Can growth hormone prevent aging?. N Engl J Med 2003; 480: 779–781
- Heinemann L AJ, Zimmerman T, Vermeulen A, Theil C, Hummel W. A new ‘aging males’ symptom rating scale. Aging Male 1999; 2: 103–114
- Morley J E, Patrick P, Perry H M, III. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51: 554–559
- Tancredi A, Reginster J Y, Schleich F, Pire G, Maasen P, Luycks F, Legross J J. Interest of the Androgen Deficiency in the Aging Males (ADAM) questionnaire for the identification of hypogonadism in elderly community-swelling male volunteers. Eur J Endocrinol 2004; 151: 355–360
- Morley J E, Perry H M, II, Kevorkian R T, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006; 53: 424–429
- Tramblay R R, Gagné J -M. Can we get away from serum total testosterone in the diagnosis of andropause?. Aging Male 2005; 8: 147–150
- Black A, Day A G, Morales A. The reliability of the clinical and biochemical assessment in symptomatic late onset hypogonadism. Can a case be made for a 3 month therapeutic trial?. Brit J Urol 2004; 94: 1066–1070
- Morales A J, Nolan J J, Nelson J C, Yen S SC. Effect of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 1994; 78: 1360–1357
- Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab 2003; 88: 3190–3195
- Skolnick A A. Scientific verdict still out on DHEA. JAMA 1996; 276: 1365–1369
- Baulieu E -E, Thomas G, Legarin S, Lahlou N, Roger M, Debuire B, Fauconau V, Girard L, Hervey M -O, Latour F, Leaud M -C, Mokrane A, Pihi-Ferrandi H, Trivalle C, de a Charriere O, Nouveau S, Rokoto-Arison B, Souberbielle J -C, Raion J, LeBouc Y, Reyneaud A, Girerd X, Forette F. DHEA, DHEA sulfate and aging: Contribution of the DHEA age study to a sociobiomedical issue. Proc Natl Acad Sci USA 2000; 97: 4279–4284
- Labrie F, Belanger A, Simard J, Luu-Thee V, Labrie C. DHEA and peripheral androgen and estrogen formation: intracrinology. Ann NY Acad Sci 1995; 774: 16–28
- Simoncini T, Manella P, Fornari L, Varone G, Caruso A, Genazzani A R. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and non-genomic mechanisms. Endocrinology 2003; 144: 3449–3455
- Blevins L S. Beneficial effects of growth hormone replacement in growth hormone-deficient adults. The Endocrinologist 2002; 12: 405–411
- Colao A, Di Somma C, Spiezia S, Fillipella M, Pironella R, Lombardi G. Effect of Growth Hormone and/or testosterone replacement on the prostate of GH-deficient adult patients. J Clin Endocrinol Metab 2003; 88: 88–94
Appendix 1: Andropause assessment questionnaire
As men age, subtle changes may gradually occur in their sexual interest and functioning, energy level and mood. In some cases these changes may be the result of hormonal changes in their body. This questionnaire is designed to help you assess yourself to determine whether any of these changes have taken place.
Because these changes occur gradually and over months and years, you are asked to try to remember how you were a few years ago in order to determine whether you feel and function much differently now.
Energy level
Compared to a few years ago, at present I have:
□0 Much less energy
□1 A little less energy
□2 As much or more energy
Circle the appropriate number describing your energy level at present:
Performance
Compared to a few years ago:
I play the sports I used to play
□2 As well as before
□1 Not as well as before
□0 Don't play them anymore
My performance at work is
□2 As good as before
□1 Not as good as before
□0 Not working anymore
Erectile function assessment
Please tick the appropriate statement.
In the last 6–12 months my erections were mostly
□4 Firm and allowed easy penetration
□3 Penetration was somewhat difficult but still possible
□2 Penetration was very difficult
□1 Penetration was not possible
□0 I had no sexual contact
Compared to a few years ago, during intercourse, my erections generally last
□3 As long as a few years ago
□2 Not quite as long as a few years ago
□1 Much less long than a few years ago
□0 No sexual contact
In the last month, when masturbating, my erections were mostly
□4 Very firm
□3 Somewhat firm
□2 Somewhat weak
□1 Very weak
□0 Did not masturbate
In the last month, when masturbating, my erections generally lasted
□3 As long as a few years ago
□2 Not quite as long as a few years ago
□1 Much less long than a few years ago
□0 Did not masturbate
Sexual interest assessment
Please circle the number that best describes your frequency of sexual desire in the following areas.
0 = None of the time
1 = A little of the time
2 = Some of the time
3 = A good part of the time
5 = Most of the time
In the last month:
Did you find your thoughts drifting to sexual fantasies
0 1 2 3 4
Did you find yourself noticing and enjoying looking at attractive women
0 1 2 3 4
Did you have an urge for sex
0 1 2 3 4
In the last month:
How often did you engage in sexual intercourse
□0 Not at all
□1 Once
□2 Twice
□3 Three times
□4 Four or more
Mood
Place an X on the line describing how you felt in the last six months on the following dimensions. For example, if you felt down or low you would put an X over the line above ‘very’ beside Down, Low. Or if your mood was quite good you would put an X on the line above ‘moderate’ beside Up, High.
Start by putting your pen on the NEUTRAL answer for each question. Decide which adjective is appropriate for you, then how much (little, moderate, very). Please indicate exactly how you feel.
Sleep assessment
Are you getting as much sleep as you did 5 years ago? □2 Yes □1 No
Do you sleep less than 5 hours most nights? □1 Yes □2 No
Do you have periods during the night when you wake up and have difficulty getting back to sleep?
□3 Never
□2 Once or less per week
□1 Twice per week
□0 Three or more times per week
Do you fall asleep after supper?
□3 Never
□2 Once or less per week
□1 Twice per week
□0 Three or more times per week
Do you feel rested when you wake up in the morning?
□3 Often
□2 Sometimes
□1 Rarely
□0 Never
Short term memory assessment
To be administered and completed by the interviewer
The interviewer says the following:“I am going to say some numbers. Listen carefully and repeat them. I cannot repeat the numbers.” The interviewer says the numbers slowly with equal time and emphasis on each number. The numbers cannot be repeated even if the patient says he didn't hear them well.
Scoring: Score 1 point for each sequence correctly repeated. The maximum score is 22. Discontinue further testing as soon as the subject fails 2 in a row of the same length.
The interviewer says the following:“Now I am going to say some numbers and ask you to repeat them in the reverse order”. (Discontinue when one pair is failed.)