Abstract
Estrogens are reported to be the essential sex steroid acting on some male physiological functions, and bioavailable estrogens comprise the free and albumin-bound fractions. Moreover, most of the bioavailable sex steroid is made up of albumin-bound fraction. We examined the age-related change in serum free, albumin-bound and bioavailable estradiol levels in comparison with each fraction of testosterone and its relationship with serum albumin level in elderly men. Albumin-bound as well as bioavailable estradiol levels declined with age, and their decreases were associated much more with the decrease of albumin level than the increase of sex-hormone binding globulin (SHBG) level in sixties and seventies, and similar results were recognized in the level of each fraction of testosterone, suggesting albumin levels have an important role for maintaining bioavailable sex steroid levels in males aged over sixty. Moreover, our study showed that SHBG levels associated inversely with bioavailable sex steroid levels particularly when serum albumin level was low. It seems likely that the decrease of bioavailable estradiol as well as testosterone is induced by the decrease of albumin-bound fractions in combination with the increase of SHBG-bound fractions in males aged over sixty, and that their physical characteristics of aging could be induced by the decrease of albumin-bound fractions caused by the decrease of serum albumin regardless of total sex steroid levels.
Introduction
Sex steroids account for sexual dimorphism because they are responsible for the establishment of primary and secondary sexual characteristics, which are under the control of androgens and estrogens in men and women, respectively. Recent studies on the role of estrogens in humans showed that a great number of estrogen actions are preserved in both sexes, while there are many differences on estrogen actions among species Citation[1-5]. Moreover, estrogens are reported to be the essential sex steroid acting on some physiological functions in men Citation[2] and some conservative biological estrogen actions are found to be preserved among species and between sexes Citation[1],Citation[6].
Recent advances came out from many studies that have begun to elucidate the mechanisms of estrogen action on the male reproductive tract Citation[7],Citation[8]. Moreover, many reports exist characterizing a decline of physical, emotional, and sexual functioning in elderly men, and suggest that symptoms such as changes in psychosocial function of elderly men might be related to declining serum testosterone levels Citation[9-13]. However, the observed association between andropause symptoms and declining testosterone may be causal, or rather merely coincidental. In addition, in the recent study, endogenous bioavailable estradiol levels are reported to be inversely associated with depressive mood as well as bioavailable testosterone levels Citation[14]. Further, men also incur substantial bone loss with aging Citation[15], and osteoporosis is being recognized increasingly in men as the male population ages and lives longer, although it has previously been regarded as a women's disease Citation[16],Citation[17]. The concept that estrogens are essential for bone maturation and mineralization in both men and women is well established today Citation[2],Citation[13].
In the mean time, the bioavailable sex steroids comprise the fractions that are free or associated with albumin in the circulation Citation[18-20], and it is these fractions that have rapid access to target tissues Citation[21]. Several studies have measured circulating free testosterone as a parameter of bioactive sex steroid levels and fails to account for the 35–55% of the circulating steroids bound to albumin, which results in the vast underestimation of the proportion available to target tissues, because most of the bioavailable sex steroid is made up of albumin-bound sex steroid and the free fraction constitutes only a few per cent of the bioavailable sex steroids Citation[20],Citation[22],Citation[23].
Despite these findings, there has never been any report examining the level of albumin-bound fraction of bioavailable estradiol in elderly men, because there is considerable inter-individual variability in steroid levels and they have failed to measure levels of each circulating fraction of bioavailable estradiol and testosterone. Moreover, they failed to examine the relationship between albumin-bound sex steroids and serum albumin levels, which might have an important role in sex hormone dynamic movements.
Therefore, in the present study, we defined the age-related changes in circulating free, albumin-bound and bioavailable (free + albumin-bound) estradiol levels in comparison with each fraction of bioavailable testosterone as an initial step to elucidate the role of estrogen in elderly human male physiology. We also assessed the importance of serum albumin level in determining bioavailable sex steroid levels in Japanese elderly men and its relationship to albumin-bound sex steroid levels, which has never been examined or accounted for.
Methods
Subjects
Seven hundred and sixty-five elderly men with ages between 36 and 96 years (Mean ± SD: 69.5 ± 9.63) in this observational study have been recruited from our urological clinic. Persons with major systemic disorders such as renal, hepatic, thyroid, parathyroid, adrenal disease, or history of malignant tumour were excluded. None were taking any agent known to affect steroid metabolism, such as steroid, vitamin D, calcium, calcitonin, antiepileptics, and thiazides. The Ethical Review Committee of the Saitama Medical University approved the study, and all subjects gave their written informed consent prior to participation.
Laboratory methods
Serum samples were obtained between 8:00 a.m. and 10:00 a.m. after an overnight fast. All samples were stored at −80°C until analysed. Fasting serum samples were assayed by commercial kits for radioimmunoassays to determine the serum concentrations of estradiol (Diagnostic Systems Laboratories, Webster, TX; interassay coefficient of variation was 11%) and testosterone (Diagnostic Products Corp., Los Angeles, CA; interassay coefficient of variation was 11%). Serum LH and FSH were measured by immunoradiometric assays (Diagnostic Products Corp.; interassay coefficients of variation were 13% for LH and 11% for FSH). Serum albumin was measured by commercially available kit for ELISA (Funakoshi, Tokyo, Japan) and serum SHBG was measured by commercially available kit for radioimmunoassay (Wien Laboratories, Succasunna, NJ; interassay coefficient of variation was 6%).
Free testosterone and estradiol levels were measured by an equilibrium dialysis method Citation[24-26]. Dialysis cells, originally designed for measuring free T4 in serum Citation[27], were used to separate the dialysable (free) and bound fractions of testosterone and estradiol. Two hundred μL of serum in the inner compartment were dialysed against 2.4 mL dialysis buffer that was designed to approximate the composition of a protein-free ultrafiltrate of normal human serum (131 mmol Na, 4.3 mmol K, 1.9 mmol Ca, 1.0 mmol Mg, 98 mmol Cl, 1.3 mmol PO4, 1.3 nmol SO4, 5.4 nmol lactate, 3.3 nmol glutamate, and 8 mmol urea). Dialysis was performed overnight for 16 hours at 37°C. Four hundred μL of the dialysate from the outer compartment were assayed in duplicate for testosterone and estradiol by RIA, using 125I-labelled ones purchased from ICN Pharmaceutical Co. (Irvine, CA). Then the results were multiplied by 12 and the free sex steroid concentrations were represented as pg/mL of serum.
Bioavailable (non-SHBG bound) fractions of testosterone and estradiol were determined by the modified technique Citation[18],Citation[19]. Briefly, tracer amounts of tritium-labelled testosterone or estradiol were added to serum aliquots. An equal volume of a saturated solution of ammonium sulphate (final concentration; 50%) was added, which effectively precipitates the beta-globulin fraction, including SHBG with its specifically bound steroid. Separation of the SHBG fraction was performed by centrifugation at 1100 × g for 30 min at 4°C. The percentage of labelled steroid remaining in the supernatant (free and albumin-bound fractions) was then calculated. The bioavailable steroid concentration was obtained by multiplying the total steroid concentration, as determined by RIA.
Albumin-bound fractions of testosterone and estradiol levels were obtained by subtraction free levels from bioavailable testosterone and estradiol levels.
Statistical analysis
Pearson correlations were used to summarize relationships between the various continuous variables.
Results
Age-related changes in serum sex steroids levels
Descriptive data of the hormone measurements are shown in . shows the age-related changes in serum sex steroid levels in elderly men. Total estradiol declined only marginally with age (0.077%/year), whereas free, albumin-bound and bioavailable (free + albumin-bound) estradiol decreased by 0.862, 0.907 and 0.905%/year, respectively. The decline in the free, albumin-bound and bioavailable testosterone (approximately 0.8%/year) were almost twice that in total testosterone. The albumin-bound estradiol and testosterone levels decreased with age slightly faster than free estradiol and testosterone levels in a manner similar to bioavailable estradiol and testosterone levels.
Table I. Summarized values of sex steroid, albumin and SHBG levels in serum from 765 elderly men.
Table II. Correlation coefficients for association of age with sex steroid levels and serum albumin and SHBG concentrations.
The age-related decreases in sex steroids were accompanied by parallel increase in serum LH and FSH levels (). Serum LH level was inversely correlated with serum free, albumin-bound and bioavailable estradiol and testosterone levels, while serum LH in men did not correlate with serum total estradiol or testosterone levels (r = −0.02 and −0.01, respectively). The albumin-bound estradiol and testosterone levels associated with serum LH levels (r = −0.43 and r = −0.31; both p < 0.001, respectively) in a manner similar to bioavailable estradiol and testosterone levels (r = −0.41 and r = −0.33; both p < 0.001, respectively), while the free estradiol and testosterone levels associated with LH levels slightly weaker than the albumin-bound and bioavailable estradiol and testosterone levels (r = −0.26 and r = −023.; both p < 0.01, respectively). However, serum FSH was only weakly inversely correlated with total estradiol and testosterone levels (r = −0.14; p = 0.05; and r = −0.14; p = 0.05, respectively).
Relationship between sex steroid levels and serum concentration of albumin and SHBG
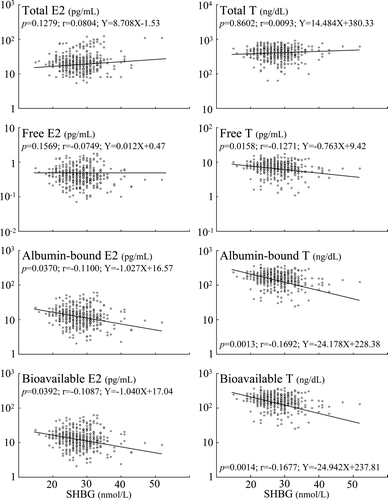
shows the age-related decrease in serum albumin and increase in SHBG. The age-related decreases in sex steroids were accompanied by parallel decrease in serum albumin level and increase in serum SHBG level (). The age-related decrease in the serum albumin levels were comparable to the decreases in free, albumin-bound and bioavailable estradiol and testosterone levels, while increase in SHBG levels were inversely correlated with their decreases. and show the correlation coefficients between serum albumin and SHBG concentrations and sex steroids in elderly men. The albumin-bound estradiol and testosterone levels associated with serum albumin level in a manner similar to bioavailable estradiol and testosterone levels, while the free estradiol and testosterone levels associated with serum albumin level slightly weaker than the albumin-bound and bioavailable estradiol and testosterone (). On the other hand, the albumin-bound estradiol and testosterone levels inversely correlated with serum SHBG level in a manner similar to bioavailable estradiol and testosterone levels, while the free estradiol did not correlate with serum SHBG level and the free testosterone level associated with serum SHBG level slightly weaker than the albumin-bound and bioavailable testosterone (). In addition, the serum albumin level associated with the albumin-bound and bioavailable estradiol and testosterone levels stronger than the serum SHBG level ( and ). Moreover, the percentage annual decreases of free, albumin-bound and bioavailable sex steroid levels were comparable to the sum of the percentage annual decreases in serum albumin level and the percentage annual increase in serum SHBG level (approximately 0.8), and the percentage annual decrease in serum albumin level was one third of the percentage annual increase in serum SHBG level (). In addition, serum albumin and SHBG levels did not correlate with total estradiol and testosterone levels.
Figure 1. The correlation coefficients between serum albumin concentrations and sex steroid levels in elderly men.
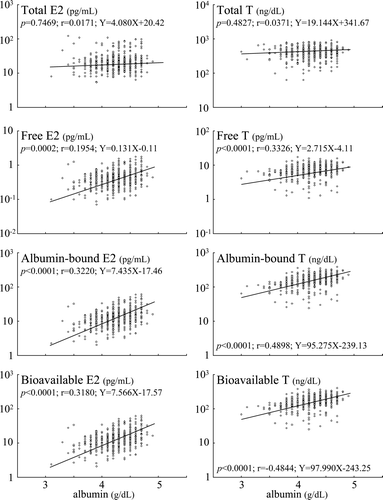
Figure 2. The correlation coefficients between serum SHBG concentrations and sex steroid levels in elderly men.
The relationships between sex steroid levels and serum concentration of albumin and SHBG were also analysed by age in five groups; Group 1: 36–49 years old (n = 114), Group 2: 50–59 (n = 178), Group 3: 60–69 (n = 191), Group 4: 70–79 (n = 153) and Group 5: 80–96 (n = 129). The association of albumin-bound and bioavailable estradiol levels with serum albumin level became significant in Group 3 (age between 60–69) and the significance increased in Group 4 (age between 70–79), although disappeared in Group 5 (age between 80–96). On the other hand, the associations of albumin-bound and bioavailable testosterone levels with serum albumin level became slightly apparent in Group 2 (age between 50–59), although their association was not statistically significant, and the strong correlation was recognized in Group 3 (age between 60–69) and the correlation became stronger in Group 4 (age between 70–79), but slightly weakened in Group 5 (age between 80–96) ().
Table III. Age related changes of the relationship between sex steroid levels and serum albumin concentration.
Although the association of the albumin-bound and bioavailable estradiol levels with serum SHBG level was not statistically significant in all age groups as well as free one, the tendency of inverse correlation between the albumin-bound and bioavailable sex steroid levels with serum SHBG level was highest in Group 3 (age between 60–69) (). Then, the relationships between sex steroid levels and serum concentration of SHBG at different levels of serum albumin were also analysed in 4 groups; Group A: 3.0–3.5 g/dL (n = 83), Group B: 3.6–4.0 (n = 144), Group C: 4.1–4.5 (n = 433) and Group D: 4.6–5.0 (n = 105). The association of free, albumin-bound and bioavailable testosterone levels with serum SHBG levels was significant in Group A and B (albumin concentration: less than 4.0 g/dL) but not in Groups C and D (albumin concentration: more than 4.1 g/dL). On the other hand, the association of free, albumin-bound and bioavailable estradiol levels with serum SHBG levels have slight negative associations in Groups A and B, although their associations were not statistically significant ().
Table IV. Age related changes of the relationship between sex steroid levels and serum SHBG concentration.
Table V. The relationship between sex steroid levels and SHBG concentration at different albumin concentrations.
Discussion
We defined the age-related changes in circulating free, albumin-bound and bioavailable (free + albumin-bound) estradiol levels in comparison with each fraction of testosterone levels. We also assessed the importance of serum albumin levels in determining each fraction of bioavailable sex steroid levels in Japanese elderly men.
Free, albumin-bound and bioavailable (free +albumin-bound) estradiol and testosterone levels all declined relatively much more with age compared with total estradiol and testosterone. Our findings that free and bioavailable testosterone decrease with increasing age are in agreement with previous studies Citation[10],Citation[28],Citation[29]. To our knowledge, however, no reference values for the albumin-bound fraction of estradiol and testosterone exist in the elderly men. Moreover, our study demonstrated that circulating levels of free, albumin-bound and bioavailable (free + albumin-bound) estradiol and testosterone all declined relatively much more with the decrease of serum albumin level than the increase of serum SHBG level, and SHBG concentration associated negatively with bioavailable sex hormones particularly when serum albumin level is low. These results give rise to the possibility that one of the most important factors for the age-related decrease of bioavailable estradiol and testosterone is the decrease of albumin bound estradiol and testosterone with age in combination with the increase of SHBG-bound fractions.
Until recently, not much attention has been paid to the role of estrogens in elderly men. The studies that have been reported so far show no change of total estradiol levels with age in men Citation[13],Citation[30],Citation[31] or a decrease of estradiol levels at old age Citation[32]. The earlier studies also reported a decrease of bioavailable estradiol Citation[13],Citation[33],Citation[34]. In women, estrogen deficiency is the major cause of early post-menopausal, and perhaps also the subsequent phase of late post-menopausal physical changes Citation[15],Citation[33],Citation[35]. This leads to a conceptual problem in defining the cause of age-related physical changes in men because serum levels of total estradiol and testosterone decline only minimally with age Citation[36]. Moreover, previous epidemiological studies have found either no association Citation[36],Citation[37] or even a negative association between serum total testosterone levels and physiological changes in aging men Citation[23]. The latter study did note a positive association between serum estradiol levels and bone mineral density in elderly men Citation[23], but it was difficult to attribute bone mineral density loss with aging in men to estrogen deficiency as serum total estradiol levels remain relatively constant over the life span in men as shown in the present study. Our data for each fraction of bioavailable estradiol and testosterone levels may help to resolve these issues. In the present study, the free, albumin-bound and bioavailable (free + albumin-bound) estradiol concentrations decreased with age to an even greater extent than total estradiol. Together with the results of the present study, it is possible that age-related bone loss in men seems to be attributed to the decrease in bioavailable estradiol. By directly measuring free and bioavailable estradiol and testosterone levels, we are able to demonstrate that elderly men have age-related decrease in both free, albumin-bound and bioavailable (free + albumin-bound) estradiol levels as well as testosterone levels. Taken together, our data are consistent with the hypothesis that age-related decrease in estradiol bioavailability could at least in part account for physical changes in elderly men.
Moreover, our results suggest that the decrease of bioavailable estradiol and testosterone is mainly due to the age-related decrease of albumin-bound ones. Earlier study suggested that albumin-bound sex steroid is available for uptake by most tissues, whereas SHBG-bound sex steroid is not Citation[38]. Considering the fact that bioavailable sex steroid as well as the albumin-bound fraction are strongly related to the physical characteristics of aging Citation[34], it is possible that the albumin-bound fraction of sex steroid is available for uptake by tissues and can exert biological effects. In the present study, albumin-bound estradiol and testosterone levels as well as bioavailable estradiol and testosterone levels have stronger relations to ages than total estradiol and testosterone levels, and also have slightly stronger relations than free estradiol and testosterone levels. Previous study showed that bioavailable sex steroid seems to be the best parameter for serum levels of bioactive sex steroid, which seems to play a direct role in the various changes that occur during aging Citation[34]. Therefore, the decrease of albumin-bound estradiol and testosterone with age seems to be the best parameter for the decrease of circulating bioactive sex steroid levels. Moreover, serum albumin concentration has an important role for maintaining serum levels of bioactive sex steroids and SHBG levels is also one of the important determinants of bioavailable sex steroids particularly when serum albumin concentration is low.
In addition, it is noteworthy that the relationships of the albumin-bound and bioavailable sex steroid levels with serum albumin level are strongest in males in their seventies followed by sixties, and that the tendency of inverse correlation between albumin-bound and bioavailable sex steroid levels with serum SHBG level is strongest in males in their sixties, while their relationships with serum SHBG levels are not statistically significant in all ages. These results give rise to the possibility that albumin level is a much more important factor than SHBG level to influence bioavailable sex steroid levels in males in their sixties and seventies.
In conclusion, the age-related changes in circulating free, albumin-bound and bioavailable (free + albumin-bound) estradiol levels were established as well as each fraction of testosterone levels. Our results raise the possibility that the decrease of albumin-bound fraction in combination with the increase of SHBG-bound fraction is the important determinant of the decrease of bioavailable estradiol as well as testosterone, and that albumin levels affect albumin-bound sex steroid levels especially in males in their sixties and seventies and SHBG levels affect bioavailable sex steroid levels particularly when serum albumin level is low. Therefore, the decreases of serum albumin levels, which are caused by undernourished state, illness or aging, might induce symptoms of sex hormone deficiencies in sixties and seventies regardless of total estradiol and/or testosterone levels. We need to reevaluate the traditional belief in the effects of sex steroids on the human male physiology age by age in relation to the albumin-bound estradiol and testosterone levels.
References
- Carani C, Qin K, Simoni M, Faustini-Futini M, Serpente S, Boyd J, Korach K S, Simpson E R. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 1997; 337: 91–95
- Faustini-Futini M, Rochira V, Carani C. Oestrogen deficiency in men: where are we today?. Eur J Endocrinol 1999; 140: 111–129
- Grumbach M M, Auchus R J. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 1999; 84: 4677–4694
- Rochira V, Balestrieri A, Faustini-Futini M, Borgato S, Beck-Peccoz P, Carani C. Pitutary function in a man with congenital aromatase deficiency: effect of different doses of transdermal E2 on basal and stimulated pituitary hormones. J Clin Endocrinol Metab 2002; 87: 2857–2862
- Swaab D F. The complex effects of sex hormones on the brain it all depends on the species, sex, age and cell type. XX vs XY 2003; 1: 3–4
- Couse J F, Korach K S. Estrogen receptor null mice: what have we learned and where will they lead us?. Endocr Rev 1999; 20: 358–417
- Luconi M, Muratori M, Forti G, Baldi E. Identification and characterization of a novel functional estrogen receptor on human sperm membrane which interferes with progesterone effects. J Clin Endocrinol Metab 1999; 84: 1670–1678
- Raman J D, Schlegel P N. Aromatase inhibitor for male infertility. J Urol 2002; 167: 624–629
- Gray A, Berlin J, McKinlay J, Longcops C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol 1991; 44: 671–684
- Vermeulen A. Androgens in the aging male. J Clin Endocrinol Metab 1991; 73: 221–224
- Vermeulen A, Kaufman J M, Giagulli V A. Influence of some biological indices on sex hormone binding globulin an androgen levels in aging and obese males. J Clin Endocrinol Metab 1996; 81: 1821–1827
- Vermeulen A. Androgen replacement therapy in the aging male. A critical evaluation. J Clin Endocrinol Metab 2001; 86: 2380–2390
- Sternbach H. Age-associated testosterone decline in men: clinical issues for psychiatry. Am J Psychiatry 1998; 155: 1310–1318
- Barrett-Connor E, Von Muhlen D G, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 1999; 84: 573–577
- Riggs B L, Melton L J, III. Involutional osteoporosis. N Engl J Med 1898; 314: 1676–1686
- Gullberg B, Johnell O, Kanis J A. World-wide projections for hip fracture. Osteoporos Int 1997; 7: 407–413
- Stock H, Schneider A, Strauss E. Osteoporosis: a disease in men. Clin Orthop 2004; 425: 143–151
- O'Connor S, Baker H WG, Dulmanis A, Hudson B. The measurement of sex steroid binding globulin by differential ammonium sulphate precipitation. J Steroid Biochem 1973; 4: 331–339
- Tremblay R R, Dube J Y. Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception 1974; 10: 599–605
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17beta to human plasma proteins at body temperature. J Steroid Biochem 1982; 16: 801–810
- Manni A, Pardridge W M, Cefalu W, Nisula B C, Bardin C W, Santner S J, Santen R J. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab 1985; 61: 705–710
- Drinka P J, Olson J, Bauwens S, Voeks S, Carlson I, Wilson M. Lack of association between free testosterone and bone density in elderly men. Calcif Tissue Int 1993; 52: 67–69
- Slemenda C W, Longcope C, Zhou L, Hui S L, Peacock M, Johnston C C. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest 1997; 100: 1755–1759
- Moll G W, Jr, Rosenfield R L, Helke J H. Estradiol-testosterone binding interactions and free plasma estradiol under physiological conditions. J Clin Endocrinol Metab 1981; 52: 868–874
- Bhasin S, Storer W, Berman M, Callegari C, Clevenger B, Phillips J, Lee W P, Bunnell T J, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996; 335: 1–7
- Vermeulen A. Reflections concerning biochemical parameters of androgenicity. The Aging Male 2004; 7: 280–289
- Nelson J C, Tomei R. Direct determination of free thyroxin in undiluted serum by equilibrium dialysis/radioimmunoassay. Clin Chem 1988; 34: 1737–1788
- Warner B A, Dufau M L, Santen R J. Effects of aging and illness on the pituitary testicular axis in men: qualitative as well as quantitative changes in luteinizing hormone. J Clin Endocrinol Metab 1985; 60: 263–268
- Korenman S G, Morley J E, Mooradian A D, Davis S S, Kaiser F E, Silver A G, Viosca S P, Garza D. Secondary hypogonadism in older men: its relation to impotence. J Clin Endocrinol Metab 1990; 71: 963–969
- Davidson J M, Chen J J, Crapo L, Gray G D, Greenleaf W J, Catania J A. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab 1983; 57: 71–77
- Labrie F, Belanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab 1997; 82: 2403–2409
- Baker H W, Burger H G, de Kretser D M, Hudson B, O'Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennis G D. Changes in the pituitary-testicular system with age. Clin Endocrinol 1976; 5: 349–372
- Khosla S, Melton L J, III, Atkinson E J, O'Fallon W M, Klee G G, Riggs B L. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998; 83: 2266–2274
- van den Beld A W, de Jong F H, Grobbee D E, Pols H AP, Lamberts S WJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 2000; 85: 3276–3282
- McKane W R, Khosla S, Risteli J, Robins S P, Muhs J, Riggs B L. Role of estrogen deficiency in pathogenesis of secondary hyperparathyroidism and bone turnover abnormalities in elderly women. Proc Assoc Am Physicians 1997; 109: 174–180
- Meier D E, Orwoll E S, Keenan E J, Fagerstrom R M. Marked decline in trabecular bone mineral content in healthy men with age: lack of association with sex steroid levels. J Am Geriatr Soc 1987; 35: 189–197
- Murphy S, Khaw K-T, Cassidy A, Compston J E. Sex steroids and bone mineral density in elderly men. Bone Miner 1992; 20: 133–140
- Pardridge W M. Serum bioavailability of sex steroid hormones. J Clin Endocrinol Metab 1986; 15: 259–278