Abstract
Objective. The aim of the present study was to analyse the effect of testosterone therapy on bone mineral density in healthy elderly men who had low levels of total testosterone.
Design. Randomized, double-blind, placebo-controlled study.
Participants. Forty-eight men over 60 years old with decreased testosterone levels (≤320 ng/dL) comprised the study. Twenty-five out of 48 received intramuscular injections of testosterone enanthate every three weeks during 12 months; the remaining 23 participants formed the control group. All participants had measurements of bone mineral density (BMD) in both lumbar spine and hip before and at the end of the study as well as testosterone and 17-β estradiol levels.
Results: Testosterone treated group exhibited a significant (p < 0.05) increment (from 1.198 ± 0.153 to 1.240 ± 0.141 g/cm2) in lumbar BMD in parallel with a significant (p < 0.001) increment (from 301 ± 32 to 471 ± 107 ng/dL) in testosterone concentrations, whereas no significant change occurred in femoral neck BMD.
Conclusions. Testosterone therapy elicited a positive effect only in lumbar BMD in elderly men with diminished testosterone serum levels.
Introduction
As men age there is a physiological decline in androgen which might induce unfavourable changes in organs upon which androgens act Citation[1-2]. In consequence, it has been suggested that the decrement in testosterone levels could be associated with low bone mineral density (BMD) in elderly males Citation[3-5].
On the basis of these observations, the use of testosterone therapy has been proposed to be beneficial on BMD in elderly men with reduced testosterone concentrations Citation[6-7]; however, few controlled randomized trials have been addressed to assess whether testosterone therapy in older men might increase bone density. At present, three placebo-controlled randomized trials in older men Citation[8-10] reported imprecise effects on BMD. In one study, intramuscular testosterone treatment increased significantly lumbar spine and hip BMD Citation[9]. Conversely, in others studies with transdermal testosterone therapy have not been convincingly established improvement in bone density Citation[8]. Moreover, it was suggested an improvement in bone density only in subjects with pretreatment testosterone levels ≤200 ng/dL Citation[10]. Therefore, questions still exist about the potential benefit of testosterone treatment on BMD in elderly men with low normal testosterone levels.
The aim of the present study was to evaluate the effect of intramuscular testosterone therapy on BMD in elderly men with low total serum testosterone concentrations.
Methods
Participants
Forty-eight subjects over 60 years of age were included in the study, and they were recruited from November 2002 to January 2005, in the General Hospital 25 of the Instituto Mexicano del Seguro Social in Mexico City. All participants had total serum testosterone levels below 320 ng/dL. Low normal testosterone level was defined as a value under 1 standard deviation (SD) of the mean in normal men aged between 50 to 70 years Citation[11]. None of them had received hormone therapy prior to this study. Participants were interviewed for medical history and underwent a physical examination, including a digital rectal examination for prostatic abnormalities.
Subjects were not included when they had a history or presence of any malignancy and prostate hypertrophy, a history of recent cardiovascular disease, liver or renal disease, haematological abnormalities, diabetes and increased (≥4.0 ng/mL) prostate-specific antigen (PSA). Participants were excluded if they were taking any medications affecting bone metabolism, or more than 30 mL of alcoholic drinks a day.
Design
This study had a randomized, double-blind, placebo-controlled design. Forty-eight subjects were eligible for randomization. A randomization without stratification was computer-generated and randomization numbers were assigned to the subjects in orders of enrolment into the trial. Twenty-five out of the 48 subjects received testosterone treatment and 23 comprised the placebo group. Enanthate testosterone was administered IM at a dose of 250 mg every 21 days during a period of 12 months. Those in the placebo group received sham injections. The placebo injections were identical in appearance to the testosterone injections. Participants were clinically evaluated every 21 days in the outpatient clinic where the injections were applied by a nurse. Pharmacy personnel labelled the injections for the participants and provided the medication upon prescription of the physician. Only the pharmacist knew of the randomization, neither the participants nor the radiologist and laboratory technicians knew the content of the injections until the entire study was completed.
Measurements of biochemical markers such PSA, haematocrit and hepatic transaminases were carried out every two months. The study was approved by the ethics committee of the Instituto Mexicano del Seguro Social, the volunteers were informed about the trial, and gave written consent to participate.
Hormone analysis
At baseline and the end of the study, fasting blood samples were obtained from an antecubital vein between 7:30 and 8:30 a.m. using vacuum tubes, these samples were centrifuged at 400 g for 15 minutes. The serum was aliquoted and stored at −70°C until assayed in a single run for each hormone assay. Serum testosterone levels were measured by a specific solid-phase radioimmunoassay using commercial kits from Diagnostic Products Corporation (Los Angeles, CA). The intra- and inter-assay coefficients of variation (CVs) were 7.3% and 7% respectively. The analytical sensitivity of this assay was 4 ng/dL. Serum 17-β estradiol levels were measured by a solid phase radioimmunoassay with a kit from Diagnostic Products Corporation (Los Angeles, CA). The intra- and inter-assay CVs were 4.0 and 8.6%, respectively, the sensitivity of this assay was 10 pg/mL. The reference normal values for testosterone in men aged 50 to 70 years in our laboratory is 300 to 700 ng/dL, whereas the reference range for estradiol is 10 to 40 pg/mL.
Bone mineral density
Bone mineral density (BMD) was estimated at baseline and after 12 months of treatment, BMD was measured at the lumbar spine (L1-L4 together) and the hip (femoral neck) by dual energy X-ray absorptiometry using a Lunar DPX densitometer scanner (GE Lunar Corporation, Madison, WI). Scanning was performed according to the instructions of the manufacturer; the CV for this technique was 1.0% in the lumbar spine and 1.3% in the femoral neck. Calibration was performed routinely every morning using the standard provided by the manufacturer. BMD was expressed as raw bone density as well as in standardized deviation scores compared with a young adult (T score) reference populations as calculated by the commercial protocols. All subjects were tested on the same machine and by the same operator.
Statistical analysis
Statistical power calculations performed to detect a difference in BMD after treatment of 3% revealed the need for 21 participants per group to achieve a power of 80%. Intention-to-treat analysis was performed; for 6 subjects who did not complete the protocol the baseline observation of BMD was carried forward to final time points. Data are expressed as means (± SD) and a parametric statistical analysis was used. Comparison of change in serum hormones concentrations basal and after treatment was done using the paired t test. The significance of the change between groups was tested using the independent sample t test. Correlation between testosterone, estradiol and BMD was calculated with Pearson coefficients. Comparisons resulting in P ≤ 0.05 were considered statistically significant. Analysis was performed using the computer program statistical software SPSS version 12.0.
Results
Main baseline characteristics of participants are shown in , they were similar in both groups. The age of the participants was 63.15 ± 7.9 years. Three out of the total 48 participants had testosterone level below 200 ng/dL, the other 21 patients had values between 201 to 300 ng/dL, and the remaining 24 had concentrations between 301 and 320 ng/dL.
Table I. Baseline characteristics of participants
Forty-two subjects completed the entire 12 months of the study. Six subjects did not complete the study for the following reasons: increment of PSA (three were in the treated group) and lost to follow-up (two were in the control group, and one was in the treated group).
Hormonal levels
The treated group exhibited a significant (p < 0.001) increment in testosterone concentrations (from 301 ± 32 ng/dL to 471 ± 107 ng/dL), at the end of the 12 months study. Total testosterone levels during the treatment reached values within normal range and neighbouring to mean for the age-matched (504 ± 169 ng/dL) male in our laboratory. By contrast, testosterone levels remained similar in the control group and there was a significant (p < 0.05) difference between groups in testosterone values at the end of the study (). Collaterally 17-β estradiol levels increased significantly (p < 0.01) in the treated group and these values had a positive correlation (r = 0.221, p = 0.014) with testosterone concentrations.
Table II. Hormonal levels before and after 12 months of testosterone therapy
Bone mineral density
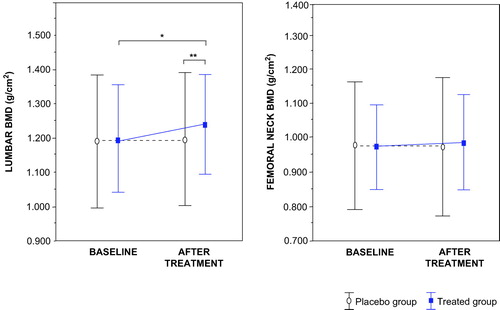
Baseline BMD of the lumbar spine and hip were similar in both groups, but lumbar BMD had a significant (p < 0.05) increment (from 1.198 ± 0.153 to 1.240 ± 0.141 g/cm2) after testosterone therapy (). Lumbar spine BMD increased by 0.042 ± 0.011 g/cm2 (+3.5% of initial value) in the treated group; in addition a significant (p = 0.05) difference was observed between the two groups at the term of the study. By contrast, femoral BMD was similar in both groups after treatment.
Figure 1. Mean (±SD) lumbar and femoral bone mineral density (BMD) at baseline and after testosterone treatment. At the end of the study, there was a difference statistically significant in lumbar BMD between both groups. *P < 0.05 compared whit baseline, by paired t test analysis. **P < 0.05 between groups, by unpaired t test.
The increment in lumbar BMD after testosterone therapy correlated significantly (r = −0.201, p < 0.05) with baseline 17-β estradiol, that is, the lower the pretreatment estradiol level, the greater the effect of testosterone treatment on BMD. This correlation was not significant with testosterone levels.
Side effects
During the study three out of 25 treated patients had a moderate elevation in PSA levels (between 4.3 and 5.0 ng/mL). These participants were excluded from the study; after discontinuation of the testosterone treatment, PSA level normalized, and they were thoroughly evaluated by the consultant urologist who ruled out malignancy in the prostate.
Haematocrit values showed a significant (p < 0.05) increment in the group compared with the placebo group (from 44.7 ± 5.2% to 46.4 ± 4.3%) but within the normal range [12). No subject experienced an increase above the upper limit of normal (52%); neither required suspension of therapy Citation[12-13]. Serum liver enzymes remained unchanged during the study.
Discussion
Aging men with low levels of either testosterone or 17-β estradiol concentrations are more likely to be osteoporotic Citation[3],Citation[14-15]. Furthermore in young men with a diagnosis of hypogonadism, the use of testosterone improves bone formation markers and trabecular bone density Citation[16-17], and these effects are also present in older men with low testosterone levels Citation[6],Citation[18-19].
In the present study a significant increase in lumbar BMD after 12 months of testosterone therapy in men with age-related decline of testosterone levels was observed. The increment in the lumbar spine BMD was similar to that previously reported Citation[9]. In a study by Amory et al., spine and hip BMD increased significantly after intramuscular testosterone Citation[9]. In our study, femoral BMD was unchanged after 12 months of intramuscular testosterone treatment; by contrast in the Amory study, the hip BMD increased after 18 and 24 months of testosterone therapy Citation[9]. The failure of hip BMD to increase during testosterone treatment in present study could be at least partly related to the slower response of hip to changes in testosterone Citation[10]. Similarly, in postmenopausal women, the bone density of the hip does not increase as much in response to estrogen therapy as does that of the spine Citation[10],Citation[20]. In another recent study, 207 older men who were treated with testosterone did not increase BMD after six months Citation[21], although the intervention period was relatively short for detecting bone changes.
Two randomized clinical trials of transdermal testosterone therapy in older men have been also published Citation[8], Citation[10]. In the first study, testosterone treatment prevented the loss of hip BMD observed in the placebo group Citation[8]. In the second study, the increments in lumbar BMD were not significantly different from placebo until a post-hoc analysis demonstrated differences when pre-treatment testosterone concentrations were below 200 ng/mL Citation[10].
In part, the varying effects of testosterone therapy on bone density can be explained by the type of testosterone preparation, since it has been suggested that testosterone concentrations achieved with intramuscular preparation could be higher than those achieved with transdermal administration, yielding a greater effect on bone density Citation[10],Citation[22].
Likewise, a recent meta-analysis has shown that testosterone therapy, particularly intramuscular testosterone, increases lumbar bone density in elderly men after a minimum of 12 to 36 months of treatment, but the results on femoral BMD are inconclusive Citation[21-22]. This systematic review included fewer trials of older men with age-related decline in testosterone levels, and consequently additional studies are necessary to support those assumptions Citation[21-22]. Therefore, the results of our study are important because they confirm that intramuscular testosterone therapy improves spine BMD in older men with age-related decline in testosterone concentrations.
In the present study, following therapy there was an increment in both testosterone and estradiol level. In addition, improvement in BMD scores was greater when baseline levels of estradiol were at their lowest. The effect of testosterone in BMD may be partially mediated by its peripheral conversion to estradiol as has been already suggested Citation[23-25]. Thus, it is possible that testosterone therapy has beneficial effects on BMD through both androgenic and estrogenic actions Citation[25]. However, the lack of statistical association of baseline testosterone levels and the changes in BMD needs to be interpreted cautiously, because the present study was not designed to have adequate statistical power to assess these parameters.
Moreover, it is worth noting that muscle mass is an independent predictor of bone density and it is possible that testosterone therapy also may contribute to bone density via this mechanism Citation[26]. It should be also noted that the most of participants did not have a diagnosis of osteoporosis, since the primary aim in the present study was to assess whether testosterone treatment increased bone density in men with age-related decline in testosterone levels. In addition, the diagnosis of osteoporosis in men is mainly based on the measurement of BMD, but standard parameters for male osteoporosis are still uncertain, due to lack of cut-off values for BMD score and the absence of data on fracture risk for age-stratified values of male BMD Citation[3].
We must consider important issues in evaluating testosterone treatment. First, there is no certain diagnosis of testosterone deficiency in elderly men. Most experts agree that diagnosis should be based on a combination of symptoms and low testosterone level Citation[13],Citation[27]. In addition, the levels of testosterone that should indicate testosterone treatment in older men are unknown, but it has been suggested to consider this treatment in elderly men with clinically significant symptoms of androgen deficiency and testosterone levels below the normal ranges observed in young men Citation[13],Citation[28]. Nonetheless, current scientific evidence does not allow recommending testosterone treatment to promote bone health in older men unless it is accompanied by clinical and biochemical hypogonadism Citation[13],Citation[29]; however, testosterone administration might be indicated to men who have both low levels of testosterone and an additional increase in the risk of osteoporosis.
In sum, this randomized clinical trial showed that treatment with testosterone for 12 months was associated with an increment in lumbar mineralization in elderly men presenting diminished levels of serum total testosterone. Further studies are needed to establish the efficacy and safety of testosterone therapy in males with low BMD and diminished testosterone concentrations.
Acknowledgements
This work was supported by a grant from the Mexican Institute of Social Security (FOFOI). L.B. was the recipient of an educational grant from the National Council of Science and Technology (CONACYT). The authors thank the nursing staff of General Hospital 25, IMSS, for their contributions to this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kaufman M, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005; 26: 833–876
- Gennari L, Merlotti D, Martini G, Gonneli S, Franci B, Campagna S, Lucani B, Dal Canto N, Valenti R, Gennari C, Nuti R. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 2003; 88: 5327–5333
- Rochira V, Balestrieri A, Madeo B, Zirilli L, Granata A RM, Carani C. Osteoporosis and male age-related hypogonadism: Role of sex steroids on bone (patho)physiology. Eur J Endocrinol 2006; 154: 175–185
- Vanderschueren D, Vandenput L, Boonen S, Lindberg M K, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev 2004; 25: 389–425
- Falahati-Nini A, Riggs L, Atkinson E J, O'Fallon W M, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 2000; 106: 1553–1560
- Tenover J S. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 1992; 75: 1092–1098
- Snyder P J, Peachey H, Berlin J A, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow D A, Holmes J H, Kapoor S C, Atkinson L E, Strom B L. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 2670–2677
- Kenny A M, Prestwood K M, Gruman C A, Marcello K M, Raisz L G. Effect of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001; 56: M266–272
- Amory J K, Watts N B, Easley K A, Sutton P R, Anawalt B D, Matsumoto A M, Bremner W J, Tenover J L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 2004; 89: 503–510
- Snyder H PJ, Peachey H, Hannoush P, Berlin J A, Loh L, Holmes J H, Dlewati A, Staley J, Santanna H, Kapoor S C, Attie M F, Haddad J G, Strom B L. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab 1999; 84: 1966–1972
- Saucedo R, Fonseca M E, Basurto L, Ochoa R, Sánchez M, Zárate A. Decremento en los andrógenos circulantes en el hombre durante la senescencia. Gac Med Mex 2000; 136: 335–339
- Ruiz-Argüelles G J, Sanchez-Medal L, Loria A, Piedras J, Cordova M S. Red cell indices in normal adultsresiding at altitudes from sea level to 2670 m. Am J Hematol 1980; 8: 265–267
- Bhasin S, Cunningham G R, Hayes F J, Matsumoto A M, Snyder P J, Swerdloff R S, Montori V M. Testosterone therapy in adult men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2006; 91: 1995–2010
- Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F. Bioavailable estradiol may be an important determinant of osteoporosis in men: The MINOS study. J Clin Endocrinol Metab 2001; 86: 192–199
- Mellstrom D, Johnell O, Ljunggren O, Eriksson A L, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 2006; 21: 529–535
- Behre H M, Kliesch S, Leifke E, Link T M, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab 1997; 82: 2386–2390
- Benito M, Gomberg B, Wehrli F W, Weening R H, Zemel B, Wright A C, Song H K, Cucchiara C, Snyder P J. Deterioration of trabecular architecture in hypogonadal men. J Clin Endocrinol Metab 2003; 88: 1497–1502
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto A M, Snyder P J, Weber T, Berman N, Hull L, Swerdloff R S. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 2004; 89: 2085–2098
- Katznelson L, Finkelstein J S, Schoenfeld D A, Rosenthal D J, Anderson E J, Klinbanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996; 81: 4358–4365
- Duan Y, Tabensky A, DeLuca V, Seeman E. The benefit of hormone replacement therapy on bone mass is greater at the vertebral body than posterior processes or proximal femur. Bone 1997; 21: 447–451
- Emmelot-Vonk M H, Verhaar H JJ, Pour H RN, Aleman A, Lock T MT, Bosch J LH, Grobbee D E, van der Schouw I T. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men. A randomized controlled trial. JAMA 2008; 299: 39–52
- Tracz M J, Sideras K, Boloña E R, Haddad R M, Kennedy C C, Uraga M V, Caples S M, Edwin P J, Montori V M. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled. J Clin Endocrinol Metab 2006; 91: 2011–2016
- Khosla S, Melton L J, III, Riggs B L. Estrogen and the male skeleton. J Clin Endocrinol Metab 2002; 87: 1443–1450
- Compston J. Local biosynthesis of sex steroids in bone. J Clin Endocrinol Metab 2002; 87: 5398–5400
- Meier C, Liu P Y, Handelsman D J, Seibel M J. Endocrine regulation of bone turnover in men. Clin Endocrinol (Oxf) 2005; 63: 603–613
- Proctor D N, Melton L J, Khosla S, Crowson C S, O'Connor M K, Riggs B L. Relative influence of physical activity, muscle mass and strength on bone density. Osteoporosis Int 2000; 11: 944–952
- Allan C A, McLachlan R I. Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol (Oxf) 2004; 60: 653–670
- Kelleher S, Conway A J, Handelsman D J. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89: 3813–3817
- Liu P Y, Swerdloff R S, Veldhuis J D. The rationale, efficacy and safety of androgen therapy in older men: Future research and current practice recommendations. J Clin Endocrinol Metab 2004; 89: 4789–4796
