Abstract
Recent work shows a high prevalence of low testosterone and inappropriately low luteinizing hormone (LH) and follicle stimulating hormone (FSH) concentrations in type 2 diabetes. This syndrome of hypogonadotrophic hypogonadism (HH) is associated with obesity in patients with type 2 diabetes. However, the duration of diabetes or HbA1c are not related to HH. Furthermore, recent data show that HH is not associated with type 1 diabetes. C-reactive protein concentrations have been shown to be elevated in patients with HH and are inversely related to plasma testosterone concentrations. This inverse relationship between plasma free testosterone and C- reactive protein concentrations in patients with type 2 diabetes suggests that inflammation may play an important role in the pathogenesis of this syndrome. This is of interest since inflammatory mechanisms may have a cardinal role in the pathogenesis of insulin resistance. It is also relevant that in the mouse, deletion of the insulin receptor in neurons leads to HH in addition to a state of systemic insulin resistance. It has also been shown that insulin facilitates the secretion of gonadotrophin releasing hormone (GnRH) from neuronal cell cultures. Thus, HH may be the result of insulin resistance at the level of the GnRH secreting neuron. Low testosterone concentrations are also related to an increase in total and regional adiposity. This review discusses these issues and attempts to make the syndrome relevant as a clinical entity. Clinical trials are required to determine whether testosterone replacement alleviates insulin resistance and inflammation. In addition, low testosterone levels are associated with an increase in cardiovascular events. Testosterone therapy may therefore, reduce cardiovascular risk. This important aspect requires further investigation.
Introduction
Large epidemiological studies have demonstrated the association of low testosterone (T) with type 2 diabetes (T2D) Citation[1,2]. In these studies T was treated as a biological marker which was found to be frequently low in males with T2D, often in association with dyslipidaemia and abdominal adiposity Citation[1]. It was also shown that insulin resistance and upper abdominal adiposity were features associated with low T Citation[3,4]. These studies did not report sex hormone binding globulin (SHBG), free T (FT) or gonadotrophin concentrations and thus could not make a comment on the mechanism underlying low T concentrations, nor did they place low T concentrations in a clinically relevant context.
In view of the above and our own recent work demonstrating the frequent occurrence of hypogonadotrophic hypogonadism (HH) in men with T2D, the inverse relationship between BMI and low T Citation[5] and the marked increase of C-reactive protein (CRP) in HH Citation[6], there is potentially an important role for a low T in T2D. In addition, type 1 diabetes(T1D) is not commonly associated with HH Citation[7]. Thus, clearly HH is a feature related to obesity, insulin resistance and T2D rather than hyperglycaemia per se. This growing field in endocrinology needs an organized systematic investigation including prospective controlled studies on the treatment of HH in T2D to determine whether clinical features associated with hypogonadism can be successfully reversed with T replacement. A review of the currently available literature in this area is thus clearly required.
The cross-sectional association of hypogonadism with type 2 diabetes
Association with type 2 diabetes
T concentrations in men have been found to be inversely related to insulin, independently of age and obesity Citation[8]. Several investigators have studied the association of low total T with diabetes Citation[1,2],Citation[9,10],Citation[15-19]. In a population-based cohort study of 985 men (40–79 years), 110 men with T2D had lower mean T and SHBG concentrations Citation[2]. These differences were reduced but not eliminated after adjustment for age and BMI. Of diabetic men, 21% versus 13% of controls had a low T concentration. T was inversely related to the degree of glycaemia in the whole cohort; throughout the whole range of plasma glucose, there was a stepwise decrease in mean T per categorical increase in FPG, apparent in both diabetics and non-diabetics. Anderson et al. also found low T and SHBG concentrations in male T2D Citation[10]. T concentrations were inversely related to fasting insulin concentrations. In a community study, comprising 1,408 older subjects (775 men) with mean age of 73 years, Goodman et al. found that in sex-specific age and BMI adjusted analyses, men with impaired glucose tolerance had significantly lower total T than those with normal glucose tolerance and that T was inversely related to fasting plasma glucose Citation[11]. A recent study in 1,200 men with erectile dysfunction found low total T in 24.5% of T2D as compared to 12.6% in non-diabetic patients; differences in the prevalence of hypogonadism retained significance after adjustment for age and BMI Citation[12].
All these studies measured total T and SHBG but not FT because reliable assays for FT have become available only recently. SHBG concentrations are decreased in obesity. T2D men have even lower SHBG levels as compared to age and BMI matched non-diabetics Citation[2]. It was therefore not clear whether the lower SHBG levels in T2D could account for the difference observed in total T levels between diabetics and non-diabetics.
Total T concentrations are determined to a large extent by circulating SHBG concentrations. In the blood of normal men, 44% of total T is bound to SHBG, 2% is unbound (FT) and 54% circulates bound to albumin and other proteins Citation[13]. Since albumin bound T has 1,000 times lower affinity than SHBG, it can freely disassociate in capillaries. Virtually all the non-SHBG bound T (also called bioavailable T) is therefore available for tissue uptake Citation[14]. Circulating SHBG concentrations are also dependent upon a number of factors, the most important association being with obesity. SHBG concentrations decrease in obesity and increase with aging. A complete assessment of hypogonadism should therefore include measurement of FT. FT measured by radioimmunoassay is considered unreliable because it represents a variable fraction (20% to 60%) of the FT measured by equilibrium dialysis Citation[15-17]. Equilibrium dialysis is considered to be the gold standard for measuring FT. FT measured by this technique represents 1.5% to 4% of total T and is not dependent upon SHBG concentrations Citation[18]. Equilibrium dialysis is a delicate, tedious and time-consuming technique and therefore may not suitable for population-based or large-sized studies. FT can also be calculated from SHBG and T using the method of Vermeulen et al. Citation[16]. This calculated FT has been shown to correlate very well with FT measured by equilibrium dialysis Citation[15] and is well suited for epidemiological studies.
The first study to report the prevalence of hypogonadism in T2D based on appropriately FT measured by equilibrium dialysis was published in 2004 Citation[5]. This study included 103 males with T2D, aged between 31 and 75 years, referred to a tertiary diabetes centre in Buffalo, NY. The prevalence of hypogonadism in T2D men was 33%. The plasma concentrations of LH and FSH were inappropriately low in men with subnormal FT. Indeed, LH and FSH were significantly lower than those in patients with normal FT. In addition, GnRH induced LH and FSH release was normal in all those who were tested. This pointed to a hypothalamo-hypophyseal lesion rather than a testicular defect. Magnetic resonance imaging (MRI) in 10 randomly selected hypogonadal patients revealed no abnormality in brain or the pituitary. There was an inverse relationship of total T and FT with BMI. Thus, the more obese the patient, the lower were total T and FT. HH was also reported in male T2D with erectile dysfunction Citation[19]. Another study has also found high prevalence of hypogonadism in male T2D (50% on that basis of calculated FT concentrations) Citation[20]. Forty-two per cent of T2D patients had symptoms of hypogonadism along with low FT concentrations. BMI and waist circumference were negatively related to total and bioavailable T. Three quarters of the men with hypogonadism had low or inappropriately low gonadotrophin concentrations. Pituitary MRI did not reveal any abnormalities in men with low gonadotrophins Citation[20]. A recently concluded multicentre study (Hypogonadism in Males (HIM)), designed to determine the prevalence of hypogonadism in males over the age of 45 years (mean age: 62 years) in the United States, found the prevalence of hypogonadism to be 38.4%Citation[21]. The study defined hypogonadism as total T < 300 ng/dL. The prevalence in male diabetics (over the age of 45 years) was 50%. Although no distinction was made between T1D and T2D diabetics, it is reasonable to conclude that the overwhelming majority of these patients had T2D. The patients with a low T had a greater prevalence of hypertension, hyperlipidaemia, obesity, inadequate sexual function and libido than patients with normal gonadal function.
What comes first? Hypogonadism or T2D?
Review of the literature clearly shows that hypogonadism is associated with T2D in a substantial number of men. Obesity might be the most important contributor to the presence of hypogonadism in T2D, and the linear inverse relationship of T with BMI is preserved in the presence of T2D Citation[8],Citation[10]. The relationship of T2D and obesity with low T is probably bidirectional. Low T can predispose to obesity while obesity perpetuates hypogonadism. But what comes first? Several epidemiological studies have looked at the predictive value of hypogonadism on the development of T2D over a number of years in men. Studies from Barrett-Conner et al. have shown that low T in men and high T in women predict insulin resistance (IR) and T2D in older adults Citation[22]. A case control study of 176 non-diabetic men followed up for 5 years showed that low levels of SHBG and T at baseline were associated with the development of T2D in men Citation[23]. A recent analysis from the NHANES III Citation[24] showed that low androgens were a risk factor for diabetes in men. In multivariable models adjusted for age, race/ethnicity, and adiposity, men in the lowest tertile of FT concentrations were four times more likely to have diabetes compared with men in the third tertile (odds ratio 4.12 [95% CI 1.25–13.55]). These associations persisted even after excluding men with low total or FT concentrations (suggesting that the risk was not completely driven by hypogonadal men).
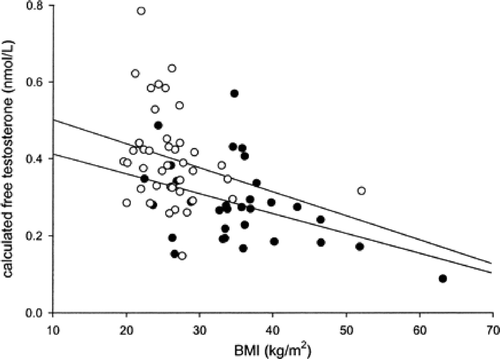
The high prevalence of hypogonadism in T2D raises several questions: first and foremost, does this defect also occur in T1D? We compared the prevalence of hypogonadism in age matched (mean age 43 years) T1D and T2D men. Six per cent of T1D mean were hypogonadal as compared to 26% of T2D men Citation[7]. SHBG in T1D was higher than that in T2D men. Thus, HH does not occur commonly in T1D, and so is not a function of diabetes or hyperglycaemia per se. Interestingly, however, TT and FT are inversely related to BMI even in T1D Citation[7]. The next question that arises is whether HH is related to insulin resistance, especially since there is an inverse relationship of TT and FT with BMI in both T1D and T2D (, correlation of calculated free testosterone with BMI in type 1 and type 2 diabetic subjects). In addition, previous studies have shown that hypogonadism is associated with upper abdominal adiposity, hyperinsulinaemia and insulin resistance Citation[1],Citation[3,4],Citation[10],Citation[22],Citation[25-33]. Thus, there is prima facie evidence that HH may be related to insulin resistance, and perhaps hyperglycaemia in addition to insulin resistance makes the defect more frequent and possibly more severe. With a prevalence of approximately one third in T2D, HH is a potential candidate for being the commonest complication in males with T2D (through association rather than causality at this time) and requires further assessment in terms of the etiology of the defect and the possible consequences, complications and treatment.
Hypogonadism and insulin sensitivity
Relationship between hypogonadism and insulin sensitivity
Insulin sensitivity is inversely related to intramuscular, intramyocellular and intra-abdominal adipose tissue. Hypogonadism is also associated with increased subcutaneous (both truncal and appendicular), intra-abdominal and intramuscular adipose tissue Citation[34,35]. Epidemiological studies clearly show an inverse relationship between T and insulin resistance, probably mediated by total or abdominal adiposity Citation[36]. Insulin resistance and visceral obesity is associated with low SHBG and low total T levels in men Citation[1],Citation[3,4],Citation[10],Citation[22],Citation[25-33],Citation[37,38]. A study on patients with Klinefelter's syndrome found decreased insulin sensitivity which correlated with truncal obesity Citation[39]. Serum LDL-C, triglycerides and CRP concentrations were also markedly increased. These abnormalities were reversed partially after replacement with T, although the doses of T given for replacement were small. We have recently evaluated the relationship of FT concentrations with body composition as measured by DEXA in men with T2D Citation[40]. FT concentrations were negatively related to all measures of fat mass (appendicular, trunk and total fat mass) and positively related to arm lean mass. The relationship between insulin resistance and hypogonadism may be bidirectional: insulin resistance may promote hypogonadotrophism and hypogonadism may promote obesity and insulin resistance Citation[41,42] Decrease in insulin resistance by rosiglitazone leads to an increase in T concentrations in T2D men Citation[43].
Interestingly, the effect of testosterone on insulin sensitivity may also be mediated by mechanisms other than change in adiposity. Pitteloud et al. looked at the relationship between testosterone levels, insulin sensitivity, and mitochondrial function in 60 men and found that T correlated with VO2 max and oxidative phosphorylation gene expression independent of BMI Citation[38]. A recent trial studied the effect of withdrawal of T therapy on insulin resistance in 12 men with idiopathic HH who were on chronic replacement with T Citation[44]. Stopping T therapy for only two weeks worsened insulin sensitivity by 40% compared to baseline. Insulin sensitivity was measured by HOMA-IR in the study. This was also associated with an increase in serum IL-6 concentrations but a decrease in TNF-α.
Studies in androgen receptor knock out (ARKO) mice show that male ARKO mice develop obesity while female ARKO mice do not Citation[45]. The obesity in male ARKO mice is not due to hyperphagia but due to decreased spontaneous activity and decreased overall oxygen consumption Citation[46]. Interestingly, these mice had preserved insulin sensitivity, possibly due to elevated adiponectin secretion. Serum estrogen concentrations were normal in ARKO mice. A mouse model of estrogen deficiency, aromatase deficient (ARKO) mouse, also demonstrates obesity and hepatic steatosis in males but not in females Citation[47].
Effect of testosterone replacement on insulin sensitivity
T replacement leads to a dose-dependent decrease in adipose tissue and an increase in muscle mass and strength Citation[34],Citation[48,49]. This effect appears to be more pronounced if the population studied has higher amounts of adipose tissue. The mechanism by which T replacement produces these effects is not well understood. Inhibition of lipoprotein lipase activity in intra-abdominal adipose tissue and the differentiation of pluripotent mesenchymal precursor cells preferentially into myogenic lineage instead of adipocytic lineage may play a role Citation[50,51]. Marin et al. demonstrated a decrease in visceral adipose tissue and an improvement in insulin sensitivity with oral and transdermal T treatment in middle-aged obese non-diabetic men Citation[52,53]. The subjects were not hypogonadal but insulin sensitivity improved more in subjects whose T concentrations were in the low normal range at the start of the study. The improvement in insulin sensitivity and basal plasma T concentrations were inversely related. In one of these studies, oral T undecanoate 80 mg twice a day (which increased serum T to 500–600 ng/dL for a few hours after the dosing) or placebo given for 8 months reduced visceral body fat (measured by CT scan) and total body fat by approximately 6%. Subcutaneous adipose tissue did not change. Glucose disposal rate (measured by hyperinsulinaemic-euglycaemic clamp) increased by 20%. Change in glucose disposal rate was related to baseline T levels (r = −0.65, P < 0.05). In another publication Citation[53] using a similar population, transdermal T for 3 months (which doubled T from 418 ng/dL at baseline to 820 ng/dL) increased glucose uptake in hyperinsulinaemic-euglycaemic clamp by 17% and decreased waist/hip circumference ratio. However, studies done in non-obese men have failed to show an improvement in insulin sensitivity Citation[54-56]. The effect of T on adipose tissue, muscle mass and insulin sensitivity seems to depend on the population studied, the dose used for T replacement and the duration of therapy. It is possible that the improvement in insulin sensitivity after T replacement is due to an increase in lean body mass and a decrease in adipose tissue mass, or more specifically by a decrease in visceral or intramyocellular adipose tissue, and that this effect is more pronounced in the hypogonadal patients.
No large studies have been done to evaluate the effect of T on insulin sensitivity in T2D patients. A small study in 24 hypogonadal type 2 diabetics showed improvement in Homeostasis Model Assessment (HOMA)-IR, HgbA1c and waist-hip ratio after treatment with intramuscular T for 3 months Citation[57]. The effects of T replacement in T2D subjects, a population that is likely to benefit from a reduction in adipose tissue and an increase in muscle mass, would be of great interest. It clearly needs to be done in view of the frequency of HH in T2D.
Pathophysiological mechanisms underlying hypogonadotrophic hypogonadism in insulin resistance
Insulin resistance and hypogonadotrophic hypogonadism
Animal data
The best insight into the mechanism underlying HH with a probable defect in GnRH secretion from the hypothalamus is obtained from the mouse with an insulin receptor deletion in neurons (NIRKO), as described by Bruning et al. Citation[58]. The selective deletion of the insulin receptor from neurons led to HH leading to a reduction in LH concentrations by 60%–90%. These animals responded to GnRH challenge by normal or supra normal release of LH. These data imply that insulin action in hypothalamic neurons may facilitate GnRH and thus gonadotrophin secretion. These mice also had atrophic seminiferous tubules and absence of mature spermatozoa in these tubules. In female NIRKO mice, interestingly, there was an absence of follicles in the ovary while the ovaries were smaller and had more fibrous tissue. Mice with IRS-2 deletion (in all tissues) are known to develop T2D and are profoundly insulin resistant and atherogenic. In addition, they are obese, have impaired pituitary development, have low gonadotrophin and have small, anovulatory ovaries with a reduced number of follicles Citation[59]. Insulin has other actions on the hypothalamus: to mediate suppression of appetite through an action on the arcuate nucleus and to suppress hepatic glucose production mediated through the vagus. Furthermore, it is known that inflammatory mediators, inflammatory cytokines, tumour necrosis factor(TNF)-α and interleukin(IL)- 1β, have been shown to reduce hypothalamic GnRH and LH secretion in animals and in vitroCitation[60,61].
Human studies
In 1993, Vermeulen et al. found attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency in hypogonadal obese men, suggesting a likely hypothalamic GnRH or a pituitary secretory defect Citation[62]. In this study, LH pulsatility over 12 hours was compared in obese and lean men. The mean integrated LH levels over 12 hours were significantly lower in obese men. FT concentrations correlated positively with the sum of LH pulse amplitudes in each individual. Further work from the same group showed low FT, LH and LH pulse amplitude in severely obese (BMI > 40kg/m2) while these parameters were normal in milder obesity Citation[63]. One study suggested that obesity can also blunt the responsiveness of pituitary gonadotrophin secretion to GnRH stimulation Citation[64]. Other studies showed a rise in LH or FSH levels after weight loss Citation[65,66].
It should also be noted that LH/hCG-induced T secretion by Leydig cells is inversely correlated with insulin sensitivity (as measured by hyperinsulinaemic-euglycaemic clamp) among men with varying degrees of glucose tolerance Citation[37]. Insulin receptors are present on Leydig cells Citation[67]. Thus, the lesion resulting in hypogonadism in obesity and T2D may occur at multiple levels of hypothalamic-pituitary-gonadal axis Citation[48-50]. However, the absence of hypergonadotrophinism indicates that the primary defect in T2D is at the hypothalamo-hypophyseal level.
Interestingly, the decline in T with aging appears to be associated with similar mechanisms. Mulligan et al. Citation[68] in 1999 performed a randomized double blinded placebo controlled study in which a 2-week pulsatile GnRH infusion unmasked a dual (hypothalamic and Leydig cell) defect in the healthy aging male. This study did not include obese or diabetic patients.
Role of estradiol
Elevated estradiol and leptin concentrations observed in obesity probably mediate a part of the hypogonadotrophic effect of obesity, but they do not account for the full effect of obesity on hypothalamo-hypophyseal axis Citation[66],Citation[69,70]. Testosterone and androstenedione in the male can be converted to estrogen (estradiol and estrone respectively) via aromatization in extraglandular tissues, most significantly in adipose tissue. The rate of extraglandular aromatization increases with age and obesity. Even though aromatase activity increases with age, estrogen concentrations fall with age in males Citation[71]. This is due to the fact that testosterone decreases with age and therefore is not available for conversion to estrogen.
Both testosterone and estradiol can act independently on pituitary/hypothalamus to induce negative feedback. Estradiol levels are increased in mild to moderate obesity Citation[62,63]. FT continues to fall as the obesity progresses. Treating obese men with an aromatase inhibitor leads to a reduction in serum estrone and estradiol, and an increase in serum testosterone and LH/FSH levels Citation[72]. The increase in FT is sometimes supraphysiological. Weight loss leads to an increase in testosterone and FSH concentrations; however serum estrogen levels either decrease or do not change with weight loss Citation[66],Citation[69]. This discrepancy may be related to the final weight achieved. It appears that even mild to moderate obesity can raise serum estrogen levels and achievement of normal weight normalizes serum estrogen levels. On the other hand, massively obese males may not have a significant change in their serum estrogen levels after weight loss if they are still obese at the end of a weight loss study, even though they may have an improvement in their FT levels.
It is not yet known if hypogonadal T2D males have higher or lower estrogen concentrations than eugonadal T2D males.
Role of leptin
Obesity is associated with increased plasma levels of leptin. Isidori et al. studied the relationship of leptin with sex hormones (one fasting blood sample) at varying degrees of obesity, normal (BMI < 30, n = 10) moderate obese (BMI 30–40, n = 14) and massive obese (BMI > 40, n = 14) Citation[70]. Moderately obese subjects had higher leptin than non-obese subjects but did not differ in terms of most sex hormones (FT was lower but was measured by RIA and therefore unreliable). Massively obese subjects had higher leptin and estradiol levels and lower TT, FT, and SHBG levels (as compared to normal subjects). LH/FSH concentrations were not different between the groups. In multiple regression analysis of T with estradiol, leptin, SHBG and LH, leptin was the best predictor of fasting T concentrations. When all the subjects were stimulated with a single dose of human chorionic gonadotropin (hCG), obese subjects had a lower peak T concentration than non-obese subjects. Another study also found a negative correlation between leptin concentrations and hCG stimulated T levels Citation[37]. Leptin receptors are present on Leydig cells Citation[73]. This implies that leptin may play a role in decreased Leydig cell responsiveness to gonadotrophin stimulation in obesity.
Leptin is known to play a permissive role in the regulation of reproductive axis. Absence of leptin results in HH Citation[74]. Leptin appears to serve as a signal of energy reserves to regulate the hypothalamo-pituitary-gonadal axis in relation to nutritional status Citation[75]. It is not yet clear what role leptin resistance might play in the HH associated with insulin resistance.
Role of inflammation
Both obesity and diabetes have been shown to be associated with oxidative stress Citation[76-79] and inflammation Citation[80-84]. The concentration of pro-inflammatory cytokines including TNFα is elevated in T2D Citation[82] and may contribute to HH in this condition. Obesity is also associated with an increase in pro-inflammatory cytokines like TNFαCitation[81],Citation[85,86]. This is consistent with the increase in hypogonadism in obesity and may also explain the effect of BMI on T concentrations in T2D and T1D.
The understanding of mechanisms leading to oxidative stress and inflammation is important. One possible reason why obesity and T2D are associated with oxidative stress and inflammation is the state of insulin resistance. This is due to the fact that 1) insulin resistance is associated with the presence of pro-inflammatory factors including cytokines Citation[87]. These cytokines also interfere with insulin signal transduction Citation[87] and this may also interfere with GnRH secretion since insulin is known to facilitate GnRH secretion both in vivo and in vitroCitation[88]; 2) insulin has been shown to exert an anti-inflammatory and anti-oxidant effects at the cellular and molecular level both in vitro, and in vivoCitation[89-92]. It is therefore relevant that insulin infusion induces an increase in plasma T Citation[93].
Inflammatory mediators may impair insulin mediated mechanisms which facilitate GnRH, gonadotrophin and T secretion. This impairment is likely to be at the hypothalamic level in view of the defect described above. TNFα and IL-6 are known to reduce insulin receptor tyrosine phosphorylation, and IRS tyrosine phosphorylation and the phosphorylation of AkT kinase (protein kinase B). These effects have been shown in adipocytes, human aortic endothelial cells and hepatocytes Citation[94]. TNFα has also been shown to reduce insulin receptor expression in human aortic endothelial cells Citation[67]. It is probable that insulin signal transduction related defects would also be observed in neurons. More recently, it has been shown that SOCS-3, a protein which suppresses insulin signal transduction at IRS-1 level is induced by TNFα and IL-6 Citation[95,96]. It is elevated in the obese and thus may have a role in the pathogenesis of a putative insulin resistant state at the level of GnRH neuron Citation[97].
It has been suggested that hypogonadism is associated with an increase in inflammatory mediators and treatment of hypogonadism leads to a reduction in inflammation Citation[98]. In vitro studies have demonstrated that T induces an inhibition of IL-6 production by human monocytes Citation[99]. T treatment of human aortic endothelial cells results in an inhibition of TNF-α induced vascular cell adhesion molecule (VCAM)- 1 mRNA expression and nuclear factor-κB (a key inflammatory mediator) activation Citation[100] Intramuscular T replacement in hypogonadal males results in a decrease in pro-inflammatory cytokines (IL-1 and TNF-α) and an increase in anti-inflammatory cytokine (IL-10) levels in serum Citation[101]. However, some studies were unable to demonstrate an anti-inflammatory effect of T Citation[54],Citation[102]. It is possible that the effects of T replacement on inflammation depend on the population studied. A comprehensive study on inflammation and T replacement needs to be done to clarify the issue.
Inflammation is known to be a cardinal causative factor in inducing atherosclerosis Citation[103,104]. Adherence of circulating monocytes and lymphocytes to the arterial endothelial lining is one of the earliest detectable events in human and experimental atherosclerosis. Is this adherence of monocytes reduced by T?
Type 2 diabetes, hypogonadism and atherogenesis
Epidemiological studies have shown that hypogonadism is associated with increased incidence of atherosclerosis Citation[105]. Patients with coronary artery disease (CAD) have lower testosterone levels than healthy controls, and this is inversely related to the degree of CAD Citation[106]. Hak et al. studied abdominal aortic calcifications (detected radiographically) in 504 non-smoking men in a population-based Rotterdam study Citation[107]. They found an inverse relationship between T and abdominal calcifications. The age-adjusted relative risk of having abdominal calcifications was only 0.4 in men in the highest tertile of T and BT as compared to men in the lowest tertile. A population-based prospective study over 16 years Citation[108] found that the ratio of cortisol to testosterone in men was directly related to incidence of ischaemic heart disease and to death due to ischaemic heart disease. These relationships were not significant when adjusted for HOMA-IR, BMI and triglycerides. Thus it may appear that there is an inverse relation of T with atherosclerosis, part of which may be explained by obesity. A recent population-based prospective study in elderly men found that men in the lower quartile of testosterone ( < 241 ng/dl) had 1.4 times higher risk of mortality (and 1.38 times higher risk of cardiovascular mortality) as compared to men in the highest quartile (total T > 370 ng/dl). This association was independent of adiposity but was attenuated by CRP and IL-6 Citation[109]. The results were similar with bioavailable testosterone.
In a Finnish study of middle-aged men, carotid IMT was found to correspond inversely with serum T independent of age, cholesterol levels, smoking, blood pressure and BMI Citation[110]. One study which measured total T, SHBG and calculated FT and IMT in men with mean age 60.3 years and BMI 26.1 kg/m2 found no relation of calculated FT with IMT Citation[111]. It found an inverse relation of total T and SHBG with IMT, but these relationships were not independent of BMI. However Makinen et al. Citation[110] found an inverse relation between T and carotid IMT independent of BMI. They did not measure or calculate FT. There have been no studies that have quantitatively demonstrated the differences in carotid IMT within hypogonadal and eugonadal T2D patients.
In this regard, it is interesting that T has been shown to have anti-anginal properties in humans Citation[112-114]. Animal studies have shown that high physiological doses of androgens (T or DHEA) decrease aortic atherosclerosis in cholesterol-fed castrated rabbits Citation[115]. Testosterone administration in the orchidectomized LDL-receptor deficient mouse reduces and delays atherosclerosis. Testosterone also induces VCAM-1 expression in endothelial cells. Thus, testosterone may have an anti-inflammatory and anti-atherogenic function Citation[116,117]. It is possible that treatment of hypogonadism can lead to decreased atherosclerosis in humans, but long-term studies looking at this effect need to be carried out.
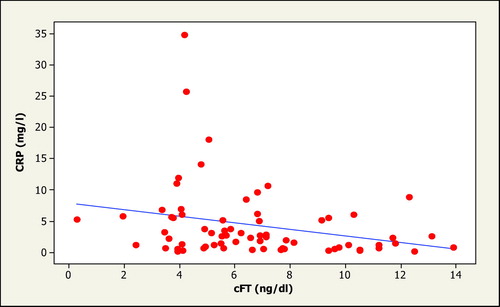
It is therefore relevant that a recent study in T2D men has found markedly increased CRP concentrations in hypogonadal T2D as compared to T2D with normal T(6.5 mg/dl versus 3.2 mg/dl) Citation[6]. The values of CRP observed in HH would place them in a group with the highest cardiovascular risk. There was an inverse relationship between CRP and calculated FT (CRP versus FT: r = −0.27; p = 0.02) (, the inverse relationship between FT and CRP). The relationship of FT with CRP concentration was independent of BMI.
It is therefore possible that a low T contributes to the overall inflammatory state of a patient with T2D. Another possibility is that the pro-inflammatory state, of which an elevated CRP concentration is an indicator, may lead to the suppression of GnRH secretion by the hypothalamic neurons and thus lead to HH. HH, which is also associated with greater BMI, may contribute to increased inflammation and atherogenesis in the long term in T2D. This group of HH males with T2D, hence, may be more vulnerable to cardiovascular events. A trial of T replacement in T2D male patients with HH is necessary to determine whether it reverses the increase in CRP and retards atherosclerosis.
Conclusion
The states of obesity and T2D, but not T1D, are clearly associated with frequent hypogonadism. In view of the increased risk of HH with an increase in BMI and the known association of hypogonadism with obesity, insulin resistance probably contributes to the pathogenesis of HH. Hyperglycaemia with insulin resistance may worsen the pro-inflammatory state, insulin resistance and HH. While the pathological defect in the secretion of T may be at several different levels, it is clear that the major/dominant defect is at the hypothalamic level since the concentrations of FSH and LH are inappropriately low and the response of these hormones to GnRH is normal. The mechanism underlying this defect is not entirely clear but insulin resistance and the associated inflammatory mediators may contribute to this defect since 1) NIRKO mice exhibit this defect and 2) insulin facilitates GnRH secretion by the GnRH secreting neurons.
Plasma CRP concentrations in HH population with T2D are markedly elevated and point to a heightened risk of cardiovascular disease. Furthermore, the increase in adiposity in this population in parallel with a reduction in FT and a simultaneous reduction in muscle mass may contribute to insulin resistance and a pro-inflammatory state. Clearly, trials of treatment with T need to be carried out to a) determine the reversibility of features related to HH and b) determine the definitive pathogenic role of T in these conditions. T replacement therapy offers opportunities in this patient population in terms of reduction of upper abdominal adiposity and an increase in muscle mass and insulin sensitivity.
Future directions
As detailed above, hypogonadism could be one of the mechanisms contributing to increased total and regional adiposity, reduced total and regional lean body mass, reduction in insulin sensitivity and increased oxidative and inflammatory stress in these subjects. It could also contribute to the higher incidence of cardiovascular disease in this population Citation[118,119]. Also, as the youngest T2D patient with HH in one study Citation[5] was 31 years old, HH could have an impact on the fertility of these patients. It is therefore extremely important that we define the effect of HH and the pathogenic mechanisms underlying it. The potential beneficial effects of the replacement of testosterone on the above parameters in T2D males need to be defined by undertaking appropriate prospective trials. It would also be important to evaluate the quality of semen and spermatozoal function. Testosterone therapy is not likely to improve this, and so gonadotrophin treatment may be required. Fertility would be more of an issue in younger patients in whom a focused study must be carried out separately. In view of the possible implications of low T in the pathogenesis of atherosclerosis and inflammation, studies directed towards these issues are also of paramount importance. The results of such trials will have a significant impact on the effect and treatment of HH in males with T2D. The findings will provide a clear rationale for future long term outcome trials of T therapy in T2D with HH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med 1992; 117: 807–811
- Barrett-Connor E, Khaw K T, Yen S S. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol 1990; 132: 895–901
- Haffner S M. Sex hormones, obesity, fat distribution, type 2 diabetes and insulin resistance: epidemiological and clinical correlation. Int J Obes Relat Metab Disord 2000; 24(Suppl.2)S56–58
- Haffner S M, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 1994; 43: 212–219
- Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004; 89: 5462–5468
- Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 2006; 29: 2289–2294
- Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P. Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care 2006; 29: 1120–1122
- Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, Papoz L. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia 1992; 35: 173–177
- Phillips G B. Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction. Proc Natl Acad Sci USA 1977; 74: 1729–1733
- Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P. Testosterone concentrations in women and men with NIDDM. Diabetes Care 1994; 17: 405–411
- Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. 2000; 23: 912–918
- Corona G, Mannucci E, Petrone L, Ricca V, Balercia G, Mansani R, Chiarini V, Giommi R, Forti G, Maggi M. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res 2006; 18: 190–197
- Dunn J F, Nisula B C, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981; 53: 58–68
- Pardridge W M. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab 1986; 15: 259–278
- Morley J E, Patrick P, Perry H M, III. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51: 554–559
- Vermeulen A, Verdonck L, Kaufman J M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84: 3666–3672
- Rosner W. An extraordinarily inaccurate assay for free testosterone is still with us. J Clin Endocrinol Metab 2001; 86: 2903
- Winters S J, Kelley D E, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful. Clin Chem 1998; 44: 2178–2182
- Tripathy D DS, Garg R, Khaishagi A, Syed T, Dandona P. Hypogondotrophic hypogonadism in erectile dysfunction associated with type 2 diabetes mellitus: a common defect?. Metabol Syndr Relat Disord 2003; 1: 75–81
- Kapoor D, Aldred H, Clark S, Channer K S, Jones T H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007; 30: 911–917
- Mulligan T, Frick M F, Zuraw Q C, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60: 762–769
- Oh J Y, Barrett-Connor E, Wedick N M, Wingard D L. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 2002; 25: 55–60
- Haffner S M, Shaten J, Stern M P, Smith G D, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol 1996; 143: 889–897
- Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson W G, Dobs A, Basaria S, Golden S H, Platz E A. Androgens and diabetes in men: results from the third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 2007; 30: 234–238
- Chang T C, Tung C C, Hsiao Y L. Hormonal changes in elderly men with non-insulin-dependent diabetes mellitus and the hormonal relationships to abdominal adiposity. Gerontology 1994; 40: 260–267
- Zumoff B, Strain G W, Miller L K, Rosner W, Senie R, Seres D S, Rosenfeld R S. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 1990; 71: 929–931
- Laaksonen D E, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T P, Salonen R, Rauramaa R, Salonen J T. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003; 149: 601–608
- Kaplan S A, Meehan A G, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men?. J Urol 2006; 176: 1524–1527, discussion 1527–1528
- Svartberg J. Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res 2007; 19: 124–128
- Laaksonen D E, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T P, Valkonen V P, Salonen J T. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005; 90: 712–719
- Laaksonen D E, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen T P, Valkonen V P, Salonen R, Salonen J T. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004; 27: 1036–1041
- Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 2005; 174: 827–834
- Haffner S M, Valdez R A, Mykkanen L, Stern M P, Katz M S. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 1994; 43: 599–603
- Woodhouse L J, Gupta N, Bhasin M, Singh A B, Ross R, Phillips J, Bhasin S. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab 2004; 89: 718–726
- Seidell J C, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990; 39: 897–901
- Tsai E C, Matsumoto A M, Fujimoto W Y, Boyko E J. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care 2004; 27: 861–868
- Pitteloud N, Hardin M, Dwyer A A, Valassi E, Yialamas M, Elahi D, Hayes F J. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 2005; 90: 2636–2641
- Pitteloud N, Mootha V K, Dwyer A A, Hardin M, Lee H, Eriksson K F, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes F J. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005; 28: 1636–1642
- Bojesen A, Kristensen K, Birkebaek N H, Fedder J, Mosekilde L, Bennett P, Laurberg P, Frystyk J, Flyvbjerg A, Christiansen J S, Gravholt C H. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care 2006; 29: 1591–1598
- Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi G, Dandona P. Effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care 2007; 30: 1860–1861
- Cohen P G. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt - a major factor in the genesis of morbid obesity. Med Hypotheses 1999; 52: 49–51
- Kapoor D, Malkin C J, Channer K S, Jones T H. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf) 2005; 63: 239–250
- Kapoor D, Channer K S, Jones T H. Rosiglitazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab Vasc Dis Res 2008; 5: 135–137
- Yialamas M A, Dwyer A A, Hanley E, Lee H, Pitteloud N, Hayes F J. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2007; 92: 4254–4259
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun 2003; 300: 167–171
- Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 2005; 54: 1000–1008
- Simpson E R, McInnes K J. Sex and fat - can one factor handle both. Cell Metab 2005; 2: 346–347
- Bhasin S, Storer T W, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell T J, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996; 335: 1–7
- Bhasin S, Woodhouse L, Casaburi R, Singh A B, Bhasin D, Berman N, Chen X, Yarasheski K E, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer T W. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 2001; 281: E1172–1181
- Singh R, Artaza J N, Taylor W E, Gonzalez-Cadavid N F, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003; 144: 5081–5088
- Marin P, Oden B, Bjorntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 1995; 80: 239–243
- Marin P, Krotkiewski M, Bjorntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med 1992; 1: 329–336
- Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, Lindstedt G, Bjorntorp P. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992; 16: 991–997
- Singh A B, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, Buchanan T A, Shen R, Bross R, Berman N, Bhasin S. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab 2002; 87: 136–143
- Liu P Y, Wishart S M, Celermajer D S, Jimenez M, Pierro I D, Conway A J, Handelsman D J. Do reproductive hormones modify insulin sensitivity and metabolism in older men? A randomized, placebo-controlled clinical trial of recombinant human chorionic gonadotropin. Eur J Endocrinol 2003; 148: 55–66
- Tripathy D, Shah P, Lakshmy R, Reddy K S. Effect of testosterone replacement on whole body glucose utilisation and other cardiovascular risk factors in males with idiopathic hypogonadotrophic hypogonadism. Horm Metab Res 1998; 30: 642–645
- Kapoor D, Goodwin E, Channer K S, Jones T H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154: 899–906
- Bruning J C, Gautam D, Burks D J, Gillette J, Schubert M, Orban P C, Klein R, Krone W, Muller-Wieland D, Kahn C R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000; 289: 2122–2125
- Burks D J, Font de Mora J, Schubert M, Withers D J, Myers M G, Towery H H, Altamuro S L, Flint C L, White M F. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000; 407: 377–382
- Watanobe H, Hayakawa Y. Hypothalamic interleukin-1 beta and tumor necrosis factor-alpha, but not interleukin-6, mediate the endotoxin-induced suppression of the reproductive axis in rats. Endocrinology 2003; 144: 4868–4875
- Russell S H, Small C J, Stanley S A, Franks S, Ghatei M A, Bloom S R. The in vitro role of tumour necrosis factor-alpha and interleukin-6 in the hypothalamic-pituitary gonadal axis. J Neuroendocrinol 2001; 13: 296–301
- Vermeulen A, Kaufman J M, Deslypere J P, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab 1993; 76: 1140–1146
- Giagulli V A, Kaufman J M, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab 1994; 79: 997–1000
- Blank D M, Clark R V, Heymsfield S B, Rudman D R, Blank M S. Endogenous opioids and hypogonadism in human obesity. Brain Res Bull 1994; 34: 571–574
- Lima N, Cavaliere H, Knobel M, Halpern A, Medeiros-Neto G. Decreased androgen levels in massively obese men may be associated with impaired function of the gonadostat. Int J Obes Relat Metab Disord 2000; 24: 1433–1437
- Strain G W, Zumoff B, Miller L K, Rosner W, Levit C, Kalin M, Hershcopf R J, Rosenfeld R S. Effect of massive weight loss on hypothalamic-pituitary-gonadal function in obese men. J Clin Endocrinol Metab 1988; 66: 1019–1023
- Lin T, Haskell J, Vinson N, Terracio L. Characterization of insulin and insulin-like growth factor I receptors of purified Leydig cells and their role in steroidogenesis in primary culture: a comparative study. Endocrinology 1986; 119: 1641–1647
- Mulligan T, Iranmanesh A, Kerzner R, Demers L W, Veldhuis J D. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 1999; 141: 257–266
- Stanik S, Dornfeld L P, Maxwell M H, Viosca S P, Korenman S G. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab 1981; 53: 828–832
- Isidori A M, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fabbri A. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84: 3673–3680
- Khosla S, Melton L J, III, Atkinson E J, O'Fallon W M, Klee G G, Riggs B L. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 1998; 83: 2266–2274
- Zumoff B, Miller L K, Strain G W. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism 2003; 52: 1126–1128
- Caprio M, Isidori A M, Carta A R, Moretti C, Dufau M L, Fabbri A. Expression of functional leptin receptors in rodent Leydig cells. Endocrinology 1999; 140: 4939–4947
- Farooqi I S, Matarese G, Lord G M, Keogh J M, Lawrence E, Agwu C, Sanna V, Jebb S A, Perna F, Fontana S, Lechler R I, DePaoli A M, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002; 110: 1093–1103
- Seth A, Stanley S, Jethwa P, Gardiner J, Ghatei M, Bloom S. Galanin-like peptide stimulates the release of gonadotropin-releasing hormone in vitro and may mediate the effects of leptin on the hypothalamo-pituitary-gonadal axis. Endocrinology 2004; 145: 743–750
- Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet 1996; 347: 444–445
- Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 2001; 86: 355–362
- Higdon J V, Frei B. Obesity and oxidative stress: a direct link to CVD. Arterioscler Thromb Vasc Biol 2003; 23: 365–367
- Vincent H K, Powers S K, Stewart D J, Shanely R A, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord 1999; 23: 67–74
- Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 1998; 83: 2907–2910
- Hotamisligil G S, Shargill N S, Spiegelman B M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91
- Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998; 83: 859–862
- Madrid L V, Mayo M W, Reuther J Y, Baldwin A S, Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 2001; 276: 18934–18940
- Davi G, Guagnano M T, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. Jama 2002; 288: 2008–2014
- Hotamisligil G S, Arner P, Caro J F, Atkinson R L, Spiegelman B M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409–2415
- Hotamisligil G S, Peraldi P, Budavari A, Ellis R, White M F, Spiegelman B M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996; 271: 665–668
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004; 25: 4–7
- Burcelin R, Thorens B, Glauser M, Gaillard R C, Pralong F P. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology 2003; 144: 4484–4491
- Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)- 1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J Clin Endocrinol Metab 2002; 87: 1419–1422
- Aljada A, Ghanim H, Saadeh R, Dandona P. Insulin Inhibits NFkappaB and MCP-1 Expression in human aortic endothelial cells. J Clin Endocrinol Metab 2001; 86: 450–453
- Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. J Clin Endocrinol Metab 2000; 85: 2572–2575
- Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect. J Clin Endocrinol Metab 2001; 86: 3257–3265
- Pasquali R, Macor C, Vicennati V, Novo F, De lasio R, Mesini P, Boschi S, Casimirri F, Vettor R. Effects of acute hyperinsulinemia on testosterone serum concentrations in adult obese and normal-weight men. Metabolism 1997; 46: 526–529
- Aljada A, Ghanim H, Assian E, Dandona P. Tumor necrosis factor-alpha inhibits insulin-induced increase in endothelial nitric oxide synthase and reduces insulin receptor content and phosphorylation in human aortic endothelial cells. Metabolism 2002; 51: 487–491
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 2000; 275: 15985–15991
- Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton D J, Hotamisligil G S, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 2001; 276: 47944–47949
- Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 2007; 50: 278–285
- Yesilova Z, Ozata M, Kocar I H, Turan M, Pekel A, Sengul A, Ozdemir I C. The effects of gonadotropin treatment on the immunological features of male patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 2000; 85: 66–70
- Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol 1996; 106: 410–415
- Hatakeyama H, Nishizawa M, Nakagawa A, Nakano S, Kigoshi T, Uchida K. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett 2002; 530: 129–132
- Malkin C J, Pugh P J, Jones R D, Kapoor D, Channer K S, Jones T H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004; 89: 3313–3318
- Ng M K, Liu P Y, Williams A J, Nakhla S, Ly L P, Handelsman D J, Celermajer D S. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol 2002; 22: 1136–1141
- Libby P, Ridker P M, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–1143
- Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med 1999; 340: 115–126
- Liu P Y, Death A K, Handelsman D J. Androgens and cardiovascular disease. Endocr Rev 2003; 24: 313–340
- Rosano G M, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res 2007; 19: 176–182
- Hak A E, Witteman J C, de Jong F H, Geerlings M I, Hofman A, Pols H A. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002; 87: 3632–3639
- Smith G D, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation 2005; 112: 332–340
- Laughlin G A, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008; 93: 68–75
- Makinen J, Jarvisalo M J, Pollanen P, Perheentupa A, Irjala K, Koskenvuo M, Makinen J, Huhtaniemi I, Raitakari O T. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol 2005; 45: 1603–1608
- Svartberg J, von Muhlen D, Mathiesen E, Joakimsen O, Bonaa K H, Stensland-Bugge E. Low testosterone levels are associated with carotid atherosclerosis in men. J Intern Med 2006; 259: 576–582
- Rosano G M, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, della Monica P L, Bonfigli B, Volpe M, Chierchia S L. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation 1999; 99: 1666–1670
- Webb C M, McNeill J G, Hayward C S, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1999; 100: 1690–1696
- English K M, Steeds R P, Jones T H, Diver M J, Channer K S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation 2000; 102: 1906–1911
- Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis: a study in castrated, cholesterol-fed rabbits. Circ Res 1999; 84: 813–819
- Mukherjee T K, Dinh H, Chaudhuri G, Nathan L. Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci USA 2002; 99: 4055–4060
- Nathan L, Shi W, Dinh H, Mukherjee T K, Wang X, Lusis A J, Chaudhuri G. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci USA 2001; 98: 3589–3593
- Makinen J, Jarvisalo M J, Pollanen P, Perheentupa A, Irjala K, Koskenvuo M, Huhtaniemi I, Raitakari O T. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol 2005; 45: 1603–1608
- Fukui M, Kitagawa Y, Nakamura N, Kadono M, Mogami S, Hirata C, Ichio N, Wada K, Hasegawa G, Yoshikawa T. Association between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetes. Diabetes Care 2003; 26: 1869–1873