Abstract
Although multiple forms of testosterone replacement therapy are available to treat hypogonadism, none is ideal. This article reports on the pharmacokinetics of an innovative nasal formulation of testosterone in hypogonadal men. The first study was undertaken in eight men with a baseline total testosterone (TT) of 130.8 ± 87.4 ng/dL and examined the pharmacokinetics of nasal testosterone given in a single dose of 7.6 mg, 15.2 mg or 22.8 mg, respectively. The second study examined the pharmacokinetics of nasal testosterone (7.6 mg) given either twice or three times a day in 21 severely hypogonadal men (baseline TT in 20 patients <50 ng/dL, in one patient 152 ng/dL) for 14 days. The steady-state concentration of testosterone was within the normal range in all treatment groups, but only in the 3-times-a-day group was the 95% confidence interval completely within the physiological range. The average DHT level did not exceed the upper range of normal. The clinical global visual analogue scale improved in the whole group receiving testosterone (p < 0.001). All adverse events in both studies were of mild to moderate intensity and were evaluated as unlikely or not related to the administered study drug. No patients dropped out during treatment.
Comparison with the normal circadian rhythm by computer modelling suggests that nasal testosterone can be used to mimic the normal diurnal pattern in eugonadal men. Thus, nasal testosterone can be administered safely to humans in doses that approximate serum concentrations in the normal physiological range.
Introduction
Androgen deficiency is now recognised to be a relatively common condition Citation[1-3]. At present none of the available replacement therapies are ideal. Testosterone injection shows wide fluctuations in circulating levels with the values often being supraphysiological Citation[4]. Testosterone patches have a high rate of skin irritation Citation[5],Citation[6]. Testosterone gels, although being the most acceptable modality in the United States, are associated with a low level of skin irritation but are not always considered convenient and bear the risk of skin-to-skin transfer Citation[7-13]. Oral testosterone undecanoate needs to be administered with a high fat meal and levels obtained are often low Citation[14-19]. The oral formulation is associated with high levels of dihydrotestosterone because of high levels of 5α-reductase in the gut Citation[20].
It is known that the nasal preparations of drugs can undergo systemic absorption Citation[21-23]. Also, some investigators have found a potential of this route for the delivery of testosterone; however, the duration of effective serum levels was too short since t1/2 was 40–50 min only Citation[24-26]. In contrast, the current report describes the pharmacokinetics of a newly developed nasal formulation that adequately replaces testosterone in men suffering from the absence of, or low, endogenous testosterone levels.
The studies reported here demonstrate the pharmacokinetics of total testosterone (TT) and dihydrotestosterone (DHT) after single application (study A) and after twice or three times daily dosing for 14 days (study B) of this nasal testosterone preparation in hypogonadal men.
Methods
The studies were carried out at the University of Medicine and Pharmacy, Institute of Endocrinology in Bucharest, Romania (C. Badiu). They were performed in compliance with current ICH Good Clinical Practice and approved by The National Ethics Committee, Bucharest, Rumania. All subjects had given written, informed consent.
Exclusion criteria included: abnormal prostate examination or serum prostate-specific antigen (PSA) above 4 ng/mL; history of testicular tumor; significant intercurrent disease of any type, in particular liver, kidney or heart disease, any form of diabetes mellitus or psychiatric illness; unstable or untreated hyperlipidemia or hyperthyroidism; history of nasal disorders; history of or current evidence of abuse of alcohol or any drug substance.
Aside from the hypogonadism, the subjects were in good health, as evidenced by medical history, physical examination, complete blood count, urinalysis, and serum biochemistry.
The subjects received no androgen treatment within 2 weeks (oral, buccal, topical), 4 weeks (intramuscular) or 12 months (implant) prior to the study. At the end of the washout period, patients had to exhibit hypogonadal morning serum TT levels of <300 ng/dL.
The test product (MPP 10, M et P Pharma AG) is a semisolid formulation containing testosterone. One applicator, intended for administration to one nostril, delivers 120 mg of gel comprising 3.8 mg of testosterone in a proprietary formulation, consisting of castor oil, a mixture of glycerides and polyethylene esters and a gelling agent. The product contains no preservatives and is filled into white unit-dose applicators that are formed using blow-fill-seal technology. One dose is given as one applicator per each nostril (i.e. a total dose of 7.6 mg of testosterone).
Study A
The objective of the study was to determine the pharmacokinetics of testosterone and DHT after single dose administration of three different doses of MPP 10, and to determine the dose level for further studies. Eight hypogonadal men between 22 and 62 years (39.3 ± 14.8 years), BMI 26.8 ± 5.4 kg/m2, were included and had morning serum TT levels at screening of 130.8 ± 87.4 ng/dL. Seven cases were with secondary hypogonadism (three prolactinoma, two non-functioning pituitary adenomas, one Kallmann's syndrome, one with idiopathic hypogonadotropic hypogonadism); one case was identified as Klinefelter syndrome.
Design
Open, sequential, single escalating dose study (three treatment days, 7 days between the treatment days as wash-out period).
Dosing
Treatment Day 1: 7.6 mg testosterone; Treatment Day 2: 15.2 mg testosterone; Treatment Day 3: 22.8 mg testosterone.
Blood sampling
0, 10, 20, 30, 40, 50 min and 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 16, 24 h post-dose.
Pharmacokinetic evaluation TT
Cmax = maximum concentration, tmax = time of maximum concentration, AUC0–24 h = area under the curve, estimated for 0–24 h using the trapezoidal rule, NAUC0–24 h = Normalised AUC, i.e. AUC/dose. For statistical comparisons (dose-linearity), baseline correction was applied by subtracting the baseline from all measured TT values before calculating Cmax and AUC. The baseline was calculated as the mean of the pre-dose and the 24 h TT value, unless these values differed for more than a factor 2. If the latter was the case, the lowest value of the pre-dose and the 24-h concentration was used as the baseline. Zero was substituted for those values that became negative after baseline subtraction.
Safety monitoring
DHT levels, ear-nose-throat (ENT) and prostate examination, adverse events, laboratory measurements, vital signs.
Study B
The objective of the study was to determine the optimum dose scheme for the treatment with MPP 10. Twenty-one hypogonadal men were included. The characteristics of the patients are given in .
Table I. Subjects' baseline characteristics in study B.
Design
Open, randomised, multiple dose, three arms, parallel-group study.
Dosing
(14 days): Group B1– 7.6 mg testosterone two times a day (08:00 h, 14:00 h), Group B2– 7.6 mg testosterone two times a day (08:00 h, 20:00 h), Group B3– 7.6 mg testosterone three times a day (08:00 h, 14:00 h, 20:00 h).
Blood sampling
Days 1–13: pre-dose sample collection (between 06:00 and 07:00 h). Day 14: blood sampling at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 6.25, 6.5, 6.75, 7, 7.5, 8, 9, 10, 12, 14, 16, 18 and 22 h after the morning administration.
Pharmacokinetic evaluation TT
Cmin = minimum concentration, Cmax, AUC0–24 h, NAUC0–24 h, Cav = average concentration, calculated as AUC0–24 h/24, PTF = peak-trough fluctuation, calculated as (Cmax − Cmin)/Cav. For comparison with Study A and for between group comparisons, AUC0–24h was calculated applying a baseline correction. As baseline values the individual pre-dose TT concentrations before first study administration were taken. This baseline was applied assuming that suppression in this study was minimal because baseline TT was virtually at castrate level for most men.
Safety monitoring
DHT levels, ENT and prostate examination, adverse events, laboratory measurements, vital signs.
Secondary parameters
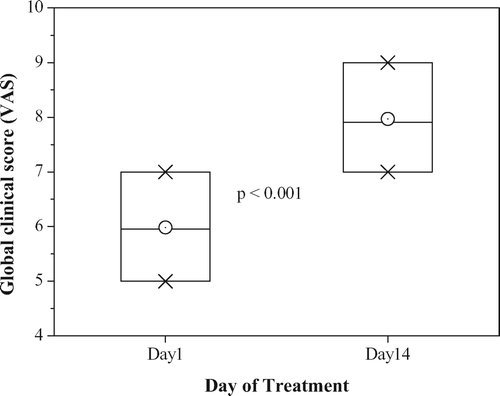
The ageing male survey (AMS) questionnaire was completed by the subjects on day 1 before the first administration and on day 14. The questionnaire was translated to Rumanian and back-translated to confirm its validity (http://www.issam.ch/AMS_English.pdf). The median decrease of the total score and the subscores were analysed using Wilcoxon signed rank test with Bonferroni adjustment and descriptive statistics. A clinical global score (CGS) was evaluated by the investigator on day 1 before the first administration and on day 14. For the CGS the following features were evaluated: skin color/turgor, posture/body tone, psychic situation/mood. The CGS was a Visual Analogue Scale (VAS), structured as 0 = worst and 10 = best. The median change of the total score was analysed using the same statistical methods as for the AMS questionnaire.
Hormone assays
The serum samples of studies A and B were analysed by Esoterix Endocrinology, Calabasas, CA, USA. At the time the studies were performed Esoterix used two different methods for TT and DHT measurement. All samples of one treatment day for an individual were measured in a single assay.
TT was measured by liquid chromatography with mass spectrometry after nonpolar solvent extraction (LOQ 3.0 ng/dL). The inter-assay coefficients of variation (CV) ranged from 7.9% (30.1 ng/dL) and 4.3% (251 ng/dL) to 5.0% (524 ng/dL). The intra-assay CV ranged from 2.6% (30.6 ng/dL) and 1.8% (263.2 ng/dL) to 1.7 (544.7 ng/dL). The normal range of TT for male adults is 350–1030 ng/dL, as determined by Esoterix Endocrinology (to convert TT to nanomoles per litre, multiply nanograms per deciliter by 0.0347).
DHT was measured by radioimmunoassay after extraction and oxidation (LOQ 2.0 ng/dL). The inter-assay CV for DHT ranged from 7.9% (10.9 ng/dL) and 7.1% at 40.8 ng/dL) to 7.4% (75.3 ng/dL). The intra-assay CV for DHT ranged from 6.7% (11.3 ng/dL) and 2.2% (37.4 ng/dL) to 4.6% (69.1 ng/dL). The normal range of DHT for male adults is 30–85 ng/dL, as determined by Esoterix Endocrinology (to convert DHT to nanomoles per liter, multiply nanograms per decilitre by 0.0345).
Statistical analyses
Data were analysed by descriptive statistical methods using TopFit version 2.1 (kinetics) and SPSS version 12.0 (statistics).
Clinical laboratory parameters
Clinical laboratory parameters were compared by means of an ANOVA test, follow-up vs. screening whenever possible.
Vital signs
Vital signs measured at screening and follow-up were compared by means of an ANOVA test, follow-up vs. screening. The vital signs measured before study medication administration in each study period were analysed only descriptively (mean, standard deviation, range).
Results
Study A
Serum T levels and pharmacokinetics
Mean concentration–time curves of testosterone after administration of the three different doses of MPP 10 are shown in . The results indicate that physiological testosterone levels can be reached with all doses applied and that a dose of 22.8 mg does not produce supraphysiological concentrations. It must be emphasised that this latter dose was mainly investigated for safety reasons, and it is important to note that even with this dose concentrations did not substantially exceed those obtained with the 15.2 mg dose of testosterone.
Figure 1. Pharmacokinetic profile of TT (solid squares) and DHT (open squares) after single-dose administration of different doses of MPP 10 in study A. 7.6 mg T (squares), 15.2 mg T (circles), 22.8 mg T (triangles). Dashed line denotes lower limits of normal range of TT based on morning serum samples. Error bars denote mean ± SD.
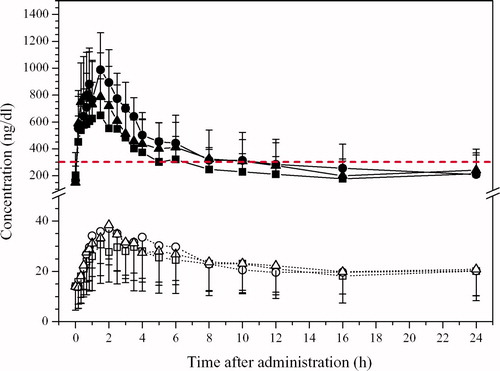
The pharmacokinetic parameters are listed in . The AUC did not linearly depend on dose. The (baseline corrected) AUC0–24 almost doubles with a dose of 15.2 mg as compared with 7.6 mg but only increases marginally when the dose is further increased to 22.8 mg. Also, the increase for Cmax is bigger between the low and medium dose than between the medium and the high dose. The maximum serum concentration is reached between 1 and 2 h after administration indicating a rapid absorption from the intranasal cavity.
Table II. Pharmacokinetic parameters of TT in study A after single escalating doses of MPP 10 in 8 men.
Serum DHT levels
The concentration of DHT is in the low physiological range for a short time, decreasing thereafter (). The risk of supraphysiological concentrations is negligible.
Adverse events
Two adverse events (fever, nausea) occurred in one patient before the first administration. This patient was excluded from the study. There were no further drop-outs.
Study B
Serum T levels and pharmacokinetics
In this study, patients with very severe hypogonadism, virtually having castrate TT levels, were included. The pharmacokinetic parameters of testosterone for the three treatment groups are compiled in , the corresponding serum concentration-vs.-time curves are shown in .
Figure 2. Pharmacokinetic profile of TT and DHT after nasal administration of MPP 10 in different dosing schemes (Study B, after 14 days of treatment). ‘Time 0’ means 08:00 h; dashed lines denote upper (TT and DHT) and lower (TT) limits of normal range based on morning serum samples. Error bars denote mean ± SD.
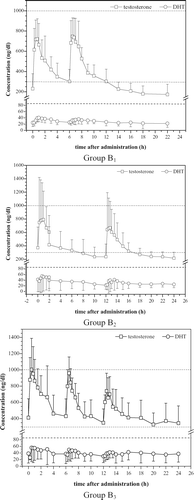
Table III. Study B.
The normalised baseline-corrected AUC values show that the extent of absorption of testosterone is virtually the same in each group. Cmin is low in all groups, but the patients started with very low baseline TT concentrations. The trough concentrations rise rapidly during the first two days from the initial low castrate TT levels to reach a new steady state between ∼200 and 400 ng/dL.
The mean of average steady-state concentrations remains within the physiological range in all treatment groups, but only in group B3 (t.i.d. administration) the 95% confidence interval is also entirely within the physiological range. In all groups Cmax values of individual patients sometimes slightly exceeded the upper limit of the physiological range. Excursions above the physiological range are short-lasting. Twelve of a total of 22 values higher than 1000 ng/dL were observed in a single patient, who started with a trough level of 900 ng/dL after 13 days of dosing with the three times daily dosing regimen.
Serum DHT levels
The pharmacokinetic parameters of DHT for the three treatment groups are shown in . These show that the average steady-state concentration of DHT did not exceed the upper limit of the physiological range (85 ng/dL), indicating no particular safety risk due to DHT pharmacokinetics. The corresponding serum concentration-vs.-time curves are shown in . The curves show that even in group B3 the risk of a DHT level above the upper limit of the physiological range for a longer time is low. The larger standard deviations in group B2 and B3 are caused mainly by the high concentrations in one patient each.
Table IV. Study B.
Adverse events
A total of 36 adverse events were observed during study B. All adverse events were of mild or moderate intensity. No serious adverse events were observed. The following organ systems were affected: psychiatric disorders, nervous system disorders, respiratory, thoracic and mediastinal disorders, gastrointestinal disorders, skin and subcutaneous tissue disorders, musculo-skeletal, connective tissue, and bone disorders, renal und urinary system disorders, general disorders and administration site condition. None of these events were evaluated as at least possibly related to drug treatment. No adverse events concerning nasal mucosa or nasal function were observed.
AMS questionnaire
The AMS questionnaire did not provide significant results but a tendency to lower values (i.e. improvement of condition) on day 14 compared with day 1 was observed. An important experience was that the AMS questionnaire was not fully compatible with the situation of the particular patients.
CGS
The overall CGS results for the total study population are given in . The overall score significantly increases (i.e. improves) after 14 days of treatment. Analysis on separate groups revealed significant increases from baseline during the treatment period in Groups B1 and B3, indicating a clinical amelioration of the condition of the patients.
Also in group B2 the CGS improved, but the change was not significant (p = 0.063).
Discussion
The results of study A indicate that testosterone is well absorbed after nasal administration of different doses of MPP 10. The maximum serum concentration of testosterone is reached between 1 and 2 h after administration. The exposure to exogenous testosterone increases approximately linearly between 7.6 and 15.2 mg but levels off with the higher dose of 22.8 mg. At dose levels above 15 mg absorption may be limited due to the restricted volume for nasal application of 0.05–0.15 mL Citation[27]; because of the low solubility of testosterone the medium and the high dose had to be administered by increasing the application volume to 0.24 and 0.36 mL, respectively. Thus, the lack of a further linear increase of testosterone levels with higher doses may reflect a saturation phenomenon at the site of absorption.
As high circulating DHT concentrations might be a risk factor for prostate safety the steady-state concentrations and the pharmacokinetics of DHT were important safety criteria for this study. Although the importance of DHT for prostate safety is still unknown, higher than physiological DHT concentrations and an increased DHT/T ratio should be avoided, especially in long-term treatment. In study A the concentrations of DHT remained within the physiological range for DHT over the observation period.
The results of the study B indicate that treatment schedule B3 is the most appropriate regimen for initial treatment of severe male hypogonadism, accompanied by a good tolerability. However, for mild or moderately hypogonadal men with higher basal TT levels, a lower dosing frequency is probably also sufficient to improve their TT levels towards a more eugonadal range. The wide confidence intervals for Cmax in Groups B2 and B3 reflect the profile of one patient in each group who started blood sampling on Day 14 with already high TT levels before the first administration of that day; 1080 and 901 ng/dL for Groups B2 and B3, respectively. Such patients would in normal practice qualify for a dose reduction.
An important observation was that the DHT concentration remained relatively low and did not exceed the physiological range for a longer time per treatment day. Also the DHT/T AUC0–24 h ratios (0.076, 0.081 and 0.074 for groups B1, B2 and B3, respectively) were in the normal range as reported before Citation[28].
The AMS questionnaire was not developed to assess effects of testosterone deficiency and replacement. The AMS, as well as the CGS, were included to test their suitability as efficacy instruments and for purposes of sample size calculation for future efficacy studies. A significant clinical response was not expected due to the low patient number and the short treatment duration. In addition, the study was an open-label study and uncontrolled. Despite these restrictions, the results showed trends towards improvement and provided valuable information about the change of the clinical state of the patients and about the suitability of these efficacy instruments.
Because none of the adverse events was judged as serious, and all adverse events were evaluated as unlikely or not related to the administered study drug, MPP 10 seems to be well tolerated. Regarding clinical chemistry and vital signs no clinically relevant changes were observed with exception of a decrease in hemoglobin concentration, hematocrit and red blood cell concentration. But this decrease may be explained by the high number of blood samples taken during the treatment interval and during the pharmacokinetic investigation on day 14.
Although MPP 10 is an oil-based formulation, there is very low risk that the vehicle might enter the lungs due to its high viscosity (∼4000 mPa × s). The results of a toxicological study (product-specific 3-months toxicity study in rabbits) did not indicate problems with the respiratory tract nor did the follow-up in patients by an ENT examination.
The WHO, NIH and FDA recommend that the major goal of therapy is to replace testosterone levels at as close to physiologic concentrations as possible. It is well-known that many endogenous hormones, including testosterone, follow a circadian rhythm. In fact many functions in humans are organised in time as biological rhythms of diverse periods, and many drugs have varying potencies and/or toxicities associated with the rhythmicity of biochemical and physiological processes Citation[29]. Application of MPP 10 results in a more pulse-like testosterone profile rather than the relatively sustained serum levels attained with transdermal administration. In studies with gels and patches it has been assumed that the upper and lower limits established were constant over the day. As an example, the often used normal reference ranges given by Meikle Citation[30] were derived by taking the 95% confidence intervals from morning hormone levels measured in normal men between 20 and 65 years old. By applying constant limits over the day however normal physiology is not followed because the secretions of the endocrine system describe a variable pattern of production with little evidence that constant secretion is the preferred option Citation[31-35]. Although the circadian fluctuation gets less in elderly men it is certainly not disappearing as was nicely shown by Diver et al. in 2003 Citation[36]. Also, many healthy men, considered to be eugonadal, have testosterone levels in the hypogonadal range during different times during the day Citation[36-38]. Therefore it must be emphasised that testosterone replacement therapy resulting in permanently increased concentrations of circulating testosterone and DHT, as is the case with transdermal gels and injectables, is significantly different from the physiological profile in eugonadal men. Because of its physicochemical characteristics and short half-life, administration of testosterone to hypogonadal men in a manner that mimics normal levels and patterns presents a therapeutic challenge. However, a challenge worthwhile facing and which may lead to a paradigm shift in HRT. In the case of estrogen, for example, pulsed therapy has shown to be highly effective, despite periods of relatively low concentrations, probably partly due to the intracellular gene activation cascades that are initiated once estrogens bind to their nuclear receptors Citation[39].
The consequences of such ‘unopposed’ high circulating levels of testosterone and DHT are unknown at present but may well result in testicular atrophy, increased prostate volume, oligo- and/ or azoospermia and infertility Citation[40-43]. To the knowledge of the authors no study has yet ascertained whether a sustained profile is a requirement for efficacy. In contrast, in mice it has been shown that testosterone production and fertility is under strict regulation of circadian clock genes Citation[44]. This emphasises that endogenous rhythms are important and should not be neglected beforehand. Therefore, for further improvement of pharmacotherapy, appropriate timing of drug performance seems mandatory Citation[45]. To evaluate the similarity of nasal administration to normal circadian rhythm, the mean curve from group B2 (dosing at 08:00 h and 2000 h, see ) is compared with the profile of a healthy young man (). As explained before there was one subject in Group B2 with high values differing significantly from the other patients in this group. Such a patient would be down-titrated in practice. For comparison this subject was left out of the mean curve for Group B2 in . It can be seen that the second dose, 12 h after the first dose, exactly coincides with the time of the physiologically lowest concentrations so that the second dosing at this time runs counter to the testosterone circadian variation. With a better timing of the doses, or with an optimised single dose, normal physiology for testosterone could probably be followed closely with this novel intranasal formulation.
Figure 4. Pharmacokinetic profile of TT in group B2 on last day of treatment (study B, day 14) in comparison with profile of a healthy young man (data taken from Diver Citation[37]; ‘time 0’ means 08:00 h). Dashed lines denote upper and lower limits of normal range based on morning serum samples.
![Figure 4. Pharmacokinetic profile of TT in group B2 on last day of treatment (study B, day 14) in comparison with profile of a healthy young man (data taken from Diver Citation[37]; ‘time 0’ means 08:00 h). Dashed lines denote upper and lower limits of normal range based on morning serum samples.](/cms/asset/a2031dab-2ccb-4251-ba16-c7f68a56d0a0/itam_a_335364_f0004_b.jpg)
In fact, PK computer modelling was performed to simulate different dosing regimens. A PK model was built that took circadian variation into account by using a mathematical model with a single cosine function of the form: B − A · cos[(t − tz) · (2π/24)] where B is the baseline value, A is the amplitude of the rhythm and tz is the time of nadir. A single cosine model is commonly applied to oscillating physiological functions Citation[36],Citation[46]. Data of 80 TT-circadian profiles in moderately hypogonadal men (screening T value <300 ng/dL, samples taken for 24 h starting at 7:00 h; internal data from the CRO that performed the PK modelling) were used to derive the model parameters of T circadian variations in this population. With this model different administration regimens of the intranasal formulation were simulated. Simulation shows that one dose at bedtime and one dose at wake up (a time interval normally associated with the TT acrophase in eugonadal men) most probably will provide testosterone exposure similar to what would be observed in a healthy population.
Taken together, the findings on pharmacokinetics of the nasal application form MPP 10 demonstrate a potential useful approach for the administration of an unmodified testosterone medication. Selected regimens in hypogonadal men can closely mimic the natural diurnal rhythm of testosterone in healthy men.
Conclusion
This study demonstrates that the novel intranasal testosterone formulation MPP 10 can be safely administered to humans. The intranasal drug delivery system represents a mechanism to more closely proximate the normal circadian variation of testosterone levels, in contrast to the abnormal steady state levels seen with transdermal products or the large fluctuations over longer periods of time seen with injections. Further studies are necessary to determine the effect of nasal testosterone application in hypogonadal men over prolonged periods of time.
Acknowledgements
C.M. is a partial owner of M et P Pharma AG. C.H. and J.E.M. consult for M et P Pharma AG. C.B. received grant support from M et P Pharma AG (at that time called Mattern Pharmaceuticals AG) to conduct the clinical studies.
References
- Wang C, Swerdloff R S. Androgen replacement therapy. Ann Med 1997; 29: 365–370
- Matsumoto A M. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Med Sci 2002; 57: M76–M99
- Haren M T, Kim M J, Tariq S H, Wittert G A, Morley J E. Andropause: a quality-of-life issue in older males. Med Clin North Am 2006; 90: 1005–1023
- Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. Clin Endocrinol (Oxf) 2006; 65: 275–281
- Tenover J L. The androgen-deficient aging male: current treatment options. Rev Urol 2003; 5(Suppl 1)S22–S28
- Jockenhovel F. Testosterone therapy – what, when and to whom. Aging Male 2004; 7: 319–324
- Wang C, Swerdloff R S, Irnamanesh A, Dobs A, Snyder P J, Cunningham G, Matsumoto A M, Weber T, Berman N, Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 3020–3023
- Swerdloff R S, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto A M, Snyder P J, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 4500–4510
- Kunz G J, Klein K O, Clemons R D, Gottschalk M E, Jones K L. Virilization of young children after topical androgen use by their parents. Pediatrics 2004; 114: 282–284
- Brachet C, Vermeulen J, Heinrichs C. Children's virilization and the use of a testosterone gel by their fathers. Eur J Pediatr 2005; 164: 646–647
- Spielberg T E. Abnormal testosterone levels in partners of patients using testosterone gels. J Sex Med 2005; 2: 278
- Merhi Z O, Santoro N. Postmenopausal virilization after spousal use of topical androgens. Fertil Steril 2007; 87: e13–e15, 976
- Bhowmick S K, Ricke T, Rettig K R. Sexual precocity in a 16-month-old boy induced by indirect topical exposure to testosterone. Clin Pediatr 2007; 46: 540–543
- Morley J E. Androgens and aging. Maturitas 2001; 38: 61–71, discussion 71–73
- Gooren L J, Bunch M C. Androgen replacement therapy: present and future. Drugs 2004; 64: 1861–1891
- Gheorghiu M, Badiu C, Caragheorgheopol A. Clinical efficacy of the long-acting intramuscular compared to oral testosterone undecanoate in adult men with central hypogonadism. Acta Endocrinol 2008; 4: 59–73
- Bagchus W M, Hust R, Maris F, Schnabel P G, Houwing N S. Important effect of food on the bioavailability of oral testosterone undecanoate. Pharmacotherapy 2003; 23: 319–325
- Haren M, Chapman I, Coates P, Morley J, Wittert G. Effect of 12 month oral testosterone on testosterone deficiency symptoms in symptomatic elderly males with low-normal gonadal status. Age Ageing 2005; 34: 123–130
- Wittert G A, Chapman I M, Haren M T, MacKintosh S, Coates P, Morley J E. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 2003; 58: 618–625
- Houwing N S, Maris F, Schnabel P G, Bagchus W M. Pharmacokinetic study in women of three different doses of a new formulation of oral testosterone undecanoate, Andriol Testocaps. Pharmacotherapy 2003; 23: 1257–1265
- Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today 2002; 7: 1184–1189
- Hussain A. Intranasal drug administration delivery. Adv Drug Del Rev 1998; 29: 39–49
- Wattanakumtornkul S, Pinto A B, Williams D B. Intranasal hormone replacement therapy. Menopause 2003; 10: 88–98
- Danner C H, Frick J. Androgen substitution with testosterone containing nasal drops. Int J Androl 1980; 3: 429–435
- Hussain A A, Kimura R, Hunag C H. Nasal absorption of testosterone in rats. J Pharmaceut Sci 1984; 73: 1300–1301
- Hussain A A, Al-Bayatti A A, Dakkuri A, OkochI K, Hussain M A. Testosterone 17β-N,N-dimethylglycinate hydrochloride: a prodrug with a potential for nasal delivery of testosterone. J Pharmaceut Sci 2002; 91: 785–789
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today 2002; 7: 967–975
- Meikle A W, Mazer N A, Moellmer J F, Stringham J D, Tolman K G, Sanders S W, Odell W D. Enhanced transdermal delivery of testosterone across non-scrotal skin produces physiological concentrations of testosterone and its metabolites in hypogonadal men. J Clin Endocrinol Metab 1992; 74: 623–628
- Ohdo S. Chronopharmacology focused on biological clock. Drug Metab Pharmacokinet 2007; 22: 3–14
- Meikle A W, Arver S, Dobs A S, Sanders S W, Rajaram L, Mazer N A. Pharmacokinetics and metabolism of a permeation-enhanced testosterone transdermal system in hypogonadal men: influence of application site – a clinical research center study. J Clin Endocrinol Metab 1996; 81: 1832–1840
- Veldhuis J D, Iranmanesh A, Keenan D M. Erosion of endogenous testosterone-driven negative feedback on pulsatile luteinizing hormone secretion in healthy aging men. J Clin Endocrinol Metab 2004; 89: 5753–5761
- Winters S J. Diurnal rhythm of testosterone and luteinizing hormone in hypogonadal men. Androl 1991; 12: 185–190
- Liu P Y, Takahashi P Y, Roebuck P D, Iranmanesh A, Veldhuis J D. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol 2005; 289: R721–R728
- Karatsoreos I N, Silver R. Minireview: the neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology 2007; 148: 5640–5647
- Kriegsfeld L J, Silver R. The regulation of neuroendocrine function: timing is everything (Review). Horm Behav 2006; 49: 557–574
- Diver M J, Imtiaz K E, Ahmad A M, Vora J P, Fraser W D. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003; 58: 710–717
- Diver M J. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem 2006; 43: 3–12
- Spratt D I, O'Dea L S, Schoenfeld D, Butler J, Rao P N, Crowley W F, Jr. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol 1988; 254: E658–E666
- Robinson J A, Spelsberg T C. Mode of action at the cellular level with specific reference to bone cells. Estrogens and antiestrogens, R Lindsay, D W Dempster, V C Jordan. Lippincott–Raven, Philadelphia 1997; 43–62
- Tarter T H, Vaughan E D, Jr. Inhibitors of 5-alpha-reductase in the treatment of benign prostatic hyperplasia. Curr Pharm Des 2006; 12: 775–783
- Bartsch G, Rittmaster R S, Klocker H. Dihydrotestosterone and the concept of 5-alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol 2002; 19: 413–425
- Mooradian A D, Morley J E, Korenman S G. Biological actions of androgens. Endocrinol Rev 1987; 8: 1–28
- Zhu Y, Zheng T, Stevens R G, Zhang Y, Boyle P. Does ‘clock’ matter in prostate cancer. Cancer Epidemiol Biomarkers Prev 2006; 15: 3–5
- Alvarez J D, Hansen A, Ord T, Bebas P, Chappell P E, Giebultowicz J M, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms 2008; 23: 26–36
- Hertoghe T. The ‘Multiple Hormone Deficiency’ theory of aging: is human senescence caused mainly by multiple hormone deficiencies. Ann NY Acad Sci 2005; 1057: 448–465
- Chakraborty A, Krzyzanski W, Jusko W J. Mathematical modeling of circadian cortisol concentrations using indirect response models: comparison of several methods. J Pharmacokinet Biopharm 1999; 27: 23–43