Abstract
Introduction: The etiology of benign prostatic hyperplasia (BPH) remains a mystery to scientists; estrogen/androgen imbalance in aged men has been implicated.
Methods: Thirty (30) apparently healthy men and newly diagnosed BPH patients were recruited from the Ghana Police Hospital. Lower urinary tract syndrome (LUTS) and prostate volume were assessed via the prostate symptom score sheet (IPSS) and abdominopelvic scan, respectively. Laboratory assays for total prostate specific antigen (tPSA) and hormones [androstenedione (AED), testosterone (T), dihydrotestosterone (DHT), androstanedioladiol (3α-adiol), androstanediol (3β-diol), estrone (E1) and estradiol (E2)] were performed via ELISA techniques. Non-parametric analyses were employed. p < 0.05 was considered significant.
Results: AED was significantly higher in controls compared to the BPH patients. AKRIC2 (3α-diol/DHT) was significantly higher in the BPH group (p < 0.001) whiles AKRIC1 (3β-diol/DHT) was significantly lower. Estradiol was significantly higher in BPH (p= 0.029). Age correlated negatively with T, while a negative correlation was observed between TIPSS and 3β-diol and AKRIC1. Also, prostate volume correlated negatively with fT.tPSA correlated positively with E2 and aromatase activity (E2/T) and negatively with fT. On multiple linear regression, DHT and 3β-diol remained independent predictors for TIPSS and fT for tPSA.
Conclusion: Estrogens and androstanediols seem to play a role in BPH development.
Introduction
Benign prostatic hyperplasia (BPH) is a common medical condition construed as normal in ageing males. BPH may cause lower urinary tract symptoms (LUTS) which affects and interferes with the quality of life of the affected patients [Citation1,Citation2]. The cause of the condition remains unresolved but age and androgens have been implicated in the etiology of the disease, largely due to the dramatic response of the hormone therapy with 5ARI and the androgen/estrogen imbalance associated with aging [Citation3].
The role of androgens in the development and progression of both PCa and BPH are well established even until it reaches advance stages. Dihydrotestosterone (DHT) is the mediator of most androgen effects in male physiology and the signaling between androgens and androgen receptors induces prostatic development and growth [Citation4]. In androgen-target tissues such as genital skin and the prostate, testosterone (T) is converted to DHT via 5α-reductase enzymes or alternatively androstanediol via 17β-hydroxysteroid dehydrogenase type 5, which bypasses T. This alternate route has been named the “backdoor” [Citation5] () [Citation6]. Androstanediol (3α-diol) can be reversibly oxidized to DHT. DHT binds to androgen receptors (AR) with an affinity about 2–5 times that of T [Citation7] while androstanediol binds with moderate affinity to either mutant or wild-type AR [Citation8].
Figure 1. Androgen biosynthesis pathway: Androgens are synthesized via a classical and non-classical pathway. Classical pathway is shown in light gray arrows while the backdoor pathway is the hatched arrows [Citation6].
![Figure 1. Androgen biosynthesis pathway: Androgens are synthesized via a classical and non-classical pathway. Classical pathway is shown in light gray arrows while the backdoor pathway is the hatched arrows [Citation6].](/cms/asset/427c47f9-510b-4084-bf5f-9f58da375fa3/itam_a_1272101_f0001_b.jpg)
In humans, serum testosterone (T) and free-testosterone (fT) levels decrease with age, but the serum estradiol remains fairly constant throughout life [Citation9] hence aging creates an estrogen dominant status. Hitherto, Ebert (2004) [Citation10] had cautioned the use of testosterone treatment in hypogonadal men for long term, however, in hypogonadal men or men with LUTS, the use of androgen treatment or 5ARI (dutastatride) produced no prostate concerns but rather improved LUTS, IPSS and other parameters associated with prostate diseases with concomitant increases in androgens [Citation11–15]. Hence, it is suggested that androgen cannot exclusively induce the condition and that estrogen may play a role in the development of the condition [Citation16]. However, reports on the association between androgen, estrogen and the risk of BPH remains diffused [Citation17,Citation18].
The prevalence of the BPH among African–Americans is higher compared to their Caucasian counterparts [Citation19]. Also, blacks seem to have high levels of hormones [Citation20] yet the interplay between sex steroid hormones and the risk of BPH development has not been ascertained in black men. This study, therefore, sought to determine the influence of sex hormones and their metabolites in the development of BPH in Ghanaian men. Besides, most studies picked out bits and pieces of the androgen biosynthetic pathway [Citation17,Citation18]. However this study ascertained the impact of these sex steroids and their metabolites in BPH as major components of both the classical and alternative pathways. Furthermore, this study demonstrated for the first time a vivid picture of the interplay between BPH development and the androgen biosynthetic pathway.
Methods
Ethics: Ethics clearance was obtained from the School of Biomedical and Allied Health Sciences. Ethics ID: SAHSET/SAHS/PSM/ML/09/AA/26A/2012–2013. The study complied with the Helsinki Declaration of 1964, with revision in October 2008.
Patients and sampling: Newly diagnosed BPH patients were recruited from the Ghana Police Hospital as well as apparently healthy controls. BPH was diagnosed histologically. Eligible healthy controls were not on steroid hormones nor did they have any medical condition and had PSA levels below 2.5 ng/ml. The participants were assessed by the international prostate symptom score sheet (IPSS), abdominopelvic scan and laboratory assays.
IPSS: The international prostate symptom score sheet assesses the severity of lower urinary tract symptoms. The IPSS has seven questions that boarder on incomplete emptying, frequency, intermittency, urgency, weak stream, straining and nocturia. Answers were assigned points 0–5, with 5 being the worst. Total IPSS scores 0–7, 8–19 and 20–35 were graded mild, moderate or severely symptomatic, respectively.
Abdominopelvic Scan: Prostate volume was obtained by abdominopelvicscan using the Sonoscape Digital Color Doppler Ultrasound system 551–6000 (Shenzhen, China). Prior to scanning, patients had to drink about 1.5 L of water and wait for 1–2 h. This was to enable better visibility of the prostate. Patients then lay on an ultrasound couch with the pelvic area exposed. To bridge acoustic impedance between patient skin and probe surface, a liquid gel was applied and the prostate and bladder volumes at full capacity were obtained. Post-void residual volume was obtained from a second scan after patients had voided urine.
Laboratory Assays: Venous blood samples (5mls) were taken into gel separator tubes from those who met the criteria and consented. This was done before 9 am as early morning sampling is recommended for androgen estimation. Samples were then centrifuged at 3500 rpm for 5 min, using Heal Force centrifuge [Shanghai Lishen Scientific equipment Co (Shanghai, China)] to obtain serum. Serum was aliquotted into micro-centrifuge tubes and stored at −70 °C until ready for analysis. All samples obtained from the subjects were assayed in the same run for each parameter to exclude inter-assay variation from changes in hormone levels within subjects. The following biochemical assays were performed: tPSA, androstenedione (AED), testosterone (T), free testosterone (fT), dihydrotestosterone (DHT), androstanedioladiol (3α-adiol), androstanediol (3β-diol), estrone (E1) and estradiol (E2). For all assays, the manufacturer’s instructions were followed accordingly.
Total and free PSA tests were performed using Monobind Inc. ELISA kits (Lake Forest, CA). The test employed a sandwich method. A stable sandwich complex formed was immobilized at the surface of the microplate well through interaction with streptavidin during the assay procedure as the enzyme-labeled antibody and native antibody in serum binds. The reaction was allowed to attain equilibrium and unbound antigen was separated by washing. The enzyme activity in the antibody bound fraction was directly proportional to the native antigen concentration. A standard curve drawn from known reference samples provided in the kits aided to extrapolate the concentrations of the unknown samples.
All hormones and metabolites were assayed using ELISA kits from Sunlong Biotech Co. Ltd. (Hangzhou, China). All the hormonal assays used the Sandwich-ELISA method. The microelisa strip plate was pre-coated with antibody specific to a particular hormone. Standards and samples were then added, antigens therein bound strongly to the antibody on the plate. A second antibody, Horseradish Peroxidase (HRP)-conjugated antibody specific to the hormone was added and incubated, followed by washing to remove unwanted components. For color development, TMB solution was added to each well. Only wells containing the hormone bound to HRP conjugated hormone antibody appeared as the final chromogen. The optical density of standards and samples were measured spectrophometrically at 450 nm.
Statistical analysis
Normality check performed with Kolmogorov–Smirnove test with Lilliefor’s correction showed deviation from Gaussian distribution. Descriptive statistics was used to characterize the study and generate distribution among the controls and cases. Non-parametric methods were employed for the analysis and correlation analyses were computed with Spearman's correlation. Backward multiple linear regression analysis was performed to ascertain whether age, hormones and metabolites and enzyme indices had independent impact on TIPSS, PV and tPSA in BPH patients after log transformation. The level of significance was 0.050. Analyses and graphs were performed with IBM SPSS Statistics (version 20).
Results
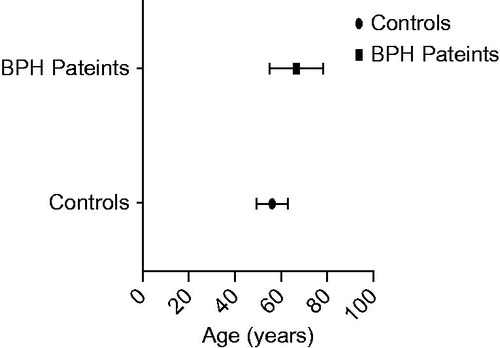
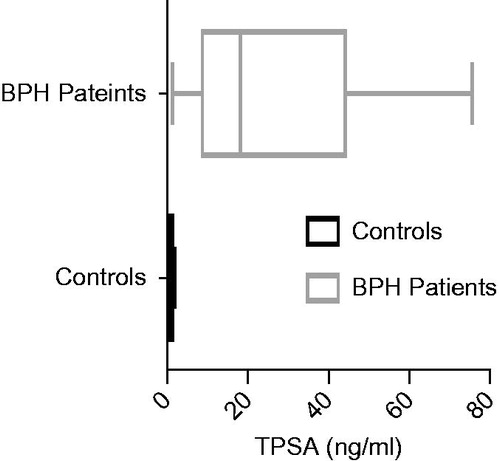
A total of 60 study participants were involved in the study; thirty (30) BPH patients and 30 apparently healthy men as controls. The BPH patients aged between 46 and 87 years while the control, between 46 and 72 years. The mean difference between their ages were statistically significant (p < 0.001) (). Total PSA (tPSA) concentrations between them was also significant (p < 0.001) (). The median tPSA estimated for patients and controls were 18.15 (9.22–42.20) ng/ml and 0.90 (0.50–1.40) ng/ml, respectively. All controls recorded tPSA values < 4.0 ng/ml. The prostate volume of the BPH patients was 99.3(66.1–127.8) cm3 and their IPSS recorded 15.5 (6.5–22.5).
Figure 3. Total PSA levels of study groups. Total PSA (tPSA) was significantly lower in control group than in BPH patients (p < 0.001).
presents the mean (standard deviation) and median (inter-quartile) concentrations of the hormones, metabolites and enzyme indices. For the androgens, none of them (T, fT or DHT) showed significant differences in the levels between the BPH patients and the controls except for AED (p values= 0.017). AED was significantly higher in the control group compared to the BPH patients. DHT metabolites, 3α-diol and 3β-diol, showed no significant difference. Both were relatively higher in the control group. 5AR inhibition activity represented by DHT/T was higher in the control group compared to the test group. AKRIC2 (3α-diol/DHT) was significantly higher in the BPH group (p < 0.001) whiles AKRIC1 (3β-diol/DHT) was significantly lower (p < 0.001). Estrone was relatively higher in BPH patients just as estradiol but significant (p = 0.029). Aromatase activity (E2/T) showed no significant difference between the two groups.
Table 1. Comparison of hormone levels and enzyme indices between BPH patients and control.
Age had a significant positive correlation with T (r = 0.268, p = 0.04). IPSS had a significant negative correlation with 3β-diol and AKRIC1. Also, prostate volume had a negative correlation with fT (r= −0.514, p = 0.01). Additionally, tPSA correlated positively with E2, aromatse activity (E2/T) and negatively with Ft (r = 0.26, p = 0.04; r = 0.27, p = 0.04; r= −0.42, p = 0.003), respectively. All other correlations were not significant (). Using backward multiple linear regression, only DHT and 3β-diol were predictors of IPSS. DHT correlated positively with IPSS while 3β-diol correlated negatively with TIPSS (). None of the hormones, metabolites or age correlated with prostate volume on multiple regression analysis. Only fT remained a possible predictor of tPSA ().
Table 2. Correlation analysis between age, clinical outcome, hormones and enzyme indices in BPH patients.
Table 3. Multiple regression analysis (R = 0.614, p = 0.048) using TIPSS as independent variable and age, hormone and metabolites as dependent variables.
Table 4. Multiple regression analysis (R = 0.136, p = 0.049) using tPSA as independent variable and age, hormone and metabolites as dependent variables.
Discussion
Androgens are perceived to be involved in the initiation and progress of BPH yet estrogens seem to play a substantial role. This study presents the involvement of sex steroid hormones in the development of BPH in black men. Androstenedione though recognized has received less attention by researchers as far as prostate disorders are concerned. This study observed that the level of androstenedione (AED) was significantly higher in BPH patients compared with controls and this was consistent with the study of Stanczyk et al. [Citation21]. The plasma dihydrotestosterone (DHT) level between the BPH patients and the controls was not significant in this study. However, intraprostatic DHT was found to be significantly increased in BPH. Additionally, serum DHT levels were found to be relatively higher in treatment group compared to placebo groups at baseline [Citation22]. In a recent study, the odds of a higher baseline level of DHT were found to increase the risk of BPH [Citation23].
This study reports a higher level of 3α-diol and 3β-diol in controls compared to BPH patients. Geller et al. [Citation24] found the levels of intraprostatic 3α-diol and 3β-diol in tissues of BPH patients to be significantly lower. To the best of our knowledge, 3α-diol and 3β-diol have not as yet been assayed in serum or plasma samples of BPH patients, although it is clearly accepted to play androgenic and estrogenic roles. In fact, tissue levels of 3α-diol correlate with that of DHT [Citation25].
This study reports a significant higher E2 in patients compared with controls as was reported by Roberts et al. [Citation26]. Estrone though higher in BPH patients, was not significant. On the contrary some studies report a significantly higher E2 level in controls compared with BPH patients [Citation18]. However, Kristal et al. [Citation17] reported no significant differences in estradiol as did Usoro et al. [Citation27] between BPH and controls. However, Skoldofors et al. [Citation28] reported a significant higher level of estrone in BPH.
In this study 5AR activity was relatively higher in BPH patients compared to controls. Liao et al. [Citation18] found a higher 5AR activity to be associated with increased risk of BPH and an association with increasing prostate volume. Aromatase converts estrogen to testosterone and its activity and was represented by E2/T. Some studies have documented an increased activity of E2/T in BPH patients [Citation17]; however, the present study did not make this observation. AKRIC1 (responsible for converting DHT to 3β-diol) was significantly higher in controls compared to cases however AKRIC2 (which converts DHT to 3α-diol) was not significant. No study has reported this finding in serum or plasma except for Geller et al. [Citation24] who reported a similar trend in prostate tissues.
Much as androgens form part of the development of BPH, this occurs in the presence of estrogens. Results from this study strongly support the androgen/estrogen hypothesis. This study observed a significant high E2 along with aromatase activity and a rather reduced activity of AKRIC1. Increased aromatse activity increases the formation of estrogen. Estrogens bind to ERα and ERβ to induce proliferation or apoptosis in prostatic cells, respectively. In BPH, ERα are up-regulated [Citation29] and the presence of an appropriate ligand E2 is likely to activate it to induce proliferation of the stromal cells. AKRIC1 was down-regulated in this study. AKRIC1 converts DHT to 3β-diol and 3β-diol binds ERβ to stimulate apoptosis. The plasma androgen levels were increased in the BPH group in this study, although this did not produce statistical significance probably due to the fact that although testosterone increases with age, DHT levels are not affected by age [Citation30,Citation31]. Controls were significantly younger in this study. BPH is thought to evolve from reduced apoptosis, increased proliferation and also high-androgenic activity [Citation32].
Age represents a singular most important factor that independently predicts the condition [Citation33]. It has been proven that as males age, the prevalence of BPH increases. This study reports a high level of tPSA levels among BPH patients. An observation consistent with other studies [Citation21]. Similar to other studies, tPSA correlated with age [Citation34]. It is established that as one ages, prostate volume increases; so does PSA.
A weak positive relationship was found between age and T and AKRIC2 in this study. In BPH, this association has not been established, yet it is suggested that men with high T are likely to experience BPH [Citation17,Citation35]. Although suggested that T decreases by 2–3% [Citation35] annually in healthy men, this has been contradicted by the study of Mustafa et al. [Citation36]. In a meta-analysis by Saad et al. [Citation31], additional androgens do not influence prostatic growth once androgen receptor binding capacity is reached. High T translates into more fT and subsequently more DHT that saturate the binding sites of AR for prostatic growth. In the formation of a massive prostate volume, high androgenic activity may be needed to stimulate prostatic growth, hence the use of fT, which forms the active component needed for the formation of the more potent androgen DHT. fT correlated negatively with both prostate volume and tPSA and appeared to independently influence tPSA; an observation consistent with Alsharef et al.[Citation37]. The observed high level of fT implies an accumulation hence less fT use. This probably gave room for prostatic growth and the release of PSA. It is noteworthy that this study recorded T concentrations of both patients and controls at values higher than the saturation point (androgen indifference) for human prostate tissues pegged at 2.31 ng/ml [Citation38].
This study found a significant inverse relationship between IPSS and 3β-diol and AKRIC1. Prostate volume correlates positively with LUTS [Citation39] yet this study did not find this association possibly due to the sample size. Voiding symptoms in BPH are largely due to bladder outlet obstruction (BOO) associated with prostatic enlargement (BPE) and failure to empty bladder has been attributed to obstruction or detrusor under-activity or a combination of both. There is a rapid proliferation of cells in the early phase of the condition, however, the disease appears to be established in the face of equal or reduced rate of cell replication. 3β-diol binds to ERβ with an equal efficacy as estradiol [Citation40]. ER induces apoptosis in luminal, basal and stromal cells of BPH tissues and cells [Citation41].
Further to this, tPSA levels correlated positively with estradiol and its corresponding aromatase activity. La Vingera et al. [Citation42] indicated that the use of HCG is good for the management of hypogonadal men as it decreased estrogens which corresponded with PSA, PV and hematocrit. Estrogens augment the apoptotic activities of caspase-3 [Citation43]. On the contrary, an Austria study of 375 non-BPH men demonstrated that estradiol had no correlation with symptoms of aging [Citation44].
Conclusion
In the presence of testosterone, estrogens and androstanediols contribute to the development of BPH.
Disclosure statement
The authors report no declaration of interest.
References
- Lephart ED. Review: anti-oxidant and anti-aging properties of equol in prostate health (BPH). Open J Endocr Metab Dis 2014;2014:1–12
- Singam P, Hong GE, Ho C, et al. Nocturia in patients with benign prostatic hyperplasia: evaluating the significance of ageing, co-morbid illnesses, lifestyle and medical therapy in treatment outcome in real life practice. Aging Male 2015;18:112–17
- Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation 2011;82:184–99
- Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. J Mol Endocrinol 2015;54:R15–29
- Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Develop Dyn 2013;242:320–9
- Mostaghel EA. Beyond T and DHT-novel steroid derivatives capable of wild type androgen receptor activation. Int J Biol Sci 2014;10:602
- Azzouni F, Mohler J. Role of 5α-reductase inhibitors in prostate cancer prevention and treatment. Urology 2012;79:1197–205
- Mohler JL, Titus MA, Wilson EM. Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone. ClinCancer Res 2011;17:5844–9
- Prezioso D, Denis LJ, Klocker H, et al. Estrogens and aspects of prostate disease. Int J Urol 2007;14:1–16
- Ebert T. Clinical experiences with testosterone therapy: prostate safety. Aging Male 2004;7:304–11
- Meuleman EJ, Legros J-J, Bouloux PM, et al. Effects of long-term oral testosterone undecanoate therapy on urinary symptoms: data from a 1-year, placebo-controlled, dose-ranging trial in aging men with symptomatic hypogonadism. Aging Male 2015;18:157–63
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016;19:64–9
- Minnemann T, Schubert M, Minnemann T, et al. A four-year efficacy and safety study of the long-acting parenteral testosterone undecanoate. Aging Male 2007;10:155–8
- Wada N, Hashizume K, Matsumoto S, Kakizaki H. Dutasteride improves bone mineral density in male patients with lower urinary tract symptoms and prostatic enlargement: a preliminary study. Aging Male 2016;19:12–14
- SwerdloffrsWang C. Three-year follow-up of androgen treatment in hypogonadal men: preliminary report with testosterone gel. Aging Male 2003;6:207–11
- Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol 2008;197:483–91
- Kristal AR, Schenk JM, Song Y, et al. Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol 2008;168:1416–24
- Liao C-H, Li H-Y, Chung S-D, et al. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40–79 years. Aging Male 2012;15:28–33
- Roehrborn C, Ray P. Efficacy and tolerability of the dual 5α-reductase inhibitor, dutasteride, in the treatment of benign prostatic hyperplasia in African-American men. Prostate Cancer Prostatic Dis 2006;9:432–8
- Richard A, Rohrmann S, Zhang L, et al. Racial variation in sex steroid hormone concentration in black and white men: a meta‐analysis. Andrology 2014;2:428–35
- Stanczyk FZ, Azen CG, Pike MC. Effect of finasteride on serum levels of androstenedione, testosterone and their 5α-reduced metabolites in men at risk for prostate cancer. J Steroid Biochem Mol Biol 2013;138:10–16
- Wurzel R, Ray P, Major-Walker K, et al. The effect of dutasteride on intraprostaticdihydrotestosterone concentrations in men with benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 2007;10:149–54
- Parsons JK, Palazzi-Churas K, Bergstrom J, Barrett-Connor E. Prospective study of serum dihydrotestosterone and subsequent risk of benign prostatic hyperplasia in community dwelling men: the Rancho Bernardo Study. J Urol 2010;184:1040–4
- Geller J, Albert J, Lopez D, et al. Comparison of androgen metabolites in benign prostatic hypertrophy (BPH) and normal prostate. J Clin Endocrinol Metab 1976;43:686–8
- Monti S, Silverio F, Toscano V, et al. Androgen concentrations and their receptors in the periurethral region are higher than those of the subcapsular zone in benign prostatic hyperplasia (BPH). J Androl 1998;19:428–33
- Roberts RO, Jacobson DJ, Rhodes T, et al. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate 2004;61:124–31
- Usoro AJ, Obot AS, Ekaidem IS, et al. Serum testosterone, 17β-estradiol and PSA levels in subjects with prostate disorders. Indian J Clin Biochem 2015;30:59–65
- Sköldefors H, Blomstedt B, Carlström K. Serum hormone levels in benign prostatic hyperplasia. Scand J Urol Nephrol 1978;12:111–14
- Royuela M, De Miguel M, Bethencourt F, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol 2001;168:447–54
- Carson C, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003;61:2–7
- Saad F, Yassin AA, Haider A, Gooren L. Effects of testosterone on the lower urinary tract go beyond the prostate: new insights, new treatment options. Arab J Urol 2011;9:147–52
- Claus S, Wrenger M, Senge T, Schulze H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol Res 1993;21:305–8
- Briganti A, Capitanio U, Suardi N, et al. Benign prostatic hyperplasia and its aetiologies. Eur Urol Suppl 2009;8:865–71
- Battikhi M. Age-specific reference ranges for prostate-specific antigen (PSA) in Jordanian patients. Prostate Cancer Prostatic Dis 2003;6:256–60
- Trumble BC, Stieglitz J, Rodriguez DE, et al. Challenging the inevitability of prostate enlargement: low levels of benign prostatic hyperplasia amongtsimane forager-horticulturalists. J Gerontol Series A: Biol Sci and Med Sci 2015;70:glv051
- Mustafa M, Horuz R, Celik M, Kucukcan A. Is there an association between serum prostate-specific antigen values and serum testosterone levels in healthy men? Korean J Urol 2014;55:465–9
- Alsharef M, Kahie A, Conradie M, et al. Association between low serum free testosterone and adverse prognostic factors in men diagnosed with prostate cancer in KwaZulu-Natal. S Afr J Surg 2012;50:40–42
- Khera M, Crawford D, Morales A, et al. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol 2014;65:115–23
- Haghsheno M-A, Mellström D, Peeker R, et al. Lower urinary tract symptoms are associated with low levels of serum serotonin, high levels of adiponectin and fasting glucose, and benign prostatic enlargement. Scand J Urol 2015;49:155–61
- Pak TR, Chung WC, Lund TD, et al. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol (3betaAdiol), is a potent modulator of estrogenreceptor-beta1-mediated gene transcription in neuronal cells. Endocrinology 2005;146:147–55
- McPherson SJ, Hussain S, Balanathan P, et al. Estrogen receptor–β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc Natl Acad Sci 2010;107:3123–8
- La Vignera S, Condorelli RA, Cimino L, et al. Late-onset hypogonadism: the advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male 2016;19:34–9
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metabol 2001;86:724–31
- Ponholzer A, Plas E, Schatzl G, et al. Association of DHEA-S and estradiol serum levels to symptoms of aging men. Aging Male 2002;5:233–8