Abstract
This study investigated the role of testosterone replacement therapy (TRT) in prostate safety and cancer progression. A cohort of 553 patients, 42 treated and 162 untreated hypogonadal men, and 349 eugonadal men were included. Pathological analysis of prostate biopsies examining the incidence and severity of PCa revealed that: 16.7% of treated hypogonadal men had a positive biopsy, a Gleason score of ≤6 in 71.4% and >6 in 28.6% of men, a predominant score of 3 and tumour staging of II in 85.7% men; 51.9% of untreated hypogonadal men had a positive biopsy, a Gleason score of ≤6 in 40.5% and >6 in 59.5% men, a predominant score of 3 (77.4%) and tumour staging of II (41.7%) or III (40.5%); 37.8% of eugonadal men had a positive biopsy, a Gleason score of ≤6 in 42.4% and >6 in 57.6% of men, a predominant score of 3 (82.6%) and tumour staging of II (44.7%) or III (47.7%). The incidence of positive prostate biopsies was lowest in hypogonadal men receiving TRT, with significantly lower severity of PCa in terms of staging and grading in the same group. These results suggest that TRT might have a protective effect against high-grade PCa.
Introduction
Numerous publications have highlighted, in recent decades, the decline in total testosterone (TT) concentrations in aging men and the consequence this has in a number of medical comorbidities. As testosterone controls a number of important functions, deficiency or absence of this hormone in combination with the characteristic symptoms of hypogonadism [Citation1], such as impaired libido, infertility, low muscle mass or decreased bone density has a significant impact on quality of life (QoL). Associated comorbidities include: depression, end-stage renal disease (ESRD), lower urinary tract symptoms (LUTS), metabolic syndrome (MS), type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), and prostate cancer (PCa) [Citation1–4].
Together with the recommendation for a healthy lifestyle, the primary approach for management of hypogonadism is physiological testosterone replacement therapy (TRT). A number of studies looking at a variety of TRT modalities in hypogonadal men, reported in the last 13 years, have proven the effectiveness of TRT in slowing or reversing the unfavorable effects related to hypogonadism [Citation5–9 and see Citation10,Citation11]. Following TRT therapy, hypogonadal men reported a general sense of well-being, improved sexual parameters (spontaneous morning erections, total erections, and ejaculations) and psychological parameters for depression, fatigue and anxiety, without any episodes of mood-swings or emotional instability. Furthermore, patients receiving TRT saw beneficial effects on both body composition and lipid profiles over time [Citation12–15].
Regardless of its widespread prevalence it is estimated that only approximately 5% of men diagnosed with hypogonadism are being treated with TRT [Citation16]. This is possibly due to increased controversy regarding the implications of TRT in CVD and involvement of the androgen receptor (AR) in PCa development and progression, and likely a limited number of clinical trials assessing long-term safety in a double-blind placebo-controlled manner.
Despite the known role of the AR in PCa and the historical use of androgen deprivation therapy (ADT) for the treatment of patients with prostatic carcinoma, an emerging body of evidence suggests that low concentrations of testosterone are associated with higher grade PCa as well as being predictive of positive margins in post-radical retro-pubic prostatectomy and higher Gleason scores [Citation17–27]. Indeed, Haider et al. [Citation28] reported that TRT does not modify levels of prostate specific antigen (PSA) and has no direct impact on PCa incidence. Furthermore, Shoskes et al. [Citation29] examined the outcome of prostate biopsies in hypogonadal men prior to and during TRT and concluded that TRT did not cause or promote PCa, although for safety reasons men receiving TRT still required regular monitoring of PSA.
One major study conducted by Muller et al. [Citation30], Reduction by Dutasteride of Prostate Cancer Events (REDUCE), considered the correlation between testosterone concentrations and development of PCa. In the placebo arm of this trial, 3255 men underwent prostate biopsies at 2 and 4 years following study entry, independent of PSA levels. Muller et al. [Citation30] concluded that there was no significant association between PCa diagnosis and serum testosterone, and that at the very highest levels of testosterone a trend towards a reduced rate of PCa diagnosis was observed. Cui et al. [Citation31], supported the hypothesis that TRT is unrelated to PCa development, in a meta-analysis of 22 randomized controlled trials (RCTs) in which 2351 men were followed for either; a maximum of 12 months or between 12 and 36 months, to determine whether men on TRT were more likely to develop PCa. Shabsigh et al. [Citation32] found, following a meta-analysis of 43 publications, no compelling evidence to suggest that TRT causes PCa.
In the present study, the incidence and severity of PCa, Gleason scores and tumor staging were assessed in prostate biopsies from hypogonadal men undergoing TRT and compared with biopsies from eugonadal men and untreated hypogonadal men.
Methods
This was a cross-sectional, descriptive, prospective study conducted between 2008 and 2013, and included 553 patients. Mean age at biopsy was 61.3 ± 4.7 (range 46–81) and median PSA concentration was 3.7 ± 1.89 μg/ml (0.28–7.23). The incidence and severity of PCa was assessed via biopsy in three patient groups presenting at our urology clinic: (a) 42 hypogonadal men (T ≤ 350 ng/dl) receiving TRT, (b) 162 untreated hypogonadal men, with a median pretreatment total T of 7.1 ± 2.3 nmol/l (3.3–11.7 nmol/l), and (c) 349 eugonadal men. Biopsies were performed when indicated and following patient consent, according to the European Association of Urology (EAU) guidelines [Citation33]. Subjects in all groups had measurements taken twice a year, therefore the number of all investigation and biopsy frequency was equal in both groups as justified by indications (EAU guidelines).
All biopsies were analysed pathologically, at the Pathology Institute, University Hospital Hamburg by the team of Prof G Sautter. Specimens were assigned a Gleason score (graded as ≤6 or >6), based on the presence and location of cancer cells in the prostate, and a tumour stage (graded between I and IV), using the TNM system which identifies size and spread of the cancer (T), lymph nodes (N), and metastasis (M). Results are presented as proportion of patients from each group, hypogonadal untreated, hypogonadal undergoing TRT and eugonadal men, which have been assigned a specific diagnosis.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) v0.11 for Windows software package (SPSS Inc., Chicago, IL). Methods of statistical description included the Z-test for proportion and the Student t-test, in order to determine statistical significance. The difference of the obtained values was considered to be significant when p < 0.05, and highly significant when p < 0.01.
Results
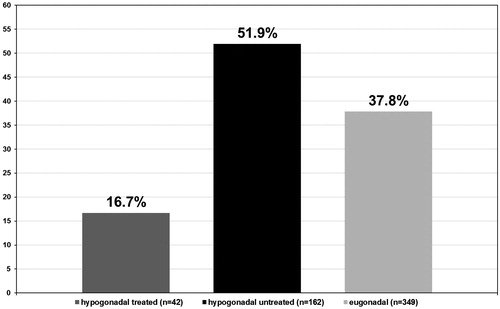
From a total of 204 hypogonadal patients, 42 received TRT of which seven (16.7%) presented with a positive biopsy for PCa. Of 162 untreated hypogonadal men, 84 (51.9%) presented with a positive biopsy for PCa. Of the 349 eugonadal men, 132 (37.8%) had a positive biopsy for PCa (p < 0.001) (see ).
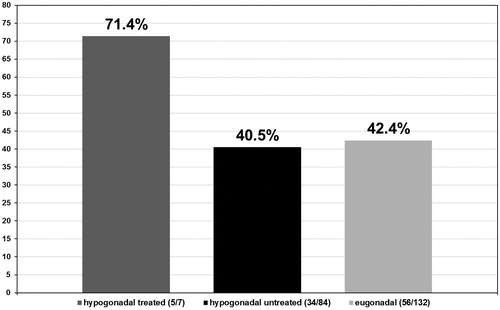
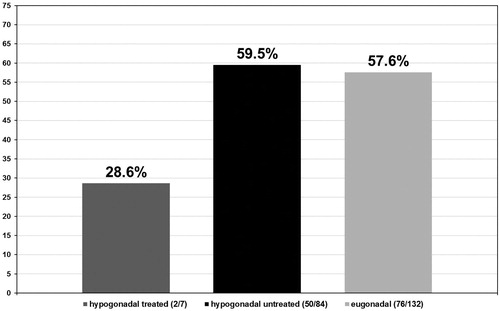
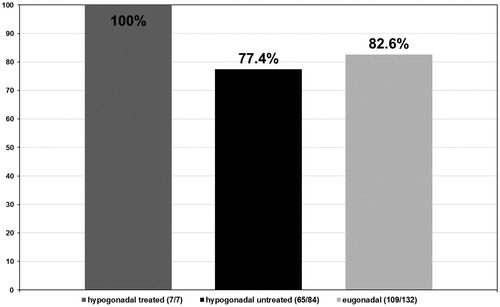
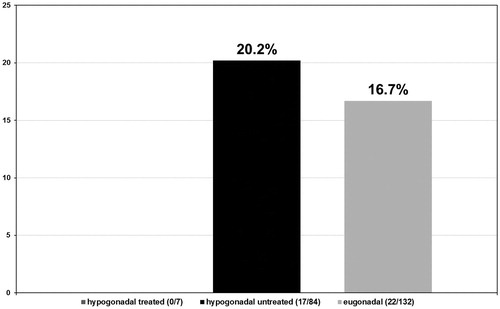
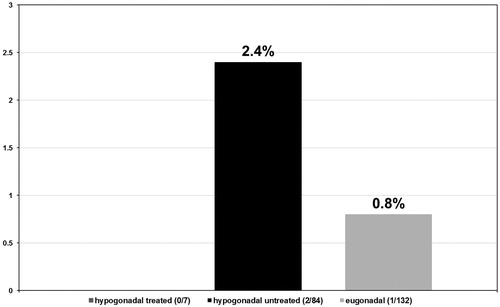
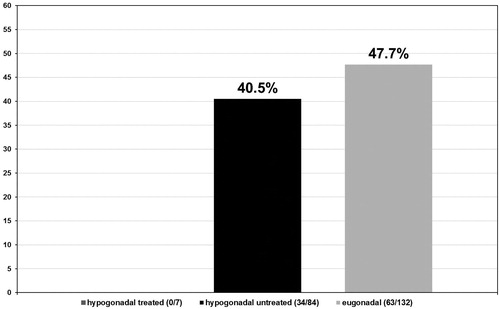
In the hypogonadal group undergoing testosterone treatment five out of seven patients had a Gleason score ≤6 (71.4%) (see ) and 2 a Gleason score >6 (28.6%) (see ). More specifically, these patients presented with a predominant Gleason score of 3 with only two patients presenting a non-dominant Gleason score of 4. In the untreated group of hypogonadal men, from the 84 patients diagnosed with PCa, 34 had a Gleason score ≤6 (40.5%) with p < 0.001 (see ) and 50 had a Gleason score >6 (59.5%) with p < 0.001 (see ). The predominant Gleason score in the untreated hypogonadal men group was 3 in 65 (77.4%) (see ), 4 in 17 (20.2%) (see ), and 5 in 2 (2.4%) (see ) men. In the group of eugonadal men, out of 132 men diagnosed with PCa, 56 had a Gleason score ≤6 (42.4%) (see ) and 76 had a Gleason score >6 (57.6%) (see ). The predominant Gleason score in the eugonadal men group was 3 in 109 (82.6%) (see ), 4 in 22 (16.7%) (see ) and 5 in 1 (0.8%) (see ) men with high statistical significance p < 0.001.
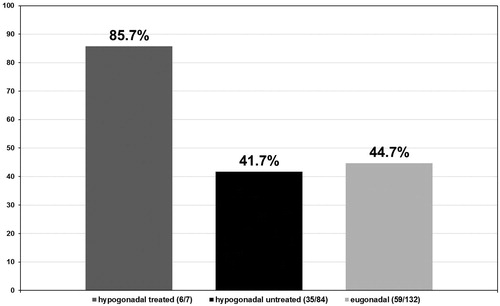
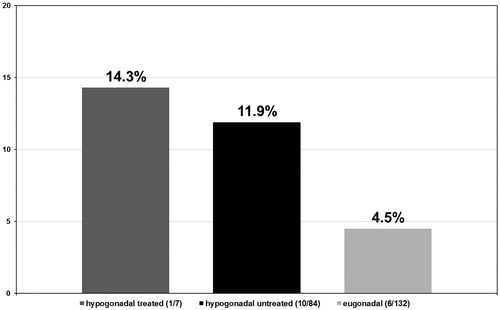
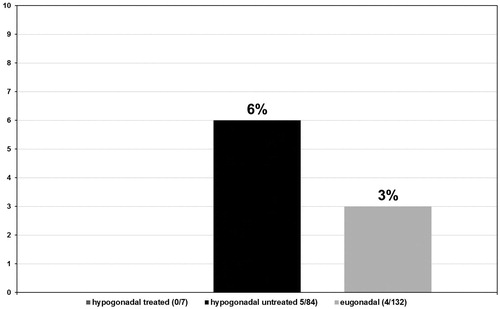
Tumor stages were assigned to all biopsies that tested positive for PCa in all three groups of men. In the group of hypogonadal men undergoing TRT, the tumor stage was II in 6/7 (85.7%) (see ) men and II–III in 1/7 (14.3%) (see ) men with no stage III or IV biopsies. In the group of untreated hypogonadal men, tumor stage was II in 35/84 (41.7%) (see ) men, II–III in 10/84 (11.9%) (see ) men, III in 34/84 (40.5%) (see ) men, and IV in 5/84 (6.0%) (see ) men. In the eugonadal men group, tumor stage was II in 59/132 (44.7%) (see ) men, II–III in 6/132 (4.5%) (see ) men, men and IV in 4/132 (3.0%) (see ) men, p < 0.001.
Discussion
The association between TRT, hypogonadism and PCa has been a subject of intense controversy over the years, due to the known role of the AR in the development of prostatic carcinoma and the success of ADT in treating patients with PCa. This led to the initiation of guidelines indicating caution when treating men at risk of PCa or men that have previously undergone PCa treatment before being cleared from the risk of recurrence. The results reported in the present study, however, demonstrate for the first time that TRT may be protective against high-grade PCa in hypogonadal men. Furthermore, these data provide additional evidence, contributing to the number of existing trials in hypogonadal patients undergoing TRT, which report no higher risk of PCa than in the general population [Citation34–36].
A number of guidelines recommend against initiating TRT in patients that are at high risk of developing PCa and in previously diagnosed or symptomatic patients, only after a prudent interval has passed with no evidence of recurrent disease following successful PCa treatment. These same guidelines advise that a high risk of developing PCa should be a contraindication against using TRT [Citation37]. Indeed, the landmark study reported by Huggins et al. [Citation38], over 70 years ago suggested that suppression of testosterone levels leads to regression of PCa, and ever since, practice has been to reduce androgen levels in the blood to castrate values in men with metastatic PCa and ultimately reduce cancer progression. Indeed, most prostate cells require testosterone for growth, and therefore TRT is not recommended in patients with or at risk of PCa, however, emerging evidence now exists which supports the so called “saturation hypothesis” which states that prostate cells require low serum testosterone concentrations for a maximal receptor engagement and thus receptor saturation will not promote PCa growth [Citation39,Citation40].
Despite these guidelines, numerous studies fail to report compelling evidence that TRT, in hypogonadal men, increases the risk of PCa above that observed in the general population [Citation41]. In contrast, a number of studies have hypothesised that untreated hypogonadal men have an increased risk of developing PCa, when compared to treated hypogonadal and eugonadal men [Citation42–44]. Results from the present study support these data, as biopsies obtained from 553 men and histological analysis revealed that only 16.7% of hypogonadal men undergoing TRT group were positive for PCa, compared to 51.9% in the untreated hypogonadal men group and 37.8% in the eugonadal men group. Furthermore, the observations from this study could indicate a somewhat protective mechanism in those hypogonadal men undergoing TRT, which would explain why none of the biopsies had advanced cell architectural changes.
Tumor stage was also investigated in the three cohorts of patients found positive for PCa. Similar to the observations reported for the Gleason scores, tumor stages were lowest in hypogonadal patients receiving TRT, thus suggesting a protective effect against tumor progression. Recent studies support these findings, one of which was a retrospective study with up to 20-year follow-up, suggesting no increased risk of PCa in hypogonadal men undergoing TRT [Citation22,Citation35]. However, inconsistent results exist with regards to testosterone concentrations and Gleason scores, with some studies reporting higher Gleason scores in men with highest baseline testosterone concentrations [Citation45], while other studies have reported no relationship between testosterone concentrations and Gleason scores [Citation46].
Previous studies have reported very heterogeneous results with regard to the association between TT concentrations and the extent of tumor progression in PCa patients. An association between low testosterone levels and high grade PCa has previously been reported in a number of reports [Citation47–49]. In one of the larger studies performed to date, Lane et al. [Citation50] reported that men with testosterone concentrations <250 ng/dl had a 2.4-fold higher chance of a Gleason score ≥4 and risk of biochemical recurrence compared to men with normal testosterone concentrations. In a study by Xylinas et al. [Citation51], low testosterone concentrations have been associated with higher prevalence of tumors with a grade higher than II, while other groups found no correlation between testosterone concentrations and cancer progression [Citation30,Citation52]. Morgentaler and Rhoden [Citation53] reported in a study of 345 hypogonadal men, that the risk of positive prostate biopsy increased in men with a testosterone concentration ≤250 ng/ml, and this was lower in men with a testosterone concentration of ≥250 ng/ml. In another prospective study, of 206 patients, the incidence of PCa was higher in men with low testosterone levels compared to men with high testosterone levels [Citation54].
In the first study addressing the effects of cumulative dose exposure of testosterone treatment, Wallis et al. [Citation55] noted that the risk of PCa in men on TRT did not increase with short-term treatment, moreover, they reported a reduced risk with prolonged TRT. The same study revealed that a short duration of TRT was associated with increased risk of mortality and CDV events, while long-term TRT would lead to reduced mortality and fewer CDV events [Citation55]. These findings are mirrored in another long-term study on patients with hypogonadism and a history of CDV diseases, which has indeed revealed that long-term TRT leads to an improvement in cardiometabolic function with no additional CDV events [Citation56].
Contrary to these data, Park et al. [Citation4] reported in a prospective study, using patient biopsies, that testosterone deficiency (<300 ng/ml) was an independent risk factor for high-grade PCa. Once more, these observations may suggest a direct correlation between testosterone concentrations and tumor progression and metastasis.
A limitation of the present study is the nature of the registry design. This single-center, open-label study was not a RCT and therefore may limit the scope of interpretation of the presented findings. However, many of the benefits (and risks) of medicines fall outside the bounds of the classical RCT and as such including data in such studies, routinely collected in the usual practice of medicine, is more reflective of the heterogeneous patients seen in real world practice settings. Other limitations include the nature of behavior of prostate volume in hypogonadal patients. It is well known, that hypogonadal subjects have smaller glands and we therefore expect to observe lower levels of PSA in this cohort [Citation57]. However, increased number of tissue cores taken upon transrectal ultrasound guided biopsies (12–14) can help to minimize the expected range of error in this respect. Furthermore, it is important to consider that secretion of PSA in tumours of untreated hypogonadal men are likely to be reduced per tumour volume than that of eugonadal men and will therefore have more pathologically aggressive tumours when they reach the given PSA-indicated biopsy threshold. Similarly, these patients are more likely to undergo biopsy for an abnormal digital rectal exam in the context of a normal PSA. These issues should be addressed in future studies.
In conclusion, data presented in this study suggest that TRT may in fact protect against high-grade PCa and together with previous literature support the “saturation theory” whereby at higher levels, when all ARs are bound, higher testosterone levels will not further stimulate prostate cells. Analysis of biopsies from three groups of men, hypogonadal men undergoing TRT, untreated hypogonadal men and eugonadal men revealed that the incidence of positive prostate biopsies was lowest in hypogonadal men receiving TRT, and the severity of PCa in terms of grading (Gleason score) and staging (tumour stage) was significantly lower in hypogonadal patients receiving TRT compared to observations made in the untreated hypogonadal and eugonadal groups.
It is well accepted that TRT is contraindicated in metastatic PCa; however, evidence suggests that TRT is advisable in hypogonadal men that have been cured of PCa with surgery. However, large, randomised and placebo-controlled trials may be necessary to elucidate the impact of TRT on the incidence of PCa in men with chronic low testosterone levels.
Declaration of interest
Aksam Yassin has occasionally received honoraria for lectures from Bayer Pharma (Germany), Ferring Pharmaceuticals (Germany), and Glaxo-Smith-Kline (Germany). No other conflicts of interest to report.
Acknowledgements
Editorial support for this manuscript was provided by Astra-Health, www.astra-health.co.uk.
References
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Behre HM, Christin-Maitre S, Morales AM, Tostain J. Transversal European survey on testosterone deficiency diagnosis. Aging Male 2012;15:69–77
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male 2014;17:161–5
- Park J, Cho SY, Jeong SH, et al. Low testosterone level is an independent risk factor for high-grade prostate cancer detection at biopsy. BJU Int 2016;118:230–5
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154:899–906
- Yassin A, Huebler D, Saad F. Long-acting testosterone undecanoate for parenteral testosterone therapy. Therapy 2006;3:709–21
- Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 2014;11:1567–76
- Saad F, Yassin A, Haider A, et al. Elderly men over 65 years of age with late-onset hypogonadism benefit as much from testosterone treatment as do younger men. Korean J Urol 2015;56:310–17
- Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gen (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin J Endocrinol Metab 2004;89:2085–98
- Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol 2013;217:R47–71
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol 2013;217:R25–45
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis 2009;207:318–27
- Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl 2009;30:726–33
- Saad F, Gooren L, Haider A, Yassin A. An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl 2007;53:353–7
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab 2007;92:3844–53
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 2009;5:427–48
- Faydaci G, Bilal E, Necmettin P, et al. Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. Aging Male 2008;11:189–92
- García-Cruz E, Piqueras M, Huguet J, et al. Low testosterone levels are related to poor prognosis factors in men with prostate cancer prior to treatment. BJU Int 2012;110:E541–6
- Grosman H, Fabre B, Lopez M, et al. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male 2016;19:40–5
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous hormones and prostate cancer collaborative group. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 2008;100:170–83
- Eisenberg ML, Li S, Betts P, et al. Testosterone therapy and cancer risk. BJU Int 2015;115:317–21
- Porcaro AB, Petrozziello A, Ghimenton C, et al. Associations of pretreatment serum total testosterone measurements with pathology-detected Gleason score cancer. Urol Int 2014;93:269–78
- Cabral PH, Iwamoto MW, Fanni VS, et al. Study of testosterone as a predictor of tumor aggressiveness in patients with prostate cancer. Int Braz J Urol 2013;39:173–81
- Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol 2000;163:824–7
- Botto H, Neuzillet Y, Lebret T, et al. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011;186:1400–5
- Morgentaler A. Turning conventional wisdom upside-down: low serum testosterone and high-risk prostate cancer. Cancer 2011;117:3885–8
- Pichon A, Neuzillet Y, Botto H, et al. Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading. Prostate Cancer Prostatic Dis 2015;18:382–7
- Haider A, Zitzmann M, Doros G, et al. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median follow-up of 3 registries. J Urol 2015;193:80–6
- Shoskes DA, Barazani Y, Fareed K, Sabanegh E. Jr. Outcomes of prostate biopsy in men with hypogonadism prior or during testosterone replacement therapy. Int Braz J Urol 2015;41:1167–71
- Muller RL, Gerber L, Moreira DM, et al. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol 2012;62:757–64
- Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2014;17:132–43
- Shabsigh R, Crawford ED, Nehra A, Slawin KM. Testosterone therapy in hypogonadal men and potential prostate cancer risk: a systematic review. Int J Impot Res 2009;21:9–23
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 2017;71:618–29
- Feneley MR, Carruthers M. Is testosterone treatment good for the prostate? Study of safety during long-term treatment. J Sex Med 2012;9:2138–49
- Raynaud JP. Prostate cancer risk in testosterone-treated men. J Steroid Biochem Mol Biol 2006;102:261–6
- Efesoy O, Apa D, Tek M, Çayan S. The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male 2016;19:79–84
- Bhasin S, Cunningham G, Hayes F, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2010;95:2536–59
- Huggins C, Stevens RE Jr., Hodges CV. Studies on prostatic cancer II: the effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941;43:209–23
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009;55:310–20
- Hassan J, Barkin J. Testosterone deficiency syndrome: benefits, risks, and realities associated with testosterone replacement therapy. Can J Urol 2016;23:20–30
- Morgentaler A. Testosterone therapy for men at risk for or with history of prostate cancer. Curr Treat Options Oncol 2006;7:363–9
- Rhoden EL, Morgentaler A. Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol 2003;170:2348–51
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 2016;19:64–9
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol 2016;84:107–14
- Yano M, Imamoto T, Suzuki H, et al. The clinical potential of pretreatment serum testosterone level to improve the efficiency of prostate cancer screening. Eur Urol 2007;51:375–80
- Sher DJ, Mantzoros C, Jacobus S, et al. Absence of relationship between steroid hormone levels and prostate cancer tumor grade. Urology 2009;73:356–61. discussion 361–2
- Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate 2001;47:52–8
- Morote J, Ramirez C, Gomez E, et al. The relationship between total and free serum testosterone and the risk of prostate cancer and tumour aggressiveness. BJU Int 2009;104:486–9
- Morgentaler A, Bruning CO, III DeWolf WC. Occult prostate cancer in men with low serum testosterone levels. JAMA 1996;276:1904–6
- Lane BR, Stephenson AJ, Magi-Galluzzi C, et al. Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology 2008;72:1240–5
- Xylinas E, Ploussard G, Durand X, et al. Low pretreatment total testosterone (<3 ng/ml) predicts extraprostatic disease in prostatectomy specimens from patients with preoperative localized prostate cancer. BJU Int 2011;107:1400–3
- Botelho F, Pina F, Figueiredo L, et al. Does baseline total testosterone improve the yielding of prostate cancer screening? Eur J Cancer 2012;48:1657–63
- Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen of 4.0 ng/ml or less. Urology 2006;68:1263–7
- Shin BS, Hwang EC, Im CM, et al. Is a decreased serum testosterone level a risk factor for prostate cancer? A cohort study of Korean men. Korean J Urol 2010;51:819–23
- Wallis CJD, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol 2016;4:498–506
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag 2016;12:251–61
- Yassin A, Saad F. Testosterone treatment in hypogonadal patients does not cause higher incidence of prostate cancer. J Urol 2008;179:301