Abstract
Objective: To analyze the impact of age, BMI and sex hormone on aging males’ symptoms (AMS) and the 5-item version of the international index of erectile function (IIEF-5) scores in middle-aged and elderly Chinese men.
Methods: A population-based cross-sectional study was conducted in Jiashan County. A total of 969 men, aged between 40 and 80 years old, were admitted. Physical examination and the sex hormones were measured, and AMS and IIEF-5 scores were assessed.
Results: The oneway ANOVA analysis indicated older age groups had higher AMS total-scores, somatic and sexual sub-scores, and lower IIEF5 scores (all p < .01). Pairwise correlation (rpairwise) analyses showed the significant associations between AMS and age or sex hormone (cFT, Bio-T, SHBG, and LH) levels, and similar for IIEF5. However, when age was adjusted, the correlation coefficients (rpartial) weakened, and correlation significance disappeared, except LH (for AMS: rpartial = 0.096, p = .009; for IIEF-5: rpartial = −0.140, p = .001). Multiple linear regressions confirmed the influence of increased age and LH on the AMS and IIEF5 scores.
Conclusion: CFT, Bio-T and SHBG failed to yield any additional predicting information when age was adjusted. To improve the male reproductive health, future research should pay more attention on aging-related comorbidities and how to improve general wellness.
Introduction
With an overall increase in life expectancy, aging is causing more severe public health issues globally. Reproductive health, as an important aspect of wellness, impacts on self-esteem, quality of life and interpersonal relationships [Citation1]. How to delay aging on reproductive function and promote healthy aging, is still a thorny problem.
In China, aging males’ symptoms (AMS) scale and the international index of erectile function (IIEF-5) were widely used in clinical or scientific research for assessing Symptomatic Late-Onset Hypogonadism (SLOH) and Erectile Dysfunction (ED), respectively [Citation2,Citation3]. Erectile dysfunction (ED) is highly prevalent in hypogonadal men [Citation4]. Although testosterone and other sex hormones were reported to be related to ED or AMS, androgen deficiency is responsible for ED or AMS only in a small subset of aging men [Citation5]. Furthermore, various researches tested different hormones, testosterone, bioavailable testosterone (Bio-T), and or calculated free testosterone (cFT), and got inconsistent results [Citation6–8]. It is imperative to make a comprehensive assessment on the relationship of sex hormone and reproductive health.
Materials and methods
Study population
In 2012, a cross-sectional study was performed in Jiashan County, Zhejiang province, which is a representative county of East China. Local male residents, aged 40 to 80 years old, were invited to volunteer to the study. Middle-aged and elderly men, who had history of psychotic, cognitive disorders, or took hormones, antiandrogen, or psychotropic agents, or had undergone pelvic surgical therapy, were excluded.
Clinical assessments
All the participants were asked to complete a uniform questionnaire, including demographic information, an abridged 5-item version of International Index of Erectile Function (IIEF-5) and Aging Males’ Symptoms (AMS) scale.
The AMS scale consists of 17 items, 5 for psychological, 7 for somatic and 5 for sexual subscores. Each item is scored as 1 to 5 points, and total score ranges from 17 to 85. The IIEF-5 includes 5 questions and each is scored on a rating scale of 0–5, for an overall score of 0–25 [Citation9,Citation10].
Endocrinology
All blood samples were drawn between 8:00 a.m. and 10:00 a.m. from overnight-fasting males to minimize the effects of diurnal variation. All hormonal assessments were conducted by the same laboratory, including total testosterone (TT), sex hormone-binding globulin (SHBG) and luteinizing hormone (LH). Serum level of calculated free testosterone (cFT) and bioavailable testosterone (Bio-T) was calculated according the equation [Citation11].
Statistical analysis
The respondents were classified into four age groups: 40 to 50, 51 to 60, 61 to 70, and 71 years or older. Body mass index (BMI) was calculated and categorized into three groups: less than 18.5, 18.5 to 24, and 24 or more. Analysis of variance (ANOVA) was applied to compare the differences of means of AMS score or IIEF5 score among age groups or BMI groups.
Pearson’s correlation analysis was used to describe associations between the age, BMI, sex hormones and AMS or IIEF5. To eliminate the influence of multi-variable, partial correlation analysis was performed.
Multiple linear regression was applied to analyze the linear relationship among variables. Considering cFT and Bio-T were calculated from TT, SHBG and serum albumin, variance inflation factors (VIFs) were estimated in the multiple linear regressions. Strong associations among independent variables may induce large VIF value. VIF >10 indicates multi-collinearity, and then the linear regression model needs to be adjusted.
All statistics were calculated by using SAS9.1.3 package (SAS Institute, Cary, NC) and Stata 14 (Stata Corporation, College Station, TX).
Results
A total of 969 men, aged 56.4 ± 9.1 years old, were admitted to the data analysis. Means of BMI, TT, cFT, Bio-T, SHBG, LH, AMS score (psychological, somatic, sexual) and IIEF score were 23.7 kg/m2, 4.1 ng/ml, 67.9 ng/l, 1.8 ng/ml, 43.3 nmol/l, 5.9 IU/l, 22.7 (5.2, 9.3, 8.2) and 17.2, respectively. The mean of each parameter was adjacent to the corresponding median ().
Table 1. Description of sex hormone levels, AMS and IIEF scores.
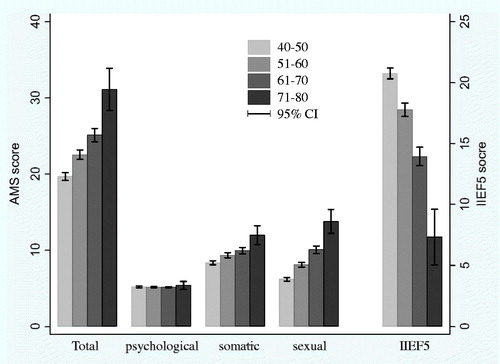
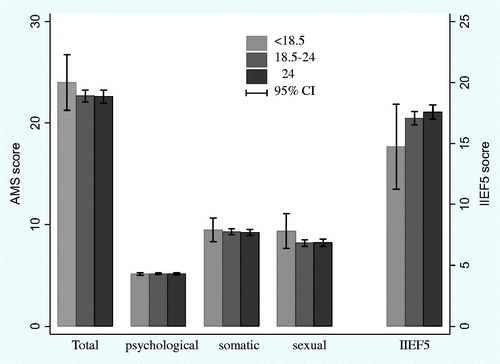
The oneway ANOVA analysis indicated total AMS scores and IIEF5 scores were significantly different among different age groups (all p < .01). Older age groups showed higher AMS scores and lower IIEF5 scores. When the AMS sub-scores were assessed, significant differences were found in the somatic and sexual sub-scores (all p < .01). However, there was no significant difference among the groups in terms of psychological sub-scores (p = .38) (). No significant difference was found in the AMS scores or IIEF5 scores among different BMI groups ().
Pairwise correlation (pairwise r) analyses showed the significant associations between AMS and age or sex hormone (cFT, Bio-T, SHBG, and LH) levels, and similar associations between IIEF5 and age or sex hormone. No correlation was found between AMS or IIEF5 and TT. However, when the age variable was adjusted, the correlation coefficients (partial r) between AMS and sex hormone (cFT, Bio-T and SHBG) or between IIEF5 and sex hormone weakened, and correlation significance disappeared, except LH. In the partial correlation analyses, age (rpartial = 0.353, p < .001) and LH (rpartial = 0.096, p = .009) showed positive and significant relationship with AMS, and negative significant relationship with IIEF (age: rpartial = −0.470, p < .001; LH: rpartial = −0.140, p = .001) ().
Table 2. Pairwise correlation and partial correlation between variables.
Multiple linear regressions confirmed the influence of increased age and LH on the AMS score and IIEF5. Each additional year of increase in age caused an increase in the AMS score of 0.324 (p < .01) and a decrease in the IIEF5 score of 0.387 (p < .01). Likewise, a 1-unit increase in LH increases AMS score of 0.182 (p < .01) and reduces IIEF5 score of 0.231(p < .01). Other sex hormones did not show such significant correlations.
Considering the association between age and sex hormones or among sex hormones, variance inflation factors (VIFs) were estimated in the multiple linear regression and indicated multi-collinearity. TT, cFT, Bio-T and SHBG were correlated. Therefore, age, BMI, and LH were kept in the model, and then only one of other sex hormones was added into the model each time. All VIFs were less than 1.5. The correlation results were similar to the formers, and only age and LH were still the significant correlated factors with AMS or IIEF5.
Discussion
In the former paper, we have found cFT and Bio-T decreased, AMS and Erectile dysfunction got more severe with aging [Citation12]. In the current paper, when AMS sub-scores were further analyzed, somatic and sexual sub-scores significantly changed with aging, but there was no impact on the psychological sub-score. The correlations were similar to other studies [Citation2,Citation13].
Pairwise correlations revealed that, in comparison to TT, Bio-T and cFT levels were more strongly associated with AMS or IIEF5. Considering the data did not completely fit normal distribution, we compared the Pearson’s correlation results and Spearman correlation (non-parametric statistical method) results. Both the correlation coefficients r and p values were similar. Therefore, we can assume that the non-normal distribution of variables have leveled off according to the law of large sample.
Liu’s and Antonio’s study also showed cFT levels were more reliable than TT levels for diagnosing LOH in middle-aged and elderly males [Citation14,Citation15]. Some studies reported testosterone treatment can significantly improve male ED and LUTS [Citation16,Citation17], however, data on the effect of exogenous testosterone on men are inconsistent [Citation18]. In sub-analysis, no correlation was observed between any hormone level and the psychological section of the AMS, whereas the somatic and sexual sections were correlated with the levels of cFT, Bio-T, SHBG and LH. This was partially supported by results of Chen W’s [Citation19]. However, when age was included into the multiple regression models, cFT, Bio-T and SHBG failed to yield any additional predicting information. Only LH was still the associated factor of AMS and IIEF5 scores. Partial correlation analysis supported these results, too. The results were supported by Kratzik’s study [Citation20]. In Tajar’s paper, normal T and elevated LH were defined as compensated hypogonadism, compensated hypogonadism was associated predominantly with physical symptoms and was considered to a genuine clinical subgroup of LOH [Citation21].
More and more research studies document the associations between AMS or erectile dysfunction and general unwellness [Citation22,Citation23]. García-Cruz E and Hamanoue N pointed out AMS values were not associated with testosterone values but rather were related to insulin resistance, particularly in subjects with moderately severe AMS values [Citation24,Citation25]. The Massachusetts Male Aging Study indicated that obesity is an independent risk factor for ED [Citation26]. However, we did not find the relationship between BMI and AMS or IIEF-5. Waist circumference may be a better predictor of hypogonadism than BMI [Citation27]. A greater severity of ED in men with LOH correlated with an increased waist circumference and a history of diabetes mellitus [Citation28]. Interestingly, testosterone treatment not only improved TT and cFT levels, but also increased lean mass and decreased fat mass [Citation29]. Corona G’s study found, testosterone treatment significantly improves erectile dysfunction in men with testosterone deficiency. However, the magnitude of the effect was lower in the presence of metabolic derangements, such as diabetes and obesity [Citation30]. In other aspects, for example, men who work nonstandard shifts and have poor sleep quality are at increased risk for hypogonadal symptoms and sexual dysfunction [Citation31]. Serum inorganic phosphorus had the highest correlation coefficient with IIEF-5 score in the middle-age [Citation32].
The limitation of our research is that we did not considering too many other influence factors on reproductive health. We are following-up these subjects and will collect more information, such as demographic statistics, living style, quality of life index, and so on.
Our research population is ordinary middle-aged and elderly men, not including many patients with severe diseases. The decreases of reproductive health were mainly induced by aging and aging-related comorbidities. Based on our study results, for ordinary middle-aged and elderly men, to improve the reproductive health, attention should not be only focused on testosterone supplement. The next step in our research will pay more attention to the effect of healthy living style, such as nutritionally balanced diet, proper exercise, regular schedule, on general wellness, as well as reproductive health.
Disclosure statement
No conflict of interest.
Additional information
Funding
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90.
- Kong XB, Guan HT, Li HG, et al. The ageing males' symptoms scale for Chinese men: reliability,validation and applicability of the Chinese version. Andrology. 2014;2:856–861.
- Tang WH, Zhuang XJ, Shu RM, et al. The prevalence of erectile dysfunction among subjects with late-onset hypogonadism: a population-based study in China. Int J Clin Exp Med. 2015;8:13901–13910.
- Corona G, Rastrelli G, Forti G, et al. Update in testosterone therapy for men. J Sex Med. 2011;8:639–654. quiz 655.
- Ahn HS, Park CM, Lee SW. The clinical relevance of sex hormone levels and sexual activity in the ageing male. BJU Int. 2002;89:526–530.
- Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025.
- Ponholzer A, Plas E, Schatzl G, et al. Relationship between testosterone serum levels and lifestyle in aging men. Aging Male. 2005;8:190–193.
- Müezzinoğu T, Gümüş B, Temeltaş G, et al. A relationship of sex hormone levels and erectile dysfunction: which tests should be done routinely? Yonsei Med J. 2007;48:1015–1019.
- Heinemann LAJ, Zimmermann T, Vermuelen A, et al. new aging males’ symptoms (AMS) rating scale. Aging Male. 1999;2:105–114.
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672.
- Li JH, Yu XH, Zheng JB, et al. The reproductive health indices and sex hormone levels in middle-aged and elderly Chinese men. Aging Male. 2016; 19:143–147.
- Ichioka K, Nishiyama H, Yoshimura K, et al. Aging Males' Symptoms scale in Japanese men attending a multiphasic health screening clinic. Urology. 2006;67:589–593.
- Liu Z, Liu J, Shi X, et al. Comparing calculated free testosterone with total testosterone for screening and diagnosing late-onset hypogonadism in aged males: a cross-sectional study. J Clin Lab Anal. 2016. Forthcoming. doi: 10.1002/jcla.22073.
- Antonio L, Wu FC, O'Neill TW, et al. Low Free Testosterone Is Associated with Hypogonadal Signs and Symptoms in Men with Normal Total Testosterone. J Clin Endocrinol Metab. 2016;101:2647–2657.
- Karabakan M, Keskin E, Akdemir S, et al. Effect of tadalafil 5 mg daily treatment on the ejaculatory times, lower urinary tract symptoms and erectile function in patients with erectile dysfunction. Int Braz J Urol. 2017;43:317–324.
- Pexman-Fieth C, Behre HM, Morales A, et al. A 6-month observational study of energy, sexual desire, and body proportions in hypogonadal men treated with a testosterone 1% gel. Aging Male. 2014; 17:1–11.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol. 2000;164:371–375.
- Chen W, Liu ZY, Wang LH, et al. Are the Aging Male's Symptoms (AMS) scale and the Androgen Deficiency in the Aging Male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16:92–96.
- Kratzik CW, Schatzl G, Lunglmayr G, et al. The impact of age, body mass index and testosterone on erectile dysfunction. J Urol. 2005;174:240–243.
- Tajar A, Forti G, O'Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818.
- Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382:1781–1794.
- Dean J, Shechter A, Vertkin A, et al. Sexual Health and Overall Wellness (SHOW) survey in men and women in selected European and Middle Eastern countries. J Int Med Res. 2013;41:482–492.
- García-Cruz E, Leibar-Tamayo A, Romero-Otero J, et al. Marked testosterone deficiency-related symptoms may be associated to higher metabolic risk in men with low testosterone levels. J Sex Med. 2014;11:2292–2301.
- Hamanoue N, Tanabe M, Tanaka T, et al. A higher score on the Aging Males’ Symptoms scale is associated with insulin resistance in middle-aged men. Endocr J. 2017;64:521–530.
- Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302.
- Yassin AA, Nettleship JE, Salman M, et al. Waist circumference is superior to weight and BMI in predicting sexual symptoms, voiding symptoms and psychosomatic symptoms in men with hypogonadism and erectile dysfunction. Andrologia. 2017;49:e12634.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Rodriguez-Tolrà J, Torremadé Barreda J, del Rio L, et al. Effects of testosterone treatment on body composition in males with testosterone deficiency syndrome. Aging Male. 2013;16:184–190.
- Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of results of testosterone therapy on sexual function based on International Index of Erectile Function Scores. Eur Urol. 2017. Forthcoming. doi:10.1016/j.eururo.2017.03.032
- Pastuszak AW, Moon YM, Scovell J, et al. Poor sleep quality predicts hypogonadal symptoms and sexual dysfunction in male nonstandard shift workers. Urology. 2017;102:121–125.
- Min SK, Choi K, Kim SK, et al. Phosphorus as predictive factor for erectile dysfunction in middle aged men: a cross sectional study in Korea. Investig Clin Urol. 2016;57:442–448.