Abstract
This observational post-marketing study of parenteral testosterone undecanoate (TU) in a non-selected population aimed to: examine the effectiveness of TU as treatment of hypogonadism; record adverse drug reactions (ADR) quantitatively particularly regarding polycythemia, prostate safety and cardiovascular-related metabolic risk factors; and verify whether recommended injection intervals apply to routine clinical practice. Eight hundred and seventy subjects from 259 outpatient units scheduled to visit the clinic six times were included. Effectiveness and tolerability of TU administration were assessed on a 4-point scale. Body weight, waist girth, blood pressure, hemoglobin levels, hematocrit, prostate-specific antigen (PSA), and digital rectal prostate examination were assessed. Over 90% of subjects completed the observational duration of 52.8 ± 9.7 weeks (mean ± SD) and 56% judged effectiveness as very good, 30.8% as good. 63.1% judged tolerability as very good, and 24.4% as good. No adverse effects on indicators of cardiovascular risk were observed. Polycythemia occurred in one subject and a supranormal hematocrit in one subject. Four subjects developed supranormal PSA levels. Prostate carcinoma was found in one subject, one subject had recurrence of a previously surgically treated prostate carcinoma, and the other two showed no indication of malignancy. Parenteral TU is safe, effective, and well-tolerated in clinical practice proving a good therapeutic option for hypogonadism.
Introduction
Aside from its well-established roles in sexual differentiation, reproductive, and sexual function in men there is increasing evidence that testosterone also has an important role in the regulation of multiple physiological processes, such as bone metabolism, body composition, glucose/lipid related metabolic processes, and hematopoiesis in adult men [Citation1]. Well-documented effects of androgen substitution in hypogonadal men are improvement in sexual function, increments in bone density, lean body mass, and erythropoiesis [Citation1,Citation2]. It is also now well established that low testosterone levels may increase the incidence of coronary artery disease (CAD) with a lack of evidence in both medium- and long-term clinical trials that testosterone replacement therapy increases risk with patients suffering from CAD [Citation3]. Testosterone formulations for intramuscular (i.m.) injection and subcutaneous application as well as for oral and transdermal administration have been approved for androgen therapy [Citation2]. To date, injectable testosterone esters are one of the most commonly used formulations. To increase serum testosterone levels to the physiological range, i.m. injections of testosterone enanthate (TE) approximately every 2 weeks are required, which lead to supraphysiological peaks shortly after administration, followed by a sharp fall in levels thereafter. Testosterone levels before the next injection are frequently in the hypogonadal range [Citation4]. In turn, the marked oscillations in serum testosterone concentration and short inter-injection intervals in this treatment regimen are associated with considerable discomfort for many patients [Citation4]. Therefore, development of longer-acting formulations, maximizing convenience, and minimizing interference with daily life represents a major improvement in testosterone therapy [Citation1].
Testosterone undecanoate (TU), an ester with a fatty acid side-chain of medium length in 17ß-position, is a long-acting formulation for i.m. injection. This requires significantly less frequent injections than other established parenteral testosterone ester formulations and has a much better pharmacokinetic profile [Citation2,Citation5–8].
Objectives
The primary aim of this post-marketing surveillance study was to examine the effectiveness of TU for the treatment of hypogonadal complaints and its safety profile in daily routine practice.
A concern of longer-term testosterone administration, particularly in elderly men, is its safety. The main concerns are polycythemia, prostate safety, and effects on metabolic factors related to cardiovascular risks. Thus, hemoglobin levels, hematocrit, prostate specific antigen, and digital rectal examination of the prostate were assessed as well as body weight and waist girth. Due to the non-interventional character of the study, laboratory results were considered only if routinely acquired in the practice. A further aim was to record adverse drug reactions (ADR) quantitatively.
The following administration regimen is recommended for TU therapy in hypogonadal men: After the first injection of 1000 mg TU, a second dose injection of 1000 mg TU is to be administered 6 weeks after the first injection (loading dose) in order to quickly reach steady state, followed by injections every 12 weeks (maintaining dose). Whilst this schedule is generally adequate, an individualization of TU therapy, based on testosterone levels measured at end of the interval or complaints of testosterone deficiency, may be desirable [Citation9]. The present study gathered information to verify whether these recommended injection intervals are confirmed in routine clinical practice given that androgen dependent functions require plasma testosterone levels in the eugonadal range [Citation10].
Subjects and methods
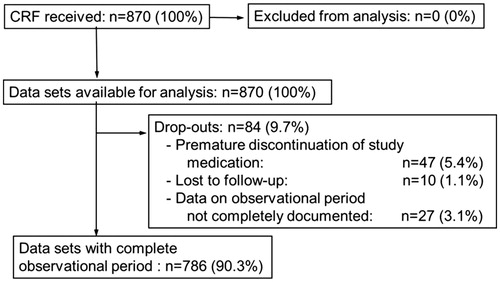
The present post-marketing surveillance study was performed in a non-selected population, largely in urology outpatient units across Germany between January 2005 and December 2006. The study was planned to include 1000 patients, although eventually 870 subjects were available for analysis (full analysis set) from 259 outpatient units (). The inclusion and exclusion criteria followed conventional clinical criteria for administration of testosterone. Study data were collected on case report forms (CRF) with the following general and safety items: Baseline demographic data and anthropometry (e.g. height, body weight, blood pressure, waist circumference), pre-existing/comorbid conditions (e.g. diabetes mellitus, hypertension, or other), pre-treatment clinical laboratory tests (including serum total testosterone, PSA, hemoglobin, hematocrit, FSH, LH), pre-treatment digital rectal examinations and any pathological findings, indication for testosterone therapy (TTh), previous TTh, including dates of start and end, route of dose, reason for switch to TU, date of start of TU therapy, and injection dates of each following injection. At each injection visit, the physician assessed effectiveness and tolerability on a 4-point scale (“Very good”, “Good”, “Satisfactory”, “Poor”). Serum prostate specific antigen (PSA) was first measured at visit 2. At visits 3 and 4 (approximately weeks 30 and 42), clinical laboratory tests (including serum total testosterone, PSA, hemoglobin, hematocrit), and a digital rectal examination were performed. From visit 3, changes of injection intervals were initiated. At visit 5 (approximately at week 54), anthropometry, clinical laboratory tests, and digital rectal examinations were repeated. This visit constituted a final assessment including information on premature discontinuation/reason for premature discontinuation, intention to continue treatment with TU, and patient satisfaction. Adverse drug reactions were collected and registered following the usual protocol of drug studies.
Figure 1. Disposition of subjects. 1000 subjects from a non-selected population were recruited across Germany between January 2005 and December 2006. In total 870 subjects were analyzed.
Data handling
The CRFs were checked for completeness and consistency. All captured untoward events (including medical terms captured in free-text) were listed and analyzed. All reported adverse drug reactions and all potentially serious untoward events were forwarded to the Drug Safety Department for evaluation.
Data analysis
Data analysis was performed descriptively, that is confirmatory statistics analysis were planned or performed. None of the subjects were excluded from analysis (). The entire set of patients with at least one injection was defined as full analysis set (FAS). In case the date of drop-out from the study was missing the date of the last visit was taken as substitute. The number of injections was derived from the injection dates on the CRF. Laboratory data were generated as part of the routine medical practice. Laboratory tests were performed in local laboratories, therefore units and laboratory ranges were not uniform and some values seemed implausible. However, none of the values were excluded from the analysis. Analyses were performed for all available laboratory parameters, that is serum testosterone, hemoglobin, hematocrit, baseline LH, FSH, and prostate-specific antigen (PSA), for all time points of sampling. The frequency of reporting (number and percent of patients), and mean, standard deviation (SD), maximum and minimum values of each parameter were evaluated. Subgroup analyses for subjects starting on TU and subjects switching from other TTh to TU were also performed.
Results
Data of 870 subjects (full analysis set; FAS) were available for analysis. Three subjects were female-to-male transsexuals. The mean age was 54.1 ± 11.8 years (mean ± SD; range: 17–85 years) at study start. 3.8% were ≤30 years and 53.8% were ≥55 years. The mean height was 178.2 ± 6.6 cm, the mean body weight 87.2 ± 11.9 kg, the mean body mass index (BMI) was 27.5 ± 3.5 kg/m2, and the mean waist circumference was 102.0 ± 12.7 cm (range: 64–145 cm).
At baseline, the mean systolic and diastolic blood pressures were 135.8 ± 13.9 and 83.3 ± 8.5 mm Hg, respectively. At study start the mean testosterone levels were 8.0 ± 5.0 nmol/l (SD) (n = 807). A total of 121 subjects (13.9%) had an orchiectomy (mostly bilateral) in their medical history. Hypertension was encountered in 238 subjects (27.4%) and diabetes mellitus in 98 subjects (11.3%). Other diseases affecting more than 2% of the patients were: status after testicular cancer (5.6%); prostate hyperplasia (3.4%), Klinefelter’s syndrome (3.2%), and coronary artery disease (2.0%); 16 subjects (1.8%) had a pituitary disorder.
Indication for treatment with TU
Male hypogonadism was reported as indication in 835 subjects (96.0%). In 376 subjects (43.2%), this was labelled as “classic” hypogonadism (hypothalamic/pituitary/testicular disease), whereas in 459 subjects (52.8%) it was classified as “age-related” hypogonadism. In 24 subjects (2.8%), “no hypogonadism” was indicated in the corresponding field, whereas for 11 subjects (1.3%) no statement was given. The most frequent conditions were status after orchiectomy (12.4%), erectile dysfunction (2.6%), Klinefelter’s syndrome (2.4%), lack of libido (2.2%), pituitary disorder (1.7%), symptoms of lack of testosterone (1.3%), lack of energy/exhaustion (1.3%), osteoporosis (0.3%), and female-to-male sex change (0.3%).
Of the 870 subjects, 408 (46.9%) were not previously receiving TTh, whereas 462 subjects (53.1%) switched to TU from a different testosterone preparation. Among those who switched, 139 (16.0%) intermitted their TTh >4 weeks whilst the other 277 (31.8%) intermitted <4 weeks. On the remaining 46 subjects (5.3%) no information was available. The majority of subjects with previous TTh (n = 305; 35.1%) had received an injectable preparation, whereas 150 subjects (17.2%) had used a testosterone gel; only 13 (1.5%) switched from capsules and 14 (1.6%) switched from patches.
Reasons for switching to TU
The most frequent reasons for choosing TU were “more comfort for the patient” (51.3%) and “steady drug levels” (49.2%). “Better compliance” (29.5%), “prevention of mood changes” (16.4%), and “less side effects” (8.0%) were less frequent reasons (multiple citing was possible). In 32% of the patients, no reason was given.
Treatment regimen
For the interval between the first administration of TU (study start) and the second administration at visit 1, the mean injection interval was 6.2 ± 1.9 weeks, thus very close to the initial 6 weeks as recommended for TU [Citation9,Citation10]. An interval of 5–7 weeks between the first and second injection was followed in 685 subjects (78.7%). The subsequent mean intervals, 11.4 ± 2.9, 12.6 ± 2.0, 12.7 ± 2.4, and 12.3 ± 2.0 weeks, were also in the recommended range of 10–14 weeks. At each visit, more than 70% of the subjects were in compliance with the product recommendations.
Assessment of efficacy and tolerability
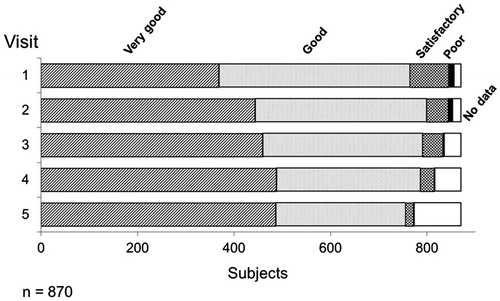
At every visit the efficacy and tolerability was assessed by the physician by means of a 4 point stage. Over the whole observational period, approximately 90% of the physicians assessed the efficacy of TU as “Good” or “Very good”. A “Poor” efficacy was reported very rarely and only at the first visit, with a frequency of 1.3%. The percentage of “Very good” assessment increased in the course of the study from 42.4% at visit 1 to approximately 56% at visits 4 and 5 ().
Figure 2. Assessment of the efficacy of Nebido at each visit. Over the time course of the observational period, over 90% of respondents described the efficacy of Nebido as very good or good with an overall increase of very good responses over the same period. Very few respondents described Nebido's efficacy as poor.
These assessments were paralleled by an increase of the mean testosterone levels during the course of the study. At study start the mean testosterone levels were 8.0 ± 5.0 nmol/l (n = 807). Given that the normal range of serum testosterone levels in eugonadal men is 12–35 nmol/l [Citation4], the means found at study start were clearly compatible with the need for hormone substitution.
During treatment, the mean trough testosterone levels ranged between 15.0 ± 6.2 (visit 3, n = 703) and 16.5 ± 6.4 nmol/l (visit 5, n = 594) ().
Table 1. Average serum testosterone levels measured at certain visits after treatment with Nebido.
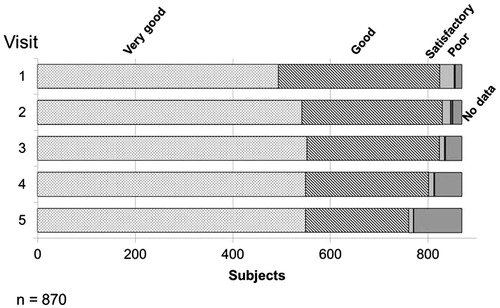
At all visits the tolerability was assessed as “Good” or “Very good” in approximately 90% of the patients. From visit 2 until the end of the observational period (visit 5), the percentage of “Very good” assessment was always higher than 60%. For 98 (11.3%) subjects no assessment was given at the end of the study (). “Poor” tolerability was reported in less than 1% of the subjects at all visits.
Figure 3. Assessment of the tolerability of Nebido at each visit. Over the time course of the observational period, over 90% of respondents described the tolerability of Nebido as very good or good with an overall increase of very good responses over the same period. Very few respondents described Nebido's tolerability as poor.
Effects on body weight, BMI, and waist circumference
The mean body weight was 87.2 ± 11.9 kg at baseline and 86.8 ± 10.5 kg at visit 5 (). This corresponded to a mean weight loss of 0.5 ± 4.2 kg within approx. 1 year. This effect on body weight was differential, that is underweight and normal weight individuals experienced slight weight increase whilst overweight men lost 0.6 ± 3.0 kg and obese men lost 2.3 ± 5.3 kg (, ). The BMI changes within the observational period were similar to the changes in body weight, that is there was a minor drop by 0.1 ± 1.3 kg/m2. The waist circumference was available for 615 (70.7%), 429 (49.3%), and 465 (53.5%) subjects at study start, visit 3, and visit 5, respectively. In order to compensate for missing data at visit 5, the value reported at visit 3 was used, if available (last-observation-carry-forward, LOCF).
Figure 4. Changes of mean body weight with 1 year of Nebido treatment. The effect on body weight was differential with underweight and normal weight patients showing modest weight gain whilst overweight and obese subjects experienced weight loss.
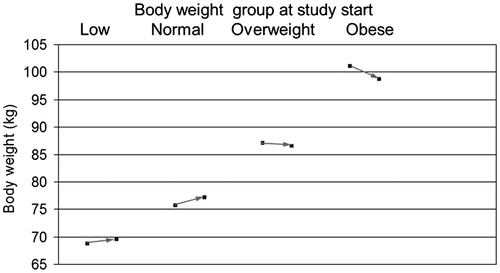
Table 2. Changes in average body weight, BMI, and waist girth during 1 year of treatment with Nebido.
Table 3. Body weight – gain and loss within 1 year on Nebido.
The mean waist circumference was 102.0 ± 12.7 cm at study start, 100.5 ± 12.3 cm at visit 3, and 100.0 ± 11.9 cm at the end of the observational period. The mean waist circumference decreased by 1.9 ± 4.3 cm at the end of the observational period ().
Effects on blood pressure
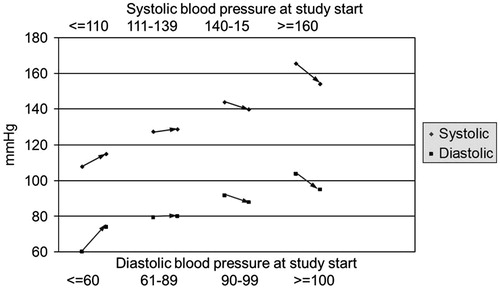
Treatment with TU was associated with minimal effects on blood pressure. The mean changes went in the direction of a decrease, if only slight (−1.6 ± 9.5 mm Hg for systolic and −0.7 ± 7.0 mm Hg for diastolic blood pressure). Again, effects on blood pressure were differential: men with low blood pressure showed an increase whilst men with elevated blood pressure had a decrease in blood pressure ().
Adverse drug reactions (ADRs) and untoward events
A total of 23 ADR forms were submitted, one additional ADR not using the ADR form was reported via client service centre. In addition, free-text entries from the main CRF were checked for further medically relevant events (“Reasons for premature discontinuation”, “Reasons for no further therapy” at the end of the observational period, and “Pathological findings at digital rectal examination and other findings”). Considering all sources of potential events, a total of 72 patients (8.3%) reported events (including some non-medical events). Multiple citing of complaints was possible, therefore the cumulative number of complaints was higher than the number of affected subjects. The most frequent type of event was insufficient efficacy, documented in 11 subjects (1.3%). Other events were (in order of frequency): pain in limbs or joints (n = 4; 0.5%), increase in PSA (n = 4; 0.5%), skin problems (n = 4; 0.5%), injection site pain (n = 3; 0.3%), weight gain (n = 3; 0.3%), and changes in liver function and/or lipid values (n = 3; 0.3%). The remaining untoward events, including prostate hyperplasia and prostate cancer, had a lower incidence (≤0.2%).
Forty-seven subjects (5.4%) stopped medication prematurely within the observational period (). This discontinuation was often paralleled by untoward events possibly associated with TU, for example four subjects (0.5%) had pain in limbs and/or joints, 4 (0.5%) had increased PSA, three (0.3%) had weight gain, and two (0.2%) had injection site pain.
Another complex of reasons for treatment discontinuation was insufficient treatment efficacy. This was the case in 10 subjects (1.1%)
Serious adverse reactions and serious untoward events
All cases of suspected serious ADRs or serious untoward events were handed over to the Drug Safety Department of Jenapharm for evaluation. After this process three events were assessed as serious: (1) Prostate cancer was reported in a 68 years old subject who completed the observational period and did not continue TU. (2) Increase of PSA in a 54-year old subject, the recurrence of prostate cancer thought to be cured [Citation11] was confirmed by biopsy. (3) Recurrence of bladder cancer, considered not related to treatment with testosterone.
Laboratory data results
Hemoglobin (normal range: 14.0–18.0 g/dl). At study start, the mean hemoglobin levels were 14.5 ± 1.8 g/dl (n = 587). During the study the mean values ranged between 14.8 ± 1.8 g/dl (n = 270) and 15.0 ± 1.7 g/dl (n = 383). In the subgroup of men whose first testosterone treatment was TU, the mean hemoglobin levels were 14.5 ± 1.6 g/dl (n = 281) at study start, and ranged between 14.8 ± 1.4 g/dl (n = 131) and 15.0 ± 1.4 g/dl (n = 189) during treatment. A single case with a supranormal value of 19.5 g/dl at visit 4 required use of a longer injection interval.
Hematocrit (normal range is 42–52%): Overall, the mean hematocrit levels were 44.4 ± 4.6% (n = 485) at study start. The levels slightly increased during the treatment period, with means ranging between 45.3 ± 4.5 (n = 368) and 45.2 ± 3.5% (n = 323) during treatment. In the subgroup of men whose first testosterone treatment was TU, the mean hematocrit levels were 43.9 ± 4.4% (n = 221) rising to 44.8 ± 4.5% (n = 110) and 45.1 ± 3.3% (n = 155).
Prostate-specific antigen (PSA; normal range <4 ng/ml): Overall, the mean levels of PSA were 1.4 ± 1.1 ng/ml (n = 755) at study start. During the observational period, the mean PSA levels ranged between 1.5 ± 1.1 (n = 548) at visit 3 and 1.7 ± 1.8 ng/ml (n = 369) at visit 4. In the subgroup of men whose first testosterone treatment was TU, the mean PSA levels were 1.5 ± 1.0 ng/ml (n = 366) at study start rising to 1.8 ± 1.6 ng/ml (n = 191) at visit 4 and declining to 1.6 ± 0.9 ng/ml (n = 269) at visit 5. In the subgroup of men who switched from a different testosterone preparation to TU, mean PSA levels were 1.3 ± 1.1 ng/ml (n = 389) at study start remaining stable at 1.3 ± 1.4 ng/ml (n = 227) at visit 2 and rising to 1.6 ± 2.0 ng/ml (n = 178) at visit 4.
In some men abnormal PSA levels (>4 ng/ml) were observed during the study, but upon evaluation only four cases were documented as ADRs or were associated with ADRs. In one patient, 68 years old, PSA values changed during TU administration from 3.38 ng/ml, 1.97 ng/ml, 2.27 ng/ml, and 5.69 ng/ml. This was considered as a reason to perform further diagnostic measures. A prostate biopsy revealed prostate cancer which was then surgically treated. One 54-year old patient had a history of prostate cancer and surgery of the prostate in July 1997 and had no signs of cancer recurrence until the study start. The first PSA value in the study was 0.5 ng/ml, followed by 0.8 ng/ml, 0.6 ng/ml, and 0.8 ng/ml at the subsequent measurements. Then PSA levels rose sharply and a diagnostic biopsy confirmed the recurrence of prostate cancer. Two patients developed supranormal PSA levels during TU administration which normalized upon discontinuation.
Compliance and adherence to therapy
The overall exposure time to TU was 880.2 patient-years. The mean duration of therapy was 52.8 ± 9.7 weeks, ranging between 0 (only one injection) and 87 weeks. More than 90% of all subjects completed the observational time until visit 5 (). Due to missing data of 27 subjects (3.1%), there was no documentation that the observational period was completed (). Altogether, 84 subjects (9.7%) did not complete the observational period. Ten subjects (1.1%) were lost to follow-up. Another 47 subjects (5.4%) stopped medication prematurely within the observational period (). At the end of the observational period 786 subjects (90.3%) decided to continue treatment with TU.
Discussion
Studies involving the use of TU have shown its applicability in treating hypogonadism and associated diseases of testosterone deficiency such as erectile dysfunction [Citation12], decreased bone mineral density associated with Klinefelter’s syndrome [Citation13], type-2 diabetes and insulin resistance [Citation14], and cardiovascular disease [Citation3]. Due to these promising results it is important to report the effectiveness and tolerability of TU. The present study was conducted in a non-selected population of patients in Germany. The proportion of subjects aged ≥55 years was 53.8%, with similar proportions of patients starting testosterone treatment with TU as changing over to TU (46.9% and 53.1%, respectively). In 96.0% of the subjects, hypogonadism confirmed by the laboratory data was the indication for treatment with TU as recommended by guidelines [Citation15].
The present study showed that the recommended injection intervals resulted in good effectiveness and tolerability of intramuscular TU. Poor effectiveness was very rarely documented. The low drop-out rate within the observation period of one year (9.7%) supports the conclusion that treatment with TU as an injection preparation with long intervals between administrations was well tolerated. There were no adverse effects on body weight, BMI, waist circumference although clinically meaningful weight loss needs to be evaluated over a longer time period and has been shown to be more effective in obese men [Citation16]. Also, blood pressure, and safety analyses showed adverse reactions that were consistent with the product information in terms of type and frequency of the side effects. Of the three serious events, only two (prostate cancer) were deemed to be causally related to TU and therefore classified as serious adverse drug reaction. Although concerns about TTh in regard to prostate cancer are still highly prevalent within the medical community [Citation17], the verdict for the relationship between TTh and prostate cancer is still outstanding. The prevalence of prostate cancer in men undergoing TTh is not above that of the general male population and recently it has been suggested that TTh may even be protective against high-grade prostate cancer [Citation18]. A very good compliance was reported. Over a mean observational time of 52.8 ± 9.7 weeks, approximately 90% of all subjects completed the study and the same percentage wished to continue TU after the study. About 1% of all subjects were lost to follow-up and 5.4% stopped medication prematurely. Our results are in line with a number of other studies, most notably the IPASS study of 1438 hypogonadal men in 23 countries [Citation19]. In this study, each subject received up to five injections over a 9- to 12-month period with a marked improvement in psychosexual parameters (libido, vigour, overall mood, and ability to concentrate) observed. The percentage of patients with “low” or “very low” levels of sexual desire/libido decreased from 64% at baseline to 10% whilst moderate, severe, or extremely severe erectile dysfunction decreased from 67% to 19%. Overall, 89% of patients stated they were “satisfied” or “very satisfied” with TU therapy after their last observation. Favourable objective parameters were also measured including a decrease in waist circumference, blood pressure, blood triglycerides, and total cholesterol with a more favourable LDL:HDL ratio observed. When the patient cohort was stratified by baseline measures of particular parameters, a trend towards a correction of blood pressure and body weight was observed following one year of treatment. Patients with low systolic and diastolic blood pressure at the start of the study saw blood pressure increase over the duration of treatment, whilst patients with high blood pressure at baseline observed a decline. A similar pattern was demonstrated with body weight. This suggests testosterone may have a “normalising” effect on blood pressure and weight in hypogonadal patients in the upper and lower quartile of each parameter. Good tolerability of TU was noted with adverse events and adverse drug reactions (ADRs) occurring in only 12% and 6% of patients, respectively. These were mainly mild to moderate with the most common ADRs been an increase in hematocrit, increase in PSA, and injection site pain (all <1%). Two cases of prostate cancer were observed in IPASS.
In the longest study of the effectiveness of TU to date in treating cardiovascular disease and type-2 diabetes, 115 hypogonadal men were followed for up to 10 years with TU injections every 12 weeks in a single-centre, cumulative, prospective, registry study [Citation20]. Similar favourable decreases in the ratio of triglycerides and HDL were observed along with a progressive decrease in waist circumference, body weight, and BMI. Similarly, fasting glucose displayed a significant decrease after the first year and continued to decrease with a decline in HbA1c (6.4–5.6% (mean <6%)) observed from year 2 onwards. A decline in CRP from 1.39 ± 0.69 to 0.62 ± 0.25 mg dl−1 was also noted with the main reduction observed in the first treatment year. No adverse cardiovascular events were noted throughout the study with the authors concluding TU to be a safe and effective treatment with maximal and sustained effects reach after 7–8 years and onwards. These favourable changes in fasting glucose and CRP are mirrored in observational, prospective, and cumulative registry studies of 156 obese hypogonadal men with T2DM followed up to a 6-year period [Citation21]. Fasting glucose declined from 7.06 ± 1.74 to 5.59 ± 0.94 mmol/l. HbA1c decreased from 8.08% to 6.14% and CRP from 3.16 ± 4.12 to 0.72 ± 0.56 U/l; blood pressure and lipid profiles including total cholesterol:HDL ratio also all improved. This study showed TU therapy resulted in a significant and sustained improvement in weight, T2DM, and other cardiometabolic risk factors in obese, diabetic men. Adherence to treatment was excellent, and TU was only discontinued in two men who were diagnosed with prostate cancer.
Another long-term (>2 years) study on TU which, in a retrospective analysis of 179 hypogonadal men, showed it to be well tolerated with 162 men (90.5%) completing 2 years of treatment, and only seven men (3.9%) stopping treatment because of adverse effects [Citation22]. In a randomised, placebo-controlled trial of Malaysian men aged 40–70 years over a 48-week period, TU therapy caused significant increases in the levels of PSA, haemoglobin, and hematocrit, however these were all within clinically safe limits. Treatment of hypogonadal men with testosterone leads to an initial rise of serum PSA, usually during the first 6 months [Citation23,Citation24]. There was no significant adverse reaction that led to the cessation of treatment [Citation25]. TU treatment of hypogonadal men with sickle cell disease over a 12-month period was deemed safe by the authors [Citation26] and a randomized, double-blind, double-dummy study of 52 hypogonadal men with metabolic syndrome found injectable TU to be more effective than oral TU at reaching and maintaining normal plasma levels of T over a 12 month period, with no difference in drop-out rate over the course of the trial compared with placebo [Citation27]. Moon et al. [Citation28] examined effectiveness and safety in 133 Korean men with TD over a 24-week period with over 75% of subjects reporting improvements in erectile dysfunction with no significant adverse reactions observed although the drop-out rate was recorded at 15%. TU’s efficacy and safety profile for treating men with ED who have type-2 diabetes has also been examined in a double-blind, placebo-controlled study with 199 subjects over a 30-week period [Citation29] with 46% reporting health improvement compared with 17% of the control group with no significant adverse reactions noted. Whilst TU was not shown to be an effective treatment in a randomized double-blind, parallel, placebo-controlled trial for treating ED in mean with type-2 diabetes (overall 88 subjects) [Citation30] this is in contrast to a long-term observational study [Citation21] and may be due to the short duration of the study and the fact that patients were well controlled and had very low levels of insulin resistance. Although the authors did note the tolerability of TU treatment with no significant adverse reactions noted [Citation30].
Finally, the effectiveness of TU therapy in the treatment of osteoporosis has also been shown in a cumulative, prospective, registry study in 45 hypogonadal men over a maximum 6-year period with a marked and progressive improvement in mineral bone density [Citation31]. As with other studies, favourable changes in objective parameters were also noted with decreases in waist circumference, body weight, BMI, and CRP and changes in blood lipid profile. Safety parameters were also recorded with no malignancies occurring in any subjects however a slight increase in prostate volume was observed thought to be due to aging. Serum PSA did not change significantly and whilst hemoglobin and hematocrit levels increased they remained within safe limits. Bone density has also been shown to increase significantly during an 8-year follow-up study on 120 men with late-onset hypogonadism (LOH) [Citation32]. Eighteen men had previously been diagnosed with osteopenia and one with osteoporosis in the lumbar spine and 35 with osteopenia and one with osteoporosis of the femoral neck, mean T scores of vertebral and femoral BMD increased significantly from 0.06 ± 1.35 and 0.55 ± 0.95 at baseline to 0.85 ± 1.33 and 0.31 ± 1.01. At the end of the study, a marked decrease in the incidence of osteopenia in the lumbar spine and femoral neck was shown with no osteoporosis reported at either of these sites This study also examined metabolic parameters, urinary symptoms, and sexual function. Whilst not all obesity parameters improved, a significant decrease (p < .005) was found in waist circumference, percentage of body fat, cholesterol, LDL, and HbA1c. The mean waist circumference decreased from 93.64 ± 9.38 to 90.70 ± 6.25 cm (p < .05). An improvement in urinary symptoms measured by mean IPSS questionnaire scores decreased significantly from 8.54 ± 6.6 at baseline to 6.78 ± 5.44 (p < .05) at the end of the 8-year study period with median prostate volume increased significantly from 24.85 cm3 at baseline to 31.53 cm3. Also, a significant improvement was noted in International Index of Erectile Function scores from 12.72 ± 6.87 and 36.18 ± 19.80 at baseline to 15.16 ± 5.38 and 44.18 ± 16.21, respectively. The authors conclude that TU treatment is safe and well tolerated as, although TU treatment significantly increased the median PSA (0.96 mg/l) and hematocrit (42.20%) levels from baseline to 1.17 mg/l and 45.05%, these changes remained within the normal range with no cardiovascular, thrombotic, or prostate cancer events reported during treatment [Citation32].
Overall, our findings along with a number of other retrospective and placebo-controlled trials have shown that a longer administration period of TU is both a safe and effective method of TTh in hypogonadal men. This conclusion is further supported by a recent meta-analysis of TU across 33 studies (including 11 placebo-controlled randomized clinical trials) since 2003 that found it to be both effective and safe in the treatment of hypogonadal men [Citation33]. Whilst observational studies have clear limitations compared with the gold standard of randomised controlled clinical trials, the cost of such trials make them prohibitively expensive; therefore evidence based medicine should be drawing on data collected from placebo-controlled trials as well as from prospective or retrospective observational studies, registries, case control studies, and meta-analyses to glean all the critical information [Citation3]. Because of their relevance to clinical practise it is important that we use these retrospective studies as a valuable source of information to evaluate the efficacy and tolerability of TTh whilst acknowledging their limitations which need to be carefully assessed before a clinical decision is made.
Disclosure statement
Michael Ernst is an employee of Jenapharm. The other authors did not report a conflict of interest.
References
- Nieschlag E, Behre HM, Bouchard P, et al. Testosterone replacement therapy: current trends and future directions. Hum Reprod. 2004;10:409–419.
- Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. Clin Endocrinol. 2006;65:275–281.
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–261.
- Behre HM, Wang C, Handelsman DJ, et al. 2004. Pharmacology of testosterone preparations. In: Nieschlag E, Behre HM, editors. Testosterone, action, deficiency, substitution. Cambridge (UK): Cambridge University Press. p. 405–444.
- Schubert M, Minnemann T, Hübler D, et al. Intramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89:5429–5434.
- Harle L, Basaria S, Dobs AS. Nebido: a long-acting injectable testosterone for the treatment of male hypogonadism. Expert Opin Pharmacother. 2005;6:1751–1759.
- Saad F, Kamischke A, Yassin A, et al. More than eight years' hands-on experience with the novel long-acting parenteral testosterone undecanoate. Asian J Andrology. 2007;9:291–297.
- Gooren LJ. Advances in testosterone replacement therapy. Front Horm Res. 2009;37:32–51.
- Moisey R, Swinburne J, Orme S. Serum testosterone and bioavailable testosterone correlate with age and body size in hypogonadal men treated with testosterone undecanoate (1000 mg IM–Nebido). Clin Endocrinol. 2008;69:642–647.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–4343.
- Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2009;181:972–979.
- Yassin AA, Saad F. Treatment of sexual dysfunction of hypogonadal patients with long-acting testosterone undecanoate (Nebido®). World J Urol. 2006;24:639–644.
- Jo DG, Lee HS, Joo YM, et al. Effect of testosterone replacement therapy on bone mineral density in patients with Klinefelter syndrome. Yonsei Med J. 2013;54:1331–1335.
- Hackett G, Cole N, Bhartia M, et al. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int J Clin Pract. 2014;68:203–215.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I–III: observational data from two registry studies. Int J Obes. 2016;40:162–170.
- Gooren L. Diagnosing hypogonadism and treating decisions in different parts of the world: shifts in patterns between 2006 and 2015. Aging Male. 2016;19:46–53.
- Yassin A, Salman M, Talib RA, et al. Is there a protective role of testosterone against high-grade prostate cancer? Incidence and severity of prostate cancer in 553 patients who underwent prostate biopsy: a prospective data register. Aging Male. 2017;20:125–133.
- Zitzmann M, Mattern A, Hanisch J, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1438 men. J Sex Med. 2013;10:579–588.
- Yassin AA, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia. 2016;48:793–799.
- Haider A, Yassin A, Doros G, et al. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515.
- Conaglen HM, Paul RG, Yarndley T, et al. Retrospective investigation of testosterone undecanoate depot for the long-term treatment of male hypogonadism in clinical practice. J Sex Med. 2014;11:574–582.
- Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol. 1994;40:341–349.
- Coward RM, Simhan J, Carson Iii CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103:1179–1183.
- Tan WS, Low WY, Ng CJ, et al. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men: a randomised controlled study. BJU Int. 2013;111:1130–1140.
- Morrison BF, Reid M, Madden W, et al. Testosterone replacement therapy does not promote priapism in hypogonadal men with sickle cell disease: 12-month safety report. Andrology. 2013;1:576–582.
- Aversa A, Bruzziches R, Francomano D, et al. Efficacy and safety of two different testosterone undecanoate formulations in hypogonadal men with metabolic syndrome. J Endocrinol Invest. 2010;33:776–783.
- Moon DG, Park MG, Lee SW, et al. The efficacy and safety of testosterone undecanoate (Nebido(®)) in testosterone deficiency syndrome in Korean: a multicenter prospective study. J Sex Med. 2010;7:2253–2260.
- Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10:1612–1627.
- Gianatti EJ, Dupuis P, Hoermann R, et al. Effect of testosterone treatment on constitutional and sexual symptoms in men with type 2 diabetes in a randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2014;99:3821–3828.
- Haider A, Meergans U, Traish A, et al. Progressive improvement of T-scores in men with osteoporosis and subnormal serum testosterone levels upon treatment with testosterone over six years. Int J Endocrinol. 2014;2014:496948.
- Permpongkosol S, Khupulsup K, Leelaphiwat S, et al. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J Sex Med. 2016;13:1199–1211.
- Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2015;15:1903–1926.