Abstract
Objective: The present subanalysis of the EARTH study investigates the effects of one year testosterone replacement therapy (TRT) on sleep disturbance among hypogonadal men without obstructive sleep apnea.
Methods: Sleep disturbance was defined as three or more points in question 4 of the aging males symptoms (AMS) questionnaire. All participants completed the AMS scale, International Prostatic Symptoms Score (IPSS), Sexual Health Inventory for Men (SHIM) and Short Form 36 (SF-36) health survey at baseline and after 12 months. Sexual symptoms were also evaluated based on three AMS subscores (Q15, 16 and 17).
Results: We identified 100 patients with sleep disturbance, of whom 48 (24 each in the TRT and control groups) were ultimately included for analysis. All SF-36 categories , AMS scale, IPSS and SHIM score subdomains were significantly worse in patients with sleep disturbance than in those without disturbance. Statistically significant differences in sleep disturbance, erectile symptoms, sexual desire and some domains of the SF-36 were observed between the TRT and control groups after 12 months.
Conclusion: Sleep disturbance may be one of the clinical signs for severe hypogonadism. Moreover, TRT improved sleep conditions, sexual function and quality of life among hypogonadal men with sleep disturbance.
Introduction
Sleep disturbance has been widely accepted as one of the most serious clinical conditions that can worsen quality of life (QOL) and vital prognosis [Citation1]. Insomnia, in particular, has been considered the most common sleep disorder. A Japanese epidemiological study showed that insomnia had a prevalence rate of 21% among adults aged 20 and over, many of whom had excessive daytime sleepiness and were regularly dependent on hypnotics or alcohol [Citation1]. Therefore, many clinicians have focused on the important issue of sleep disturbance.
Aging, stress, mental disorders, depression and lifestyle changes are well known causes of sleep disturbance. Aging, which is associated with decreased exercise, chronic diseases, lifestyle changes, nocturia and decreased melatonin secretion, has been considered the most common cause of sleep disturbance [Citation2,Citation3]. In addition, serum testosterone, which gradually decreases with age, has been reported to be associated with sleep disturbance in elderly men. Decreased testosterone levels can contribute to the development of specific signs of late-onset hypogonadism (LOH) syndrome [Citation4,Citation5], which include decreased muscle volume, erectile dysfunction (ED), obesity, hypertension, dyslipidemia and insulin resistance [Citation4–6]. Currently, testosterone replacement therapy (TRT) has been a useful treatment option to improve many of these clinical conditions while maintaining QOL in hypogonadal men [Citation4].
On the other hand, sleep disturbance is well known to be one of the common symptoms of LOH syndrome. Indeed, the aging males symptoms (AMS) scale questionnaire includes two questions on sleep disturbance: question 4 (sleep problems, difficulty in falling asleep, difficulty in maintaining sleep, waking up early and feeling tired, poor sleep and sleeplessness) and question 5 (increased need for sleep and often feeling tired) [Citation7]. However, although many studies have investigated the effects of TRT on obstructive sleep apnea (OSA), limited information on the efficacy of TRT on sleep disturbance apart from OSA has been currently available. We had recently conducted a randomized controlled trial (RCT; the EARTH study) demonstrating that TRT for one year had some positive effects in Japanese hypogonadal men, such as decreased waist circumference and serum triglycerides, significantly increased muscle mass and serum hemoglobin and improved QOL [Citation8]. Hence, the present subanalysis of the EARTH study was conducted to investigate the efficacy of TRT on sleep disturbance and QOL among hypogonadal men without OSA.
Methods
Study subjects
Among patients included in the EARTH study, we identified those with hypogonadism who suffered from sleep disturbance. The inclusion criteria were similar to those in the EARTH study [Citation8]. In addition, patients with incomplete data and those taking any medications associated with sleep conditions, such as hypnotics, anti-anxiety agents and anti-depressive agents, were excluded. Those with OSA had already been excluded in the EARTH study.
The biochemical diagnosis of hypogonadism was based on the following Japanese biochemical criteria: free testosterone (FT) ≤ 8.5 pg/mL (TRT as first-line treatment) and FT 8.5–11.8 pg/mL (TRT as a treatment option) [Citation9]. Sleep disturbance was defined as three or more points in question 4 of the AMS questionnaire.
All patients provided written informed consent before participating in the EARTH study and the protocol and study procedures were approved by the Kanazawa University Hospital Institutional Review Board.
Study protocols
A medical history that included current medications and illnesses was initially collected from all participants during the baseline visit. Blood samples from each patient were collected between 8:30 and 10:30 am, from which serum FT values were determined. FT values were measured by radioimmunoassay using DPC’s free-T kit (Mitsubishi Kagaku, Tokyo, Japan). All eligible patients completed the same questionnaires, namely, the AMS scale, International Prostatic Symptoms Score (IPSS), Sexual Health Inventory for Men (SHIM), and Short Form 36 (SF-36) health survey, at baseline and after 12 months. In addition, some sexual symptoms were also evaluated based on the following three AMS subscores: Q15 (Erectile symptoms: decrease in the ability/frequency of sexual performance), Q16 (Morning erection: decrease in the number of morning erections) and Q17 (Sexual desire: decrease in sexual desire/libido). The TRT group received intramuscular testosterone enanthate (250 mg; Enarmon Depot®; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) every fourweeks for 12 months.
Statistical analysis
Baseline characteristics of patients with and without sleep disturbance from the EARTH study, as well as those included in the TRT and control groups during the final analysis (48 participants), were compared using Student’s t-test. Differences between both groups after 12 months were compared using the Mann–Whitney U-test to evaluate the effects of one year TRT on sleep disturbance. All statistical analyses were performed using SPSS™ statistics 22 (SPSS Inc., Chicago, IL). In all analyses, p values <0.05 were considered statistically significant.
Results
A total of 100 patients with sleep disturbance (48 and 52 in the TRT and control groups, respectively) had been identified from the 255 participants enrolled in the final analysis of the EARTH study (). The mean age and FT value of the identified patients were 66.4 ± 8.6 years and 6.69 ± 2.36 pg/mL, respectively (). Those with sleep disturbance had significantly lower FT levels compared to those without sleep disturbance (p = 0.00514). All SF-36 categories and subdomains (mental, physical and sexual domains) of the AMS scale, IPSS and SHIM score were significantly worse in those with sleep disturbance than in those without.
Figure 1. Flow chart of inclusion criteria in the present study. TRT: testosterone replacement therapy; AMS: aging male symptoms.
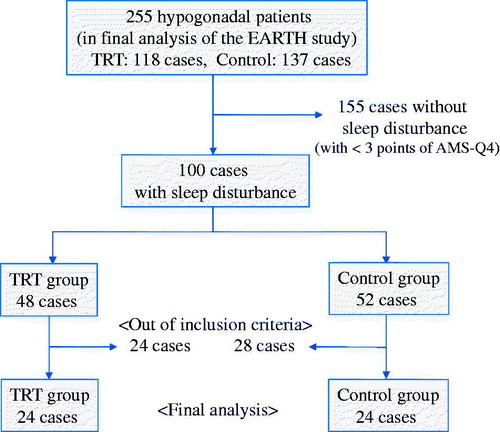
Table 1. Comparison of patients baseline characteristics between the sleep and non-sleep disturbance groups.
On the basis of the inclusion criteria, 48 patients who had hypogonadism with sleep disturbance, among whom 24 received TRT for 12 months (TRT group) and 24 received no TRT (control group), were ultimately included in the present subanalysis. A comparison of the baseline characteristics revealed that physical functioning (PF) of the SF-36 was significantly higher in the TRT group than in the control group (53.0 ± 4.3 vs. 46.1 ± 10.3, p = 0.0172; ). In addition, morning erection was slightly worse in the TRT group than in the control group (3.9 ± 0.9 vs. 3.5 ± 1.0, p = 0.0654), though the results were not significant. On the other hand, no statistically significant differences in any other baseline characteristic had been observed between the two groups.
Table 2. Patients baseline characteristics in the TRT and control groups.
Statistically significant differences in sleep disturbance (−0.5 ± 0.8 vs. −0.1 ± 0.5, p = 0.0449), erectile symptoms (−0.3 ± 0.8 vs. 0.0 ± 1.3, p = 0.0333) and sexual desire (−0.3 ± 0.7 vs. 0.1 ± 1.2, p = 0.0455), as well as role limitation because of physical programs (RP; 2.8 ± 9.3 vs. −3.1 ± 10.1, p = 0.0318) and general health (GH; 3.4 ± 6.7 vs. 0.7 ± 6.4, p = 0.0433) of the SF-36 survey, were observed between the TRT and control groups (). A slight but not significant difference in role limitations because of emotional programs (RE) was found between the two groups (3.0 ± 10.4 vs. −1.8 ± 7.7, p = 0.0518). No significant differences in other parameters were found between both groups.
Table 3. Comparison of parameter changes from baseline to 12 months between the TRT and control groups.
Discussion
The present study demonstrated that all subdomains of the SF-36 and AMS scale were worse in patients with sleep disturbance than in those without, suggesting that hypogonadal men with sleep disturbance had significantly poorer health-related QOL. Many previous studies also show that sleep disturbance often lead to daytime somnolence, general malaise, depression, nocturia and decreased concentration and vitality, which cause a decrease in health-related QOL [Citation1,Citation10]. Insomnia, especially in elderly men, can increase the risk of falling and bone fractures caused by the lack of vigilance and exercise tolerance, leading to further increases in mortality [Citation11]. Therefore, sleep disturbance may be one of the clinical signs for severe hypogonadism, as well as a critical factor to consider during the selection of therapeutic interventions.
In addition, erectile symptoms, sexual desire, morning erections and lower urinary tract symptoms (LUTS) were worse in patients with sleep disturbance, a finding that was consistent with a number of previous studies [Citation12,Citation13]. A cross-sectional study revealed that sleep quality significantly affected erectile function among 334 Chinese men [Citation12]. Another investigation found that poor sleep quality was significantly associated with an increased risk for hypogonadal symptoms and sexual dysfunction [Citation13]. Given its effects on the pituitary–gonadal axis in men, sleep disturbance can be associated with sexual functions [Citation14]. Alternatively, sleep disturbance can trigger depressive symptoms and anxiety, which can indirectly impair sexual arousal and cause ED [Citation12,Citation15]. The close link between sleep disturbance and the development of nocturia has been widely accepted. Moreover, a number of previous studies have demonstrated that sleep disturbance was significantly associated with LUTS, which include storage and voiding symptoms, irrespective of nocturia [Citation16,Citation17].
The present study found significantly lower FT levels in patients with sleep disturbance. A previous study revealed that elderly men with low testosterone levels had reduced sleep efficiency and nonrapid eye movement (non-REM) sleep and increased nocturnal awakenings [Citation18]. Conversely, sleep fragmentation and insomnia have been reported to disrupt the diurnal testosterone rhythm, leading to a decrease in nocturnal testosterone secretion [Citation19]. A cross-sectional study of 1274 elderly men in Hong Kong found that sleep disturbance could have inverse effects on testosterone levels, muscle mass and strength [Citation20]. Sleep disturbance, which lead to decreased health-related QOL, serious sexual disorders and LUTS, may also be associated with declining FT levels. Moreover, the decline in FT levels may lead to depression and anxiety, further aggravating sleep conditions. Therefore, sleep disturbance, sexual function, LUTS and AMS are closely interrelated critical factors that affect health-related QOL in hypogonadal men.
One year TRT significantly improved sleep disturbance in hypogonadal men without OSA. Indeed, a number of previous reports have shown that testosterone deficiency could be associated with sleep disturbance [Citation18–21]. However, little information on the effects of TRT on sleep disturbance apart from OSA among hypogonadal men has been currently available. To our knowledge, this is the first RCT to demonstrate the efficacy of one year TRT on sleep disturbance in hypogonadal men without OSA. Only experimental data demonstrated that the quality and quantity of non-REM sleep were reduced in mice with decreased testosterone levels after gonadectomy, which could be corrected by testosterone administration [Citation22], suggesting that TRT may have a direct effect on sleep conditions though the central nervous system.
Although sleep disturbance is often caused by nocturia, which is one of the common symptoms developed in elderly men with LUTS, nocturia is also caused by sleep disturbance [Citation23]. Some recent reports have suggested that testosterone deficiency is significantly associated with nocturia and sleep disturbance [Citation16,Citation21,Citation23], whereas TRT was able to improve nocturia and sleep disturbance in hypogonadal men [Citation3,Citation23]. In addition, many previous studies have clarified that TRT can have some favorable effects on LUTS, including nocturia, among hypogonadal men with benign prostate hyperplasia (BPH) [Citation24–26]. Furthermore, some beneficial long-term and sustained effects of TRT for up to 10 years on LUTS and sexual functions have been demonstrated [Citation27]. In the present study, the IPSS score was significantly higher in patients with sleep disturbance than in those without disturbance. However, one year TRT failed to improve LUTS in our population. This was because not all of our participants had BPH and the severity of nocturia at baseline was only moderate.
Furthermore, one year TRT improved a number of sexual symptoms, including RP and MH of the SF-36, in hypogonadal men with sleep disturbance. The EARTH study also found that TRT contributed to some degree of improvement in erectile function and QOL among hypogonadal men [Citation8], suggesting that TRT might have a direct positive effect on QOL and sexual function. Alternatively, correcting sleep disturbance through TRT improved QOL and sexual symptoms. Nevertheless, given that hypogonadal men with sleep disturbance have more health and physical problems compared to those without disturbance, TRT should be recommended for improving sleep conditions and QOL.
In the present study, we used some alternative scales together with the SHIM score to evaluate erectile function. The TRT and control groups comprised patients with severe ED who had SHIM scores of 8.8 ± 7.1 and 9.8 ± 5.0, respectively. The SHIM is not applicable to men who have a definite decrease in sexual desire and those who do not have an opportunity to engage in sexual activity [Citation28]. Thus, we made use of the following simple AMS subscales: Q15 for erectile symptom, Q16 for morning erection and Q17 for sexual desire. Indeed, one year TRT improved erectile symptoms and sexual desire, whereas no significant changes in SHIM from baseline were found between both groups.
On the other hand, high-dose testosterone administration and abuse, which include supratherapeutic doses of testosterone, have been reported to cause reduced sleep duration, insomnia, and nocturnal awakenings [Citation21,Citation29]. A previous RCT revealed that even short-term administration of high-dose testosterone, consisting of three intramuscular injections at weekly intervals (500, 250 and 250 mg), shortened and worsened sleep apnea in older men [Citation29]. Therefore, adequate therapeutic doses of testosterone are necessary when using TRT for hypogonadal men with sleep disturbance.
The present study has several limitations. Firstly, our sample size was too small (24 patients each in the TRT and control groups). Moreover, sleep disturbance were evaluated based on only one AMS scale question. Further studies that include evaluations from some additional questionnaires, such as the Pittsburgh Sleep Quality questionnaire and the Epworth Sleepiness Scale, may be needed [Citation30,Citation31]. Furthermore, TRT is not recommended for hypogonadal men with untreated OSA. Patients with OSA were not included in the EARTH study and incidences of OSA as a result of TRT were not monitored. Actually, OSA is well known to have negative effects on sleep conditions. There have been only a few evidences regarding the adverse effects of exogenous testosterone on OSA. One previous RCT revealed that relative low-dose TRT for 12 weeks resulted in impairing sleep and breathing conditions for obese men with severe OSA [Citation32]. Another study showed that TRT did not worsen breathing condition among men with OSA, but a higher testosterone concentration had a significant correlation with overnight hypoxia [Citation33]. Alternatively, this study was a retrospective subanalysis of the EARTH study. Although a statistically significant difference in PF of the SF-36 subdomain at baseline was found between the two groups, this result is likely to be acceptable for the present analysis. Therefore, further prospective studies, including a large number of participants and some additional evaluations for sleep conditions, are certainly required to validate our conclusions.
Conclusion
Sleep disturbance may be one of the clinical signs for severe hypogonadism, as well as a critical factor to consider during the selection of therapeutic interventions. Moreover, one year TRT improved sleep conditions, sexual function and QOL among hypogonadal men with sleep disturbance.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article
References
- Liu X, Uchiyama M, Kim K, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93:1–11.
- Maggio M, Colizzi E, Fisichella A, et al. Stress hormones, sleep deprivation and cognition in older adults. Maturitas. 2013;76:22–44.
- Shigehara K, Konaka H, Koh E, et al. Effects of testosterone replacement therapy on nocturia and quality of life in men with hypogonadism: a subanalysis of a previous prospective randomized controlled study in Japan. Aging Male. 2015;18:169–174.
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male. 2005;8:59–74.
- Chen W, Liu ZY, Wang LH, et al. Are the Aging Male's Symptoms (AMS) scale and the Androgen Deficiency in the Aging Male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16:92–96.
- Konaka H, Sugimoto K, Orikasa H, et al. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: a multicenter randomized controlled trial in Japan (EARTH Study). Asian J Androl. 2016;18:25–34.
- Namiki M, Akaza H, Shimazui T, et al. Clinical practice manual for late-onset hypogonadism syndrome. Int J Urol. 2008;15:377–388.
- Krystal AD. Treating the health, quality of life, and functional impairments in insomnia. J Clin Sleep Med. 2007;3:63–72.
- Sivertsen B, Pallesen S, Glozier N, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:720.
- Cheng QS, Liu T, Huang HB, et al. Association between personal basic information, sleep quality, mental disorders and erectile function: a cross-sectional study among 334 Chinese outpatients. Andrologia. 2017;49:e12631.
- Pastuszak AW, Moon YM, Scovell J, et al. Poor sleep quality predicts hypogonadal symptoms and sexual dysfunction in male nonstandard shift workers. Urology. 2017;102:121–125.
- Luboshitzky R, Aviv A, Hefetz A, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–3398.
- Meisler AW, Carey MP. Depressed affect and male sexual arousal. Arch Sex Behav. 1991;20:541–554.
- Shimizu N, Nagai Y, Yamamoto Y, et al. Survey on lower urinary tract symptoms and sleep disorders in patients treated at urology departments. Nat Sci Sleep. 2013;5:7–13.
- Cakir OO, McVary KT. LUTS and sleep disorders: emerging risk factor. Curr Urol Rep. 2012;13:407–412.
- Barrett-Connor E, Dam TT, Stone K, et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–2609.
- Luboshitzky R, Zabari Z, Shen-Orr Z, et al. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–1139.
- Auyeung TW, Kwok T, Leung J, et al. Sleep duration and disturbances were associated with testosterone level, muscle mass, and muscle strength: a cross-sectional study in 1274 older men. J Am Med Dir Assoc. 2015;16:630.e1–e6.
- Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl. 2014;16:262–265.
- Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463:239–243.
- Shigehara K, Izumi K, Mizokami A, et al. Testosterone deficiency and nocturia: a review. World J Mens Health. 2017;35:14–21.
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14:53–58.
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–246.
- Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008;11:146–149.
- Haider KS, Haider A, Doros G, Traish A. Long-term testosterone therapy improves urinary and sexual function and quality of life in men with hypogonadism: results from a propensity-matched subgroup of a controlled registry study. J Urol. 2017; [Epub ahead of print].
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–319.
- Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–3613.
- Buysse DJ, Reynolds IIICF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28:121–193.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545.
- Hoyos CM, Killick R, Yee BJ, et al. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol. 2012;77:599–607.
- Killick R, Wang D, Hoyos CM, et al. The effects of testosterone on ventilatory responses in men with obstructive sleep apnea: a randomised, placebo-controlled trial. J Sleep Res. 2013;22:331–336.
