Abstract
Background: The aim of this study was to evaluate the relationship between mean platelet volume (MPV) and vitamin D levels according to ED severity.
Methods: Between October 2015 and September 2017, patients who applied to the andrology outpatient clinic with an ED complaint were retrospectively reviewed. Patients with diabetes, hypertension, hyperlipidemia, malignancy, late-onset hypogonadism and smokers were not included in the study. The International Erectile Function Index-Erectile Function (IIEF-EF) questionnaire was used to assess the levels of erectile function. According to this scoring system, patients were divided into two groups. IIEF score: between 17 and 25 = mild ED (Group 1) and IIEF score between 16 and 0 = moderate–severe ED (Group 2). Blood samples of the patients were taken from antecubital vein and MPV and 25-hydroxyvitamin D [25(OH)D] levels were evaluated.
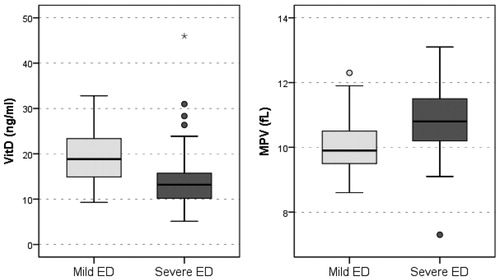
Results: Ninety patients were included in the study (Group 1: n = 41, Group 2: n = 49). The mean age of the patients was 41.07 ± 8.56 and the mean body mass index (BMI) was 27.59 ± 3.91. 25(OH)D levels were found to be statistically lower in Group 2 (18.85 ± 6.09; 13.98 ± 7.10; p = .001). MPV levels were found to be statistically higher in Group 2 (10.05 ± 0.81; 10.78 ± 1.16; p = .001). Correlation between IIEF-EF scores and 25(OH)D levels was positive (p = .03, r = 0.22). There was negative correlation between IIEF-EF scores and MPV and between 25(OH)D levels and MPV levels [p = .003 for IIEF-EF/MPV, p = .04, r = −0.23 for 25(OH)D/MPV].
Conclusion: There is a significant positive correlation between ED severity and 25(OH)D levels and there is a significant negative correlation between ED severity and MPV levels.
Introduction
Erectile dysfunction (ED), defined as the inability to develop and/or maintain penile erection, is one of the most common sexual disorders in men [Citation1]. ED is associated with increasing age as well as metabolic syndromes and various cardiovascular risk factors such as diabetes mellitus, hypertension and hyperlipidemia [Citation2]. Vascular ED is the most commonly observed dysfunction among the organic forms of ED, and it is mainly caused by underlying atherosclerosis and/or endothelial dysfunction [Citation3]. ED and atherosclerotic cardiovascular diseases have many risk factors in common. Therefore, ED can be considered as a marker of cardiovascular diseases that can identify individuals at an increased risk of atherosclerotic cardiovascular disease [Citation4].
Platelets, a component of human blood, play an important role in the pathophysiology of atherothrombosis. Mean platelet volume (MPV) is frequently used to predict the functional changes and activation of platelets. MPV levels tend to increase in various cardiovascular diseases, peripheral artery disease, and cerebrovascular diseases [Citation5,Citation6]. Moreover, MPV is related to various cardiovascular conditions such as smoking, obesity, hyperlipidemia, hypertension, coronary artery disease, metabolic syndrome, statin and anti-hypertensive use, atrial fibrillation and systemic inflammation, thyroid diseases, diabetes mellitus and alcohol use [Citation7].
Despite the established role of vitamin D in bone and calcium metabolism, its deficiency has become more prevalent in recent years. Low 25-hydroxyvitamin D [25(OH)D] levels are also associated with an increased risk of clinical atherosclerotic cardiovascular diseases. It is believed that suboptimal vitamin D levels affect the risk of atherosclerotic cardiovascular disease as well as lead to the development of conditions such as hypertension, diabetes, inflammation and endothelial dysfunction, which are predominant vascular risk factors [Citation8]. Furthermore, it is considered that similar to leading to the development of risk factors associated with cardiovascular diseases, low vitamin D levels may contribute to the development of ED via causing the development of risk factors such as endothelial dysfunction, inflammation, impaired glucose homeostasis and atherosclerosis [Citation9]. On the other hand, there is a positive correlation between vitamin D levels and testosterone levels [Citation10]. This is correlated with the relationship between systemic inflammation and erectile function and testosterone levels [Citation11]. Recently, it has been demonstrated that MPV and 25(OH)D levels are negatively correlated [Citation12]. To date, there are no studies in the literature analyzing the relationship between ED and MPV and 25(OH)D levels.
The purpose of the present study is to evaluate the relationship between MPV and 25(OH)D levels according to ED severity.
Methods
Medical records of patients between the ages of 18 and 65 years who were admitted to the Andrology Outpatient Clinic at Okmeydani Training and Research Hospital between October 2015 and September 2017 were retrospectively examined. Detailed medical and sexual histories of the patients were examined, and physical examination findings were recorded. International Index of Erectile Function–Erectile Function (IIEF-EF) questionnaire, which consists of six questions, was used to evaluate the erectile function [Citation13]. The ED area in the scale consists of first, second, third, fourth, fifth, and fifteenth questions. These questions classify the level of ED as mild, moderate, and severe. Patients were divided into two groups according to this scoring system: 17–25 points: mild ED and 0–16 points: moderate and severe ED.
Patients who had congestive heart failure, kidney failure, acute infection, rheumatic diseases, coronary artery disease, hypertension, diabetes mellitus, dyslipidemia, malignancy, testosterone deficiency, chronic obstructive lung disease and hepatopathy history and patients who were smoking or taking vitamin D supplements were excluded from the study.
Blood samples of the patients were obtained from the antecubital vein between 08:00 and 10:00 am after nightlong fasting. Haematologic tests measuring MPV were performed using the Cell-Dyn Ruby analyzer (Abbott Diagnostics, Abbott Park, IL, USA). Serum 25(OH)D levels were measured using Architect-I 2000 system (Abbott Diagnostics) via chemiluminescence microparticle immunoassay.
Statistical analysis
SPSS 22.0 program (SPSS, Chicago, IL) was used in the analyses. In the descriptive statistics of the data, mean, standard deviation, median lowest, highest, frequency and percentages were used. The distribution of the variables was measured using the Kolmogorov–Smirnov test. In the analyses of quantitative independent data, independent sample t-test and Mann–Whitney U-test were used. In the analyses of qualitative independent data, chi-square test was used. When the data did not meet the test criteria for chi-square test, Fischer’s exact test was used. Spearman correlation analysis was used in the correlation analysis. A p values of <.05 was considered to be statistically significant.
Results
Ninety patients were included in the study (Group 1: n = 41, Group 2: n = 49). Between the mild and severe ED groups, there was no statistically significant difference with respect to age, age of the partner, height, weight and BMI level, serum total testosterone levels (p > .05) (). In the severe ED group, 25(OH)D values were significantly lower compared with those in the mild ED group (p < .001). In the severe ED group, MPV was significantly higher compared with that in the mild ED group (p = .001) ().
Table 1. Comparison of mild and severe ED group.
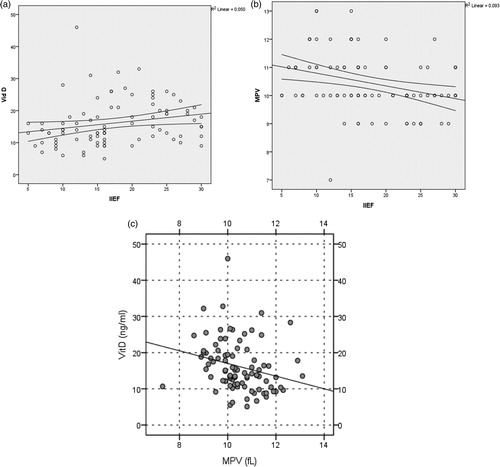
There was positive correlation between IIEF-EF scores and 25(OH)D levels (p = .03, r = 0.22). There was negative correlation between IIEF-EF scores and MPV and between 25(OH)D and MPV levels (p = .003, r = −0.31 for IIEF-EF/MPV; p < .001, r = 0.395 for 25(OH)D/MPV) ().
Figure 2. (a) Serum vitamin D levels were positively correlated with IIEF scores. (b) Serum MPV levels were negatively correlated with IIEF scores. (c) Serum MPV levels were negatively correlated with vitamin D levels.
When dividing patients into mild and severe ED groups, the activity of vitamin D of <15 ng/ml [area under the curve 0.723 (0.615–0.831)] was considered to be significant. When dividing patients into mild and severe ED groups, the activity of MPV of >10 fl [area under the curve 0.699 (0.587–0.811)] was considered significant (). When dividing patients into mild and severe ED groups, the coexistence of the activities of MPV > 10 fl and 25(OH)D of <15 ng/ml, respectively [area under the curve 0.764 (0.662–0.865)] was considered significant ().
Figure 3. The activities of MPV > 10 fl and 25(OH)D of <15 ng/ml, respectively [area under the curve 0.764 (0.662–0.865)] was considered significant.
![Figure 3. The activities of MPV > 10 fl and 25(OH)D of <15 ng/ml, respectively [area under the curve 0.764 (0.662–0.865)] was considered significant.](/cms/asset/391a0302-5ba2-49ff-a39e-df85bf8f8471/itam_a_1459544_f0003_b.jpg)
Table 2. ROC curve analysis for Vitamin D and MPV levels.
Discussion
In our study, MPV values in moderate and severe ED patients were significantly higher compared with those in mild ED patients, and 25(OH)D levels were significantly lower in the moderate and severe ED patients compared with those in the mild ED patients. Moreover, there was a moderate negative correlation between 25(OH)D levels and MPV values. Cut-off value for MPV was 10 fl, whereas that for 25(OH)D was 15 ng/ml.
Today, one of the most common sexual dysfunctions, ED, is considered to be an important concern among the healthy ageing population [Citation14]. The most prevalent type of ED, organic ED, is generally associated with endothelial dysfunction related to vascular pathologies [Citation15]. Endothelial dysfunction plays an important role in cardiovascular and peripheral vascular diseases due to the systemic and subclinical inflammatory response characterized by atherosclerosis [Citation16]. Impairment of endothelium caused by cardiovascular risk factors not only affects coronary arteries but also affects many other arteries in the body, including the corpus cavernosum [Citation17].
The effect of increased platelet (PLT) activation on vascular disorders has been demonstrated in many studies [Citation18]. PLTs play an important role in the pathogenesis of atherosclerotic complications. They contribute to thrombosis formation and apoptosis following plaque rupture. MPV is an indicator of PLT function. Large PLTs contain denser granules and produce more thromboxane A2 [Citation19]. Larger thrombocytes tend to get denser and include more α-granules, which may release prothrombotic materials such as chemotactic and mitogenic factors that contribute to thrombocyte factor 4, P-selectin, and platelet-derived growth factor, i.e. vascular neointimal, proliferation [Citation20]. MPV, routinely evaluated in the clinical practice during an automated blood count, is considered a possible initial expression of platelet activation and represents a marker of cardiovascular risk. La Vignera et al. [Citation21] showed that increased MPV and percentage of a vitronectin receptor (Vb3)-expressing elements that may be envisioned as marker of cardiovascular dysfunction in arterial ED patients [Citation21]. In many studies, increased MPV values were shown to be associated with cardiovascular risk factors such as smoking, diabetes, obesity, hypertension and hyperlipidemia [Citation19,Citation22].
In addition, there are studies demonstrating that MPV is significantly higher in ED patients compared with healthy subjects [Citation23]. In a meta-analyses wherein seven studies were included, it was detected that MPV was significantly higher in vasculogenic ED patients compared with healthy controls [mean difference (MD) = 0.681, 95% CI: 0.444, 0.918] and non-vasculogenic ED (MD = 0.706, 95% CI: 0.410, 1.002) [Citation23]. These findings show that MPV may be an indicator of vascular pathologies in ED patients. In our study, it was found that MPV values are positively correlated with the severity of ED.
It was reported that vitamin D particularly modulates endothelial function [Citation24]. Endothelial cells synthesize vitamin D and express vitamin D receptors. Observational data associates low serum vitamin D levels with coronary artery calcification, cardiovascular acute and chronic incidents, stroke and general mortality [Citation25,Citation26]. It was suggested that low 25(OH)D contributes to ED via leading to the development of various risk factors such as endothelial dysfunction, inflammation, impaired glucose homeostasis and atherosclerosis [Citation9]. Basat et al. [Citation27] demonstrated that the strong relationship between vitamin D levels and ED in patients with type 2 diabetes mellitus. The effects of vitamin D on testosterone hormone are also known. Studies have shown that testosterone replacement improves erectile function and increases penile arterial blood flow [Citation28–30]. Along with that, vitamin D replacement improved the serum testosterone levels of middle-aged men and improved erectile function [Citation31].
In vitamin D deficiency, there is an increase in the level of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [Citation32]. High cytokine levels cause increased MPV [Citation33]. In a study, Cure et al. [Citation12] demonstrated that vitamin D deficiency caused an increase in MPV, which in turn increased the risk of cardiovascular diseases. This change can be observed in patients with vitamin D deficiency as a combined effect of increased TNF-α and IL-6 levels and increased release of adhesion molecules with an increase in MPV levels. In our study, there was a negative correlation between vitamin D levels and MPV values.
There are a couple of limitations to this study. First, the study was a retrospective study. Second, the number of patients included in the study was low. Finally, the Doppler ultrasound findings of the patients were not recorded.
There is a statistically significant relationship between vitamin D levels and ED severity. This relationship is moderately correlated with the increase in MPV values. Vitamin D deficiency and increase in MPV values play a role as factors affecting ED severity. Further studies are required for the confirmation of these findings.
Acknowledgements
Informed consent was obtained from all the patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165.
- Banks E, Joshy G, Abhayaratna WP, et al. Erectile dysfunction severity as a risk marker for cardiovascular disease hospitalisation and all-cause mortality: a prospective cohort study. PLoS Med. 2013;10:e1001372.
- Shah NP, Cainzos-Achirica M, Feldman DI, et al. Cardiovascular disease prevention in men with vascular erectile dysfunction: the view of the preventive cardiologist. Am J Med. 2016;129:251–259.
- Chung RY, Chan D, Woo J, et al. Erectile dysfunction is associated with subsequent cardiovascular and respiratory mortality in cohort of 1,436 Chinese elderly men. J Sex Med. 2015;12:1568–1576.
- SansanayudhAnothaisintawee N, Muntham T, McEvoy D, et al. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2014;175:433–440.
- Bath P, Algert C, Chapman N, et al. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626.
- Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44:805–816.
- Lutsey PL, Michos ED. Vitamin D, calcium, and atherosclerotic risk: evidence from serum levels and supplementation studies. Curr Atheroscler Rep. 2013;15:293.
- Sorenson M, Grant WB. Does vitamin D deficiency contribute to erectile dysfunction? Dermatoendocrinology. 2012;4:128–136.
- Park SG, Yeo JK, Cho DY, Park MG. Impact of metabolic status on the association of serum vitamin D with hypogonadism and lower urinary tract symptoms/benign prostatic hyperplasia. Aging Male. 2017. DOI: 10.1080/13685538.2017.1311857
- Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. Aging Male. 2016;19:239–243.
- Cumhur Cure M, Cure E, Yuce S, et al. Mean platelet volume and vitamin D level. Ann Lab Med. 2014;34:98–103.
- Rosen RC, Allen KR, Ni X, et al. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016.
- YuanZhang J, Yang RZ, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63:902–912.
- Maas R, Schwedhelm E, Albsmeier J, et al. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7:213–225.
- Vlachopoulos C, Rokkas K, Ioakeimidis N, et al. Inflammation, metabolic syndrome, erectile dysfunction, and coronary artery disease: common links. Eur Urol. 2007;52:1590–1600.
- Uslu N, Eren M, Gorgulu S, et al. Left ventricular diastolic function and endothelial function in patients with erectile dysfunction. Am J Cardiol. 2006;97:1785–1788.
- Hansen GA, Vorum H, Jacobsen C, et al. Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Mol Cell Biochem. 2009;320:25–33.
- Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156.
- Bayraktar Z, Albayrak S. Blood platelet activity in men with vasculogenic erectile dysfunction. Arch Ital Urol Androl. 2017;89:51–54.
- La Vignera S, Condorelli RA, Burgio G, et al. Functional characterization of platelets in patients with arterial erectile dysfunction. Andrology. 2014;2:709–715.
- Coban E, Ozdogan M, Yazicioglu G, et al. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–982.
- Ren ZJ, Ren PW, Yang B, et al. Mean platelet volume, platelet distribution width and platelet count in erectile dysfunction: a systematic review and meta-analysis. Andrologia. 2017;49. DOI: 10.1111/and.12777
- Tarcin O, Yavuz D, Ozben GB, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030.
- Giovannucci E, Liu Y, Hollis BW, et al. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180.
- Karakas M, Thorand B, Zierer A, et al. Low levels of serum 25-hydroxyvitamin D are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA Augsburg case-cohort study. J Clin Endocrinol Metab. 2013;98:272–280.
- BasatSivritepe S, Ortaboz RD, et al. The relationship between vitamin D level and erectile dysfunction in patients with type 2 diabetes mellitus. Aging Male. 2017. DOI: 10.1080/13685538.2017.1379488
- Canguven O, Talib RA, El-Ansari W, et al. RigiScan data under long-term testosterone therapy: improving long-term blood circulation of penile arteries, penile length and girth, erectile function, and nocturnal penile tumescence and duration. Aging Male. 2016;19:215–220.
- Yassin AA, Nettleship JE, Salman M, et al. Waist circumference is superior to weight and BMI in predicting sexual symptoms, voiding symptoms and psychosomatic symptoms in men with hypogonadism and erectile dysfunction. Andrologia. 2017;49. DOI: 10.1111/and.12634
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.
- Canguven O, Talib RA, El Ansari W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017;20:9–16.
- Di Rosa M, Malaguarnera G, De Gregorio C, et al. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43.
- Cure E, Balik MS, Cumhur Cure M, et al. Is the mean platelet volume predictive of hip fractures in the elderly? Ann Lab Med. 2013;33:367–370.