Abstract
Objectives: To investigate the prevalence and severity of varicocele in adult population over the age of 40. We also measured testicular size, consistency, and total testosterone levels with an aim to observe the effect of varicocele on testis as men age.
Methods: Two hundred twenty-four patients with varicocele, 241 patients without varicocele who admitted to our clinic were enrolled in the study. We stratified participants by four age groups (40–49, 50–59, 60–69, >70 yr). Patients were grouped according to varicocele grade and laterality. The morning testosterone level was drawn. The subgroups were compared with each other.
Results: Overall, varicocele prevalence was 48%. Of the patients, 104 had unilateral, 120 had bilateral varicocele. Of the patients with varicocele, 62 (13.30%) were found as grade 3, 99 (21.10%) were grade 2, and 63 (13.60%) were grade 1. The percentages of smaller testes in grade 1, grade 2, and grade 3 varicocele group were 20.60, 79.80, and 88.70 and a significant association was detected. Age stratification of the data revealed the smaller and soft testis prevalence as well as higher grade varicocele prevalance increased in older age groups.
Conclusions: Varicocele presence is associated with lower testicular size, softer testicular consistency, and lower testosterone levels, especially in older patients with bilateral and high-grade varicocele.
Introduction
Varicocele is an abnormal dilatation of internal spermatic veins within the pampiniform plexus. Varicoceles are a frequent scrotal finding in normal males. Although the pathogenesis and natural history remain controversial, varicocele is thought to contribute to the risk of infertility in men. Furthermore, two population-based studies indicate that 85% of men with varicocele have fathered children suggesting that its effect on paternity is less clear [Citation1,Citation2]. The underlying pathologic process is not well known, but varicoceles have been associated with turbulent venous flow related to the right angle insertion of the left testicular vein into the left renal vein, which could be an explanation why left-sided varicocele is observed more frequently. In addition, the nutcracker phenomenon, defined as the compression of the left renal vein between superior mesenteric artery and aorta, may contribute to the pathogenesis of varicocele [Citation3,Citation4].
The leading theory as to how varicocele affects testicular cells is based on the knowledge that the testicular processes are entirely temperature dependent. Elevated intrascrotal temperature caused by varicocele results in injury to Leydig cells, thus reducing testosterone levels. It also affects germinal cell membranes and reduces Sertoli cell function [Citation5]. Furthermore, varicocele ligation has been demonstrated to be associated with reduction in intrascrotal temperatures [Citation6]. The other proposed mechanism results in the alteration of the microenvironment of testicular cells including free reflux of adrenal and renal metabolites from the left renal vein. It has also been indicated that the level of seminal oxidative stress correlates with varicocele grades and improves with the treatment of varicocele [Citation7].
The prevalence of varicocele has been widely studied in adolescents and young adults and found to be approximately 15% but ranges from 3% to 43% in some studies [Citation8–10]. However, researches are insufficient about the prevalence of varicoceles in adult populations. In this study, our goal was to detect the prevalence, severity of varicocele and its effect on testicular consistency, size and T-testosterone levels in the adult population providing a descriptive analysis of aging male varicocele characteristics.
Materials and methods
Between January and October 2014, 465 patients aged 40 or more were enrolled in this cross-sectional study. Institutional review board approval was obtained before the start of the study. All participants signed the informed consent before being enrolled in the study. First, we divided the participants into three groups including unilateral, bilateral, and varicocele-free groups; then varicocele patients were classified into three grades considering the severity of the disease: severe (grade 3 ), moderate (grade 2), and mild (grade 1). We stratified the patients according to age category: 40–49, 50–59, 60–69, and ≥70 years old. For detecting varicocele, the patients were examined in a warm room in supine and standing positions. The spermatic cord was palpated at rest and during Valsalva maneuver. Each side was evaluated using following standard grading system; grade 1, palpable only with Valsalva; grade 2, easily palpable but not visible and grade 3, easily visible. To prevent inter-observer bias, the same experienced physician performed all examinations. Scrotal ultrasound was not delivered to detect varicocele; only physical examination was applied. Testicular size was measured with a Prader orchidometer, which is a chain of 12 solid wooden ellipsoids with volumes of 1–6,8,10,12,15,20, and 25 ml that are visually compared with the size of each testis. We grouped the participants depending on whether their testicular volume was greater than 20 ml or not. We evaluated the testicular consistency by palpation. The consistency was subjectively graded as either soft or normal. All men had testosterone levels as assessed by a peripheral venous serum sample taken between 8:00 h and 10:30 h. Plasma testosterone levels for each subject were measured by automated chemiluminescent microparticle immunoassay; intra-assay coefficient of variability (CV) was 4.4%, and inter-assay CV was 5.2%.
Statistical analysis
The statistical analyses were performed by SPSS for Windows 20.0 (SPSS Inc, Chicago, IL, USA). Based on Kolmogorov–Smirnov test used to test the parameter distribution normality, if the parameters were not normally distributed, non-parametric tests including Mann–Whitney U and Kruskal–Wallis were used. When the parameters met normality test criteria, comparisons between the groups were carried out using ANOVA or student’ t-test. Chi-square test was used for qualitative parameters. Significance determined at 0.05 was used throughout all statistical analyses. All p values reported in this paper are two-tailed unless stated otherwise.
Results
Varicocele laterality
Of the participants, 120 had unilateral, 104 had bilateral varicocele, while 241 had no varicocele (varicocele prevalence 48%). Of the 120 unilateral varicocele patients, four patients had a unilateral right varicocele, whereas remaining 116 patients had left varicocele. No underlying pelvic or retroperitoneal pathology was detected in those with unilateral right varicocele patients. The mean age of the patients with bilateral varicocele was 63.13, and those with unilateral varicocele was 61.51. BMI of three groups (none, unilateral, and bilateral) was 27.83, 27.16, and 27.44, respectively and no statistically significant difference was found. The percentages of smaller testes (<20 ml) in none, unilateral, and bilateral varicocele group were 7.10, 61.70, and 69.20 and a significant association was detected. Similarly, patients with bilateral varicocele had a higher rate of soft testis compared to unilateral and no varicocele group. Mean total testosterone levels for none, unilateral, and bilateral varicocele group were 3.52, 3.17, and 2.97 and a significant association was found (p < .001). The paternity rate of patients was comparable among groups. The detailed demographic and clinical features for varicocele laterality are illustrated in .
Table 1. Demographic and clinical features of participants stratified by varicocele laterality.
Varicocele grade
Of the participants, 63 patients had grade 1, 99 had grade 2, and 62 had grade 3 varicocele. Mean age of the patients with grade 1, grade 2, and grade 3 varicocele were 58.79, 62.22, and 65.98, respectively. BMI of three groups (grade 1, grade 2, and grade 3 varicocele) were 27.23, 27.02, and 27.82, respectively and no statistically significant difference was found. The percentages of smaller testes (<20 ml) in grade 1, grade 2, and grade 3 varicocele group were 20.60, 79.80, and 88.70 and a significant association was detected. Similarly, patients with higher varicocele grade had increased rate of soft testis compared to the lower grade of varicocele group. Mean total testosterone levels for grade 1, grade 2, and grade 3 varicocele group were 3.33, 3.33, and 2.85 and a significant association was found (p < .001). The paternity rate of patients was comparable among groups. The detailed demographic and clinical features for varicocele grade are illustrated in .
Table 2. Demographic and clinical features of participants stratified by varicocele grade.
Age stratification
The varicocele prevalence among four groups stratified by age (40–49, 50–59, 60–69 and >70) were 44%, 40%, 51%, and 66%, respectively. Patients with >70 years old had the highest prevalence of bilateral varicocele compared with younger participants.
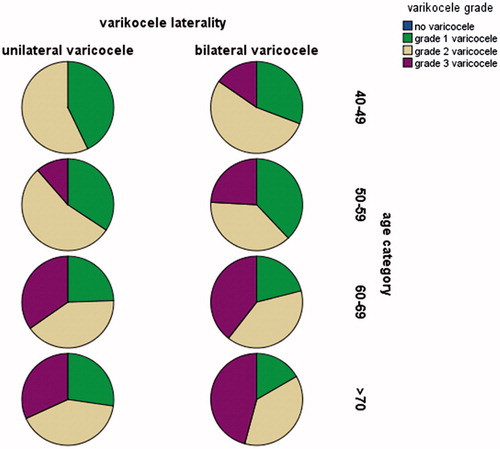
The increasing trend of grade-3 varicocele prevalence was detected for among four groups with the rates of 3.22%, 6.79%, 18.12%, and 25.71% respectively. demonstrated the distribution of varicocele grade among patients stratified by age category.
Smaller testis size prevalence of four groups were 19.35, 30.24, 38.59, and 51.42. Soft testis prevalence of four groups were 24.19, 33.33, 46.19, and 60.00 and both the findings were statistically significant among four age category. The detailed information of characteristics of patients stratified by age category was presented in . Besides, illustrated the percentage of testis volume in patients stratified by varicocele grade and age groups.
Figure 2. The percentage of testis volume in patients stratified by varicocele grade and age groups.
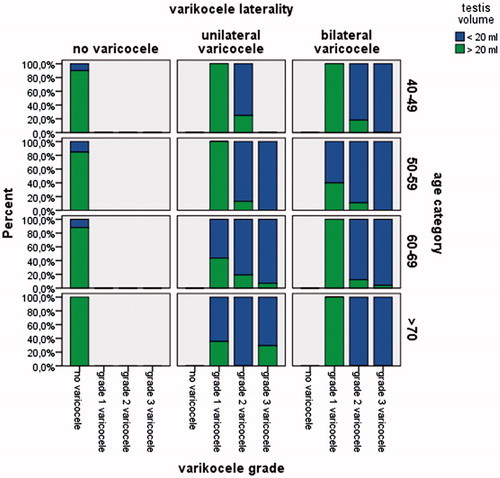
Table 3. The clinical findings stratified by age category.
Discussion
Varicocele, which is associated with time-dependent testicular growth arrest and the most correctable cause of male infertility, is an abnormal tortuosity and dilatation of the veins of the pampiniform plexus within the spermatic cord. Although it is commonly examined in adolescents and young adults, there is little knowledge regarding its prevalence and conclusions on testes in older men. The prevalence of varicocele is reported to be as high as 10–15% in the general population, 30–35% of men with primary infertility, and 69–81% of men with secondary infertility [Citation11]. Because epidemiologic studies have shown that age is a significant risk factor for the development of varicose veins [Citation12], it is reasonable to predict that increasing age may also be a risk factor for varicocele. Compared with those studies including younger population, our study demonstrated the quite higher prevalence of varicocele with 48% in 465 adult patients over the age of 40. Canales et al. [13] conducted a study in which the varicocele prevalence was 42.90% in 352 patients. Of these, bilateral and unilateral varicocele rates were 19.80% and 21.20%, respectively. This distribution was similar to our study. In another study, Levinger et al. [Citation14] found that the prevalence of varicocele increases with age with a rise of about 10% for each decade of life with the incidence reaching 75% in the eighth decade of life. Our results revealed increasing age is associated with higher prevalence of varicocele, but it did not emerge such a linear enhancement in varicocele prevalence as men age. Furthermore, our analysis included subgroups of varicocele laterality, severity, testis size, consistency stratified by age category, which is lacking in that study implying that we tried to provide a more in-depth analysis.
The association of varicocele with a decrease in volume of testis has been demonstrated in many studies [Citation15–17]. Although all these are adolescent and young adult studies, eventually the pathophysiology for elderly patients is similar to younger population and varicocele could result in testicular volume loss over time. We stratified the patients by age including 10-year periods and compared varicocele related conditions. Among the age groups, patients with higher than 70-year-old were more likely to have a higher percentage of grade 3 and bilateral varicocele as well as soft and smaller testis. Sigman and Jarrow [Citation18] conducted a study regarding testicular hypotrophy and varicocele grade and found that left testicular varicoceles were associated with decreased testicular volumes in 73, 53, and 43% in grade 3, 2 and 1 varicoceles, respectively. These are similar findings to our study. According to our study, while all grades of varicocele were associated with a decrease in testicular volume, patients with grade 2 and grade 3 varicoceles or bilateral varicocele were highly susceptible to the risk of lower testicular volume. We also observed the percentage of smaller testis size was higher in older patients. Although varicocele grade is positively associated with the risk of left testicular hypotrophy in some other studies [Citation19,Citation20], there are some investigations opposed to them [Citation21,Citation22]. At this point, it seems that varicocele could result in volume decreases in older populations over time owing to its longstanding features. These results are suggestive of the fact that varicocele is a chronic disorder that its consequences on testes are becoming more apparent by age.
We evaluated the existence and grade of varicocele by physical examination, and this may be regarded as a handicap, especially for detecting grade 1 varicoceles and testicular consistency. However, in a study evaluating the patients using scrotal ultrasound, a more objective method to assess testis volume, Sakamoto et al. [Citation23] found that left clinical varicocele was associated with significant ipsilateral testicular hypotrophy.
Although the effect of varicocele on testosterone levels is not well illustrated and most studies in this area focus on spermiogenesis in infertile younger populations, the harmful effects of the varicocele could affect not only spermatogenesis but also Leydig cell function, and could negatively affect testosterone production. In a study similarly designed to ours, Canales et al. [Citation13] concluded that while varicocele presence is not associated with decreased testosterone levels, only bilateral, soft testicular consistency was significantly correlated. However, the subgroups according to varicocele grade were not assessed in that study. According to our data, the presence of either unilateral or bilateral varicocele is associated with lower testosterone levels. Furthermore, while patients with grade 3 varicocele were likely to have lower testosterone levels the other grades were not affected. It is reasonable to assume that high-grade varicocele might create more severe damage to patients compared with lower-grade resulting in softer consistency and lower volume of the testis and ultimately reducing testosterone levels. Contrary to our findings, in a cross-sectional analysis of 875 men screened for prostate cancer, Yamacake et al. [Citation24] demonstrated that the presence of the clinical varicocele, as well as its grade, has no impact on testosterone level in elderly men. However, they did not categorize patients according to age stratification, as well as their study population was not similar to ours, which are the plausible factors for the different results. Current scientific evidence provided essential insights for the implication of varicocele in hypogonadism. In a study including a total of 130 males with varicocele complaining of infertility or scrotal discomfort and 130 age-matched healthy males, Ji B et al. [Citation25] reported TT levels, as well as grade 3 and 2 varicocele, were significant indicators for hypogonadism occurrence. In addition, varicocelectomy was shown as efficient in hypogonadic patients in some studies [Citation26–29]. A current meta-analysis of eight studies with a total of 712 male patients showed that mean testosterone among hypogonadal increased by 105.65 ng/dl after surgical treatment of varicocele [Citation30]. In addition, in a study including aging males besides the younger population, Hsiao et al. [31] revealed that microsurgical varicocelectomy resulted in significant increase in testosterone levels in all age groups, including men in the fifth and sixth decades of life. They concluded that microsurgical varicocelectomy should be offered to older men for infertility and hypogonadism. We also observed increasing age was associated with decreased testosterone levels. However, the exact implication of varicocele presence and severity in this decrease is not clear from our study as the primary purpose of our study was to provide a descriptive analysis of aging male varicocele characteristics rather than examining its association with hypogonadism.
There are several limitations to this study. Firstly, this is a cross-sectional study implying we may not conclude cause and effect relationship between varicocele and testicular deficiency and we may only propose that men with varicocele(s) are at risk for diminished testosterone levels. Secondly, we measured T testosterone levels, but we did not investigate its clinical effects. Therefore, we are unable to reach a conclusion whether patients with varicocele should be treated with testosterone supplements, varicocelectomy, etc. Thirdly, testicular consistency is an absolute subjective finding, but the physical examination of all participants was performed by a single experienced clinician to avoid the possible bias. Lastly, although it is not routinely recommended, we did not confirm our physical examination findings of varicocele with imaging studies.
Conclusions
The prevalence of varicocele was 48% in our population of older than 40 years old, and despite the deficiency of younger control groups, we are confident that our results reflect an accurate representation of our population’s clinical varicocele prevalence. In addition, bilateral and high-grade varicocele presence was mainly associated with lower size and consistency of testis, and these results are more apparent in older participants. Patients with bilateral and grade 3 varicocele were more likely to have lower testosterone levels, and it should also be taken into account while evaluating the patients for androgen deficiency syndrome.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Pinto KJ, Kroovand RL, Jarow JP. Varicocele related testicular atrophy and its predictive effect upon fertility. J Urol. 1994;152:788–790.
- Safarinejad MR. Infertility among couples in a population-based study in Iran: prevalence and associated risk factors. Int J Androl. 2008;31:303–314.
- Coolsaet BL. The varicocele syndrome: venography determining the optimal level for surgical management. J Urol. 1980;124:833–839.
- Kim WS, Cheon JE, Kim IO, et al. Hemodynamic investigation of the left renal vein in pediatric varicocele: Doppler US, venography, and pressure measurements. Radiology. 2006;241:228–234.
- Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am. 2008;35:183–189.
- Wright EJ, Young GP, Goldstein M. Reduction in testicular temperature after varicocelectomy in infertile men. Urology. 1997;50:257–259.
- Allamaneni SS, Naughton CK, Sharma RK, et al. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82:1684–1686.
- Kumanov P, Robeva RN, Tomova A. Adolescent varicocele: who is at risk? Pediatrics 2008;121:53–57.
- Zampieri N, Cervellione RM. Varicocele in adolescents: a 6-year longitudinal and follow up observational study. J Urol. 2008;180:1653–1656.
- Stavropoulos NE, Mihailidis I, Hastazeris K, et al. Varicocele in schoolboys. Arch Androl. 2002;48:187–192.
- Sabanegh E, Agarwal A. Male infertility. 10th ed. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell–Walsh urology. Philadelphia: Saunders; 2012. p. 636–637.
- Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994;81:167–173.
- Canales BK, Zapzalka DM, Ercole CJ, et al. Prevalence and effect of varicoceles in an elderly population. Urology. 2005;66:627–631.
- Levinger U, Gornish M, Gat Y, et al. Is varicocele prevalence increasing with age? Andrologia. 2007;39:77–80.
- Lyon RP, Marshall S, Scott MP. Varicocele in childhood and adolescence: implication in adulthood infertility? Urology. 1982;19:641–644.
- Kass EJ, Belman AB. Reversal of testicular growth failure by varicocele ligation. J Urol. 1987;137:475–476.
- Lipshultz LI, Corriere JN. Jr. Progressive testicular atrophy in the varicocele patient. J Urol. 1977;117:175–176.
- Sigman M, Jarow JP. Ipsilateral testicular hypotrophy is associated with decreased sperm counts in infertile men with varicoceles. J Urol. 1997;158:605–607.
- Thomas JC, Elder JS. Testicular growth arrest and adolescent varicocele: does varicocele size make a difference? J Urol. 2002;168:1689–1691.
- Zampieri N, Zuin V, Corroppolo M, et al. Relationship between varicocele grade, vein reflux and testicular growth arrest. Pediatr Surg Int. 2008;24:727–730.
- Alukal JP, Zurakowski D, Atala A, et al. Testicular hypotrophy does not correlate with grade of adolescent varicocele. J Urol. 2005;174:2367–2370.
- Kolon TF, Clement MR, Cartwright L, et al. Transient asynchronous testicular growth in adolescent males with a varicocele. J Urol. 2008;180:1111–1114.
- Sakamoto H, Ogawa Y, Yoshida H. Relationship between testicular volume and varicocele in patients with infertility. Urology. 2008;71:104–109.
- Yamacake KG, Cocuzza M, Torricelli FC, et al. Impact of body mass index, age and varicocele on reproductive hormone profile from elderly men. Int Braz J Urol. 2016;42:365–372.
- Ji B, Jin XB. Varicocele is associated with hypogonadism and impaired erectile function: a prospective comparative study. Andrologia. 2017;49:e12683.
- Abdel-Meguid TA, Farsi HM, Al-Sayyad A, et al. Effects of varicocele on serum testosterone and changes of testosterone after varicocelectomy: a prospective controlled study. Urology. 2014;84:1081–1087.
- Hsiao W, Rosoff JS, Pale JR, et al. Varicocelectomy is associated with increases in serum testosterone independent of clinical grade. Urology. 2013;81:1213–1217.
- Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int. 2011;108:1480–1484.
- Sathya Srini V, Belur Veerachari S. Does varicocelectomy improve gonadal function in men with hypogonadism and infertility? Analysis of a prospective study. Int J Endocrinol. 2011;2011:916380.
- Chen X, Yang D, Lin G, et al. Efficacy of varicocelectomy in the treatment of hypogonadism in subfertile males with clinical varicocele: a meta-analysis. Andrologia. 2017;49:e12778.
- Hsiao W, Rosoff JS, Pale JR, et al. Older age is associated with similar improvements in semen parameters and testosterone after subinguinal microsurgical varicocelectomy. J Urol. 2011;185:620–625.