Abstract
Objective: SIRT6 is a main regulator of metabolism and lifespan and its importance has been implicated in the prevention against aging-related diseases. The objective of this study was to examine the application of multivariate longitudinal models in SIRT6, FBS, and BMI analysis in the elderly men after eight weeks concurrent training with supplementation of l-arginine (l-Arg).
Methods: Thirty two elderly men with mean age of 63.09 ± 3.71 years were randomly divided into four equal-sized groups (each n = 8); Exercise + supplement (ES) group; exercise + placebo (EP) group; supplement (S) group and control (C) group. The ES and EP groups performed the eight weeks of concurrent training, three sessions per week. Group ES and group S consumed 1000 mg of l-Arg per day at 8:00 pm. Measurements of biochemical variables were done by ELISA Reader method. For analytical purposes, we used the paired sample t-test and multivariate longitudinal modeling with generalized estimating equation (GEE) methodology. All analyses have been implemented in R-3.4.1. p Values less than .05 were considered statistically significant.
Results: With respect to significant association between sirt6, FBS, and BMI, this study showed that synergy effect of training and supplementation was greater than the sum of their individual effects on SIRT6 (β = 0.79, p < .001), FBS (β = −5.56, p = .022), and BMI (β = −3.89; p = .041). Also exercise alone had a significantly larger effect than supplementation alone on responses.
Conclusions: It can be concluded that the joint usage of concurrent training and supplement of l-Arg for elderly men could improve the metabolism and body composition.
Keywords:
1. Background
Aging is a multifactorial process likely modulated by diverse molecular and cellular events, and is accompanied by the increased incidence of various diseases such as neurodegeneration, cancer, genome instability, epigenetic and transcriptional changes, molecular damage, cell death, inflammation, and metabolic disease [Citation1]. Although there is solid evidence showing that imbalance between the antioxidant enzyme system and ROS production is a contributor to some aging pathologies, lifespan extension has yet to be consistently achieved through strategies to mitigate levels of oxidative damage [Citation2]. Sirtuins have been implicated in the age-related malfunctioning of these pathways and sirtuins are able to alter the progress of aging [Citation3]. SIRT6 is a nuclear protein and recently it has been shown to act as a histone deacetylase that can influence the telomeres of human cells and increased genomic instability and sensitivity to DNA damage, glucose metabolism, stress response, life span and SIRT6 may play a pivotal role in both longevity and progeria [Citation4,Citation5]. Global SIRT6 knockout mice display hypoglycemia, premature aging, loss of subcutaneous fat, lymphopenia and lordokyphosis, dying at around 4 weeks [Citation6,Citation7]. Postprandial limb blood flow and skeletal muscle microvascular perfusion reduce with aging [Citation8] and show that pelvic floor muscle strength is affected by physical activity [Citation9]. Age is also associated with uninterrupted decline in physical activity. Regular physical exercise-mediated health promotion is a key factor in maintaining our functional autonomy. Chen et al. found that the anaerobic threshold increased with arginine and antioxidant-containing supplement in elderly male cyclists [Citation10]. Moreover, l-arginine (l-Arg) is also implicated in insulin resistance [Citation11,Citation12], heat stress [Citation13], oxidative stress [Citation14], and many other environmental stresses. These physiological functions of l-Arg may be related to its regulating effects on some aging associated genes. As, skeletal muscle is a major site of glucose utilization, exercise and resistance training can protect us from sarcopenic loss of muscle mass, also involves sirtuin-regulated pathways including the antioxidant, macromolecular damage repair, energy, mitochondrial function, and neuronal plasticity associated pathways [Citation15]. Probably, for maintaining muscle function, combination protein nutrition with exercise is considered optimal. The aging process is associated with gradual and progressive loss of muscle mass along with lowered physical endurance and strength and quality of life in seniors. It has been shown that consumption of l-Arg increases plasma levels and improves blood flow. It also increases insulin, which increases the anabolism in the elderly and can be a good protector in the human body in the fight against damage from free radicals [Citation8]. l-Arg plays a role in reducing the accumulation of lactate in the body, especially lactate due to exercise and modulation of muscle metabolism. On the other hand, regular aerobic and resistance exercise programs may be to counteract most aspects of sarcopenia. However, most of the previous study have evaluated the effect of exercise and supplements on multiple responses, do not take into account the nature of repeated measurements of correlated responses. In this study, we utilized a multivariate longitudinal modeling, to know whether concurrent training and supplementation of l-Arg can have significant effects on multiple responses of SIRT6, FBS, and BMI simultaneously.
2. Materials and methods
2.1. Study design and participants
In this quasi-experimental pre-test/post-test study, 32 elderly non-athlete men aged 60–75 years were recruited to assess the effects of concurrent training and supplementation of l-Arg on SIRT6, FBS, and BMI. The subjects were selected by convenience sampling method in Isfahan, Iran from April to June 2017. The inclusion criteria were as follows: male gender, 60 ≤ age≤75 years, no systematic strength training last 6 months (<1 session/week). Exclusion criteria were:
Having acute illness (acute articular disease and bone softness, bone fractures), disability to perform exercise, smoking and alcohol consumption, use of antioxidant supplements, unwillingness to attend a study at any time. Also, the subjects were evaluated by the physician regarding the ability to perform physical activities and use of l-Arg supplement before and during the study. The ethical aspects of this study were approved by the institutional ethics committee of Tabriz University of Medical Sciences, with code IR.TBZMED.REC.1396.1136. The written consent was obtained from all subjects.
Using NCSS PASS11 software, procedure menu, DOE submenu, 32 subjects were randomly divided into four equal-sized groups (each group n = 8); exercise + supplement (ES) group; exercise + placebo (EP) group; supplement (S) group and control (C) group. The ES group, in addition to the concurrent training, performed three sessions per week (coupled days), each received 1000 mg of l-Arg capsule daily. The EP group like the ES group had training three sessions per week (coupled days), each received 1000 mg of placebo capsules. The capsules had identical shape and appearance and contained l-Arg hydrochloride, microcrystalline cellulose, P.V.P., magnesium, stearate (Karen Pharma & Food Supplement Co., Yazd, Iran) and multi dekstrin. Researcher and subjects were blinded to participants' study groups. Also subjects were asked not to change their diet and physical activity during the study. They were followed-up every 2 weeks for receiving capsules and any possible sides effects and sport injuries.
2.1.1. Training intervention
The program training was 8 weeks concurrent training (strength + endurance training) with do the principle of overload and increased training intensity [Citation16]. Before the start of the concurrent training, subjects completed two familiarization sessions to practice the exercises and determined the 1 RM for every subject with use of brzycki formol [Citation17]. Endurance training was performed two minutes after resistance training on ergometer with intensity of 60–85% MHR that was determined by the researcher before the beginning of the exercises, during and after the operation, using the polar heart rate monitor (). Also, the Borg scale was used to control exercise intensity. The resistance program training was included of Barbell Bench Press – Medium Grip, Wide-Grip Lat Pulldown, Dumbbell Shoulder Press, Wide-Grip Standing Barbell Curl, Triceps Pushdown, Leg Extensions, Lying Leg Curls, Standing Calf Raises, Bicycling, Stationary. shows the details of program of training.
Table 1. Time and intensity of concurrent training endurance training strength training.
2.2. Assessments
All assessments were recorded at study start and at the end of the intervention.
2.2.1. Anthropometric and dietary assessments
Weight was measured to the nearest 100 g using digital scales while the individuals were minimally clothed, without shoes. Height was measured to the nearest 0.5 cm, in a standing position without shoes, using a tape measure. BMI was calculated as weight (kg) divided by the square of the height (m2). Twenty four hour dietary record (two work days and one weekend) was filled out by subject at the baseline and the end of study for dietary assessment.
2.2.2. Clinical and laboratory assessments
After 12–14 h fasting, 5 cm3 venous blood samples of subject were collected into test tubes containing EDTA. Blood samples were centrifuged and plasma separated and stored at −70 °C until biochemical analyses were performed. FBS and SIRT6 concentrations were assayed in plasma according to the manufacturers’ instructions using ELISA kits specific for human: SIRT6 (EASTBIOPHARM, Torrance, CA, with number Cat. No.: CK-E91225), CV (%) = SD/mean ×100, intra-assay: CV<%10, inter-assay: CV<%12, sensitivity: 0.23 ng/ml and assay range: 0.5 ng/ml → 40 ng/ml. Plasma glucose was determined by an enzymatic, colorimetric method (glucose oxidize-amino antipyrine [GOD-PAP] Pars Azmoun, Tehran, Iran); and the intraassay coefficient of variation and sensitivity of the method were 1.3% and 1 mg/dl, respectively.
2.3. Statistical analysis
For description purposes, the quantitative variables were summarized using statistical indices such as mean and standard deviation. For analytical purposes, in the first step, the paired sample t-test of BMI, FBS, and SIRT6 was utilized on each group (exercise + supplement; exercise; supplement, control) at two time points (baseline; 8 weeks later), separately. Moreover, because of the nature of repeated measurements is typically collected on multiple correlated responses, we used the multivariate longitudinal modeling and generalized estimating equation (GEE) methodology with AR(1) correlation structure to have valid statistical inferences. With this model, in addition to the within-subject association, multivariate response association at a specific time point is also considered to assess the effects of endurance training and supplementation of l-Arg on multivariate repeated measurements of BMI, FBS, and SIRT6 adjusting for the age of the participants. All analyses have been implemented in R-3.4.1 [Citation18]. p Values less than .05 were considered statistically significant.
3. Results
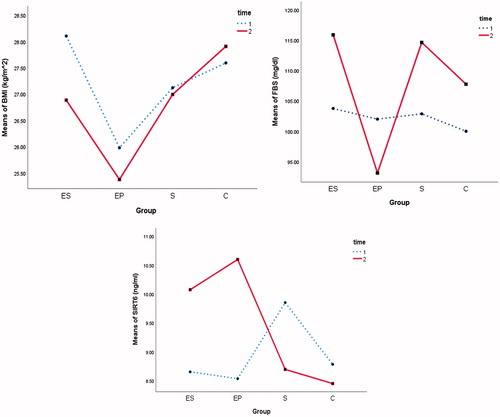
In this study, 32 participants with mean ± SD age of 63.09 ± 3.71 and weight of 79.94 ± 12.90 kg were evaluated. No significant difference was found in age, weight, and height at baseline between groups (not shown). displays the descriptive statistics for mean BMI, FBS, and SIRT at two time points in four groups. Also in this table, the results of paired sample t-test of BMI, FBS, and SIRT6 were shown on each group at two time points, separately. Result showed that the levels of SIRT6 in the ES and EP groups after eight weeks significantly increased (p = .004 and p = .006, respectively) but significantly reduced in the S group (p = .001). In the ES and EP groups, FBS (p = .044 and p = .034, respectively) and BMI (p = .002 and p = .012, respectively) significantly decreased after eight weeks.
Table 2. Comparing mean BMI, FBS, and SIRT at two time points in four groupsTable Footnotea.
To clarify the behavior of mean BMI, FBS, and SIRT of the participant at different time points they are displayed in .
In the final step of data analysis, because of the repeated and significant correlated nature of responses (BMI, FBS, and SIRT6 at different points), the multivariate longitudinal modeling with GEE methodology was utilized. The results are displayed in .
Table 3. Results of multivariate marginal model with AR (1) correlation structure.
4. Discussion
Regular physical exercise-mediated health promotion also involves sirtuin-regulated pathways, facilitates muscle glucose uptake, and modifies the heritability of BMI including the antioxidant, macromolecular damage repair, energy, mitochondrial function, and neuronal plasticity associated pathways [Citation15,Citation19]. With respect to significant association between sirt6, FBS, and BMI, in this study, we used a multivariate GEE modeling to investigate the effects of concurrent training, and supplementation of l-Arg on alters jointly of SIRT6, FBS, and BMI. This study showed that subjects who received supplement and training to gather had a higher reduction in the levels of BMI and FBS and higher increase in SIRT6 than the subject of other groups (). Especially, synergy effect of training and supplementation was greater than the sum of their individual effects on SIRT6, FBS, and BMI (). Also, according to , exercise alone had a significantly larger effect than supplementation alone on responses. These findings are in agreement with other studies that showed increase in family of sirt (sirt1–3) after exercise training and supported the evidence that suggested a pivotal role for SIRTs in mediating the adaptive response to physical exercise [Citation20,Citation21]. It has been shown that SIRT6 regulated metabolism in liver and brain [Citation5]. Functionally, SIRT6 plays an important role in DNA repair, telomerase function, genomic stability and cellular senescence [Citation22]. Cui et al. show that the mice with SIRT6 deficiency in muscle displayed impaired glucose homeostasis and insulin sensitivity, attenuated whole-body energy expenditure and weakened exercise performance [Citation5]. As the results of the present study also showed concurrent training increased sirt6 and decreased FBS and BMI (), this effect was higher in ES group. Therefore, it could be argued that concurrent training and supplementation of l-Arg have improved the metabolism. It has been reported that the fasting blood glucose level is associated with IL-18 and hyperglycemia promotes the synthesis of IL-18 and other pro-inflammatory cytokines that has been associated with the development of diabetes [Citation23]. Of course, we hypothesized that concurrent training might lead to increased oxidative stress, and so we used the help supplementation of l-Arg. It has been shown that due to the reduced AMPK activity, deletion of SIRT6 in muscle occurred. Mechanistically, decreased expression of genes involved in glucose and lipid uptake, mitochondrial oxidative phosphorylation and fatty acid oxidation in muscle cells [Citation5]. Of course Aguiar et al. show that acute l-Arg supplementation provides no ergogenic effect on blood flow and muscle performance in older women [Citation24] and as in the effect of supplement of l-Arg alone on BMI, FBS, and SIRT6 is very low. It was reported that vitamin D particularly modulates endothelial function [Citation25]. In this study, we did not measure other factors related to metabolic syndrome but some studies show that probably there is association between vitamin D and prostate enlargement. Hypogonadal men are more likely to have low vitamin D levels and increased risk for hypogonadism symptoms and related metabolic disorders [Citation26–28]. It has been suggested that there was probably relationship with exercise, l-Arg and age that needs more attention and research about it. For ordinary middle-aged and elderly men, other study shown that to improve the reproductive health, more attention should be on the effect of healthy living style, such as nutritionally balanced diet, proper exercise and regular schedule [Citation29,Citation30]. As the age increases, strength muscle and power cardiovascular decrease whereas finding of our study shows, concurrent training could result in improvement of the BMI and increased muscle mass in the elderly.
5. Conclusions
Based on the result, it can be concluded that the joint usage of concurrent training and supplement of l-Arg for elderly men could improve the metabolism and body composition. It was also found that consumption of l-Arg supplement alone is not suitable for elderly.
Acknowledgements
The authors thank the participants for taking part in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The SPSS data used to support the findings of this study are available from the corresponding author upon request.
References
- Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713.
- Pérez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014.
- Cantó C, Houtkooper RH. Sirtuins and aging. Sirtuins: Springer; 2016. p. 213–227.
- Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492.
- Cui X, Yao L, Yang X, et al. SIRT6 regulates metabolic homeostasis in skeletal muscle through activation of AMPK. Am J Physiol-Endocrinol Metabol. 2017;313:E493–E505.
- Xiao C, Kim H-S, Lahusen T, et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285:36776–36784.
- Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329.
- Mitchell WK, Phillips BE, Wilkinson DJ, et al. Supplementing essential amino acids with the nitric oxide precursor, l-arginine, enhances skeletal muscle perfusion without impacting anabolism in older men. Clin Nutr. 2017;36:1573–1579.
- Sousa LE, Figueiredo AA, Netto JMB. Correlation between pelvic floor strength and physical activity level in healthy men. Aging Male. 2018;1–5. DOI:10.1080/13685538.2018.1453797
- Chen S, Kim W, Henning SM, et al. Arginine and antioxidant supplement on performance in elderly male cyclists: a randomized controlled trial. J Int Soc Sports Nutr. 2010;7:13.
- Dioguardi FS. Wasting and the substrate-to-energy controlled pathway: a role for insulin resistance and amino acids. Am J Cardiol. 2004;93:6–12.
- de Castro Barbosa T, Poyares LL, Machado UF, et al. Chronic oral administration of arginine induces GH gene expression and insulin resistance. Life Sci. 2006;79:1444–1449.
- Zhu W, Jiang W, Wu L. Dietary l‐arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J Anim Physiol Anim Nutr. 2014;98:1124–1131.
- Cao W, Xiao L, Liu G, et al. Dietary arginine and N-carbamylglutamate supplementation enhances the antioxidant statuses of the liver and plasma against oxidative stress in rats. Food Funct. 2016;7:2303–2311.
- Radak Z, Koltai E, Taylor AW, et al. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic Biol Med. 2013;58:87–97.
- Cadore E, Pinto R, Lhullier F, et al. Physiological effects of concurrent training in elderly men. Int J Sports Med. 2010;31:689–697.
- Knutzen KM, Brilla LR, Caine D. Validity of 1RM prediction equations for older adults. J Strength Condition Res. 1999;13:242–246.
- Asar Ö, İlk Ö. mmm: an R package for analyzing multivariate longitudinal data with multivariate marginal models. Comput Methods Prog Biomed. 2013;112:649–654.
- Mustelin L, Silventoinen K, Pietiläinen K, et al. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond). 2009;33:29–36.
- Guerra B, Guadalupe-Grau A, Fuentes T, et al. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: influence of glucose ingestion. Eur J Appl Physiol. 2010;109:731–743.
- Pucci B, Villanova L, Sansone L, et al. Sirtuins: the molecular basis of beneficial effects of physical activity. Intern Emerg Med. 2013;8:23–25.
- Tennen RI, Chua KF. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem Sci. 2011;36:39–46.
- Angelova P, Kamenov Z, Tsakova A, et al. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male. 2018;21:130–137.
- Aguiar AF, Balvedi MCW, Buzzachera CF, et al. l-Arginine supplementation does not enhance blood flow and muscle performance in healthy and physically active older women. Eur J Nutr. 2016;55:2053–2062.
- Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Culha MG, Atalay HA, Canat HL, et al. The relationship between erectile dysfunction severity, mean platelet volume and vitamin D levels. Aging Male. 2018;1–6. DOI:10.1080/13685538.2018.1459544
- Canguven O, Talib RA, El Ansari W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017;20:9–16.
- Yu X-H, Zhao J, Zhang S-C, et al. The impact of age, BMI and sex hormone on aging males’ symptoms and the international index of erectile function scores. Aging Male. 2017;20:235–240.
- Salman M, Yassin D-J, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.