Abstract
Impairment of antioxidant defense system and increase in metabolic rate and production of reactive oxygen species have been demonstrated in strenuous exercise. Both at rest and during contractile activity, skeletal muscle generates a very complex set of reactive nitrogen and oxygen species; the main generated are superoxide and nitric oxide. The nature of the contractile activity influences the pattern and the magnitude of this reactive oxygen and nitrogen species (ROS) generation. The intracellular pro-oxidant/antioxidant homeostasis undergoes alteration owing to strenuous exercise and the major identified sources of intracellular free radical generation during physical activity are the mitochondrial electron transport chain, polymorphoneutrophil, and xanthine oxidase. Reactive oxygen species increased tissue susceptibility to oxidative damage and pose a serious threat to the cellular antioxidant defense system. The possible dangerous consequences of the aging process and human wellness are emphasized in this review.
Introduction
Physical inactivity (lack of physical activity) leads to increased incidence of a variety of diseases and it can be regarded as one of the endpoints of the exercise-associated hormesis curve. It has been identified as the fourth leading risk factor for global mortality (6% of deaths globally). Moreover, physical inactivity is estimated to be the main cause for approximately 21–25% of breast and colon cancers, 27% of diabetes and approximately 30% of ischemic heart disease burden [Citation1,Citation2].
On the other hand, physical activity (PA) is defined as any bodily movement produced by skeletal muscles that require energy expenditure. The benefit of exercise in promoting good health and preventing various diseases is well known. The regular exercise, with moderate intensity and duration, has a wide range of beneficial effects reducing the risk of developing heart disease, stroke, high blood pressure, some cancers, type 2 diabetes and “thinning” of the bones, called osteoporosis [Citation3–7]. The regular physical activity also helps to control weight, body composition, and metabolic function [Citation8,Citation9] and may help to ease stress, decreasing the pro-inflammatory mediators. Based on the genetic predisposition, regular moderate physical exercise/activity provides systemic beneficial effects, including improved physiological function, decreased the incidence of disease and a higher quality of life. Single bouts of exercise and regular exercise decrease the oxidative challenge to the body. Furthermore, important emerging evidence has demonstrated the remarkable health benefits for cognition and well-being in persons that are regularly active: a recent study [Citation10,Citation11] described that PA reduced depression and anxiety. Recent systematic reviews highlighted a negative relationship between PA and the incidence of Alzheimer’s disease and dementia [Citation12,Citation13] or a positive effect of routine PA participation on indices of cognitive function in young to middle-aged adults [Citation14–16].
Contrariwise, strenuous exercise bouts, excessive exercise, and overtraining lead to negative consequences on muscle strength and integrity [Citation17] and to damaging oxidative stress. Thus, are an indication of the other endpoint of the hormetic response [Citation18–23]. Furthermore, the moderate exercise up-regulates the immune system, while strenuous exercise increases the risk of infection. Moreover, strenuous chronic exercise also represents a form of oxidative stress to the organisms and, therefore, can alter the balance between pro-oxidants and antioxidants [Citation24–26].
At the light of these studies, a controversy role is reserved to PA as generating of free radicals and consequently, as cancer promoter (physical inactivity or strenuous physical activity), or cancer protector (moderate physical activity).
The aim of this review is to analyze the state-of-the-art in this thematic and its relationship with aging, trying to clarify the major aspects of the PA role in the cellular antioxidant systems.
Generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS)
The ROS and RNS are free radicals with one unpaired electron derived from molecular oxygen or nitrogen. ROS and RNS are highly reactive and toxic to cellular components such as proteins, membrane lipids, and DNA, but they also act as signal transducers in Inflammatory Immune Response (IRI). ROS and RNS can be generated by nonenzymatic (ETC) and enzymatic (reduced form of nicotinamide adenine dinucleotide phosphate [NADPH] oxidase, xanthine oxidase, myeloperoxidase) pathways and have different origins during the different phases of reperfusion. It was originally thought that only phagocytic cells were responsible for ROS production as their part in host cell defense mechanisms. Recent work has demonstrated that ROS have a role in cell signaling, including apoptosis, gene expression, and the activation of cell signaling cascades [Citation27,Citation28].
The production of oxygen-based radicals is the bane of all aerobic species. These molecules, produced during the mitochondrial electron transport of aerobic respiration or by oxidoreductase enzymes and metal-catalyzed oxidation, have the potential to cause a number of deleterious events.
During PA there is an increase of ROS production by skeletal muscle. Moreover, a significant ROS production derives from other tissues such as heart, lungs, and white blood cells. In blood and in other cells, the excessive presence of ROS can cause lipid, DNA, and protein oxidation [Citation29,Citation30].
Some study reported that the increase in ROS production derives from the common metabolic changes that occur during exercise. For example, the catecholamines production contributes to increasing the ROS production [Citation31–33].
Nowadays, it is well described that the exercise generates muscle damages and inflammatory process; both these factors are important for radical production.
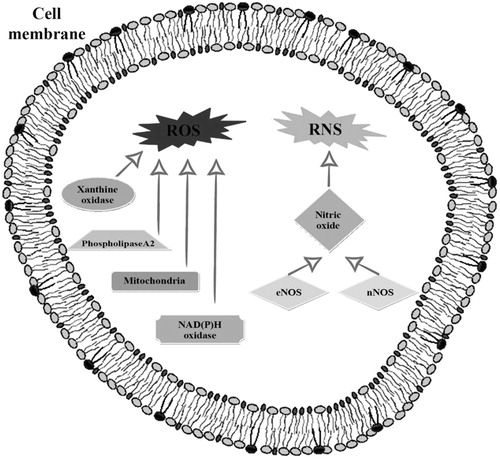
In the skeletal muscle cells, the ROS production derived from numerous systems. At mitochondrial level, about 2–5% of the total oxygen consumed may concur to intracellular ROS production. Therefore, the mitochondria are not the main production in this kind of cells [Citation30,Citation34,Citation35].
In the muscle cells other sites important in producing ROS, beyond mitochondria, are:
NAD(P)H oxidase enzymes associated with the sarcoplasmic reticulum which also release superoxide to the intracellular space [Citation36–38].
Phospholipase A, an enzyme that produces ROS; specifically, phospho-lipase A cleaves membrane phospholipids to release arachidonic acid which is a substrate for ROS-generating enzyme systems such as the lipoxygenases [Citation39–41]. Furthermore, the activation of phospholipase A can stimulate NAD(P)H oxidases [Citation42].
Xanthine oxidase, an enzyme that generates reactive oxygen species such as superoxide radicals and hydrogen peroxide when it catalyzes the oxidation of hypoxanthine to xanthine, and can further catalyze the oxidation of xanthine to uric acid [Citation42–44]. Its role is well explained in rat skeletal muscles that contain significant levels of xanthine oxidase [Citation45,Citation46], while human skeletal muscle cells possessing low amounts of xanthine dehydrogenase or oxidase [Citation47,Citation48] additional research needed to clarify their role in human muscles.
Analyzing the RNS category, one of the most important oxidants is nitric oxide produced by NOS. Skeletal muscle normally expresses neuronal NOS (nNOS) and endothelial NOS (eNOS). nNOS is strongly expressed in “white muscle fibers” (fast-twitch), while eNOS is localized in “red muscle fibers” (slow-twitch), that are rich in mitochondria [Citation49,Citation50]. Nitric oxide is generated continuously by skeletal muscles and this production is enhanced by contractions [Citation51,Citation52].
In summary, ROS and RNS production increases during exercise and these damaging molecules can be generated at various compartments within cells and by numerous organelles and enzymes, as shown in .
Cellular antioxidant defense systems
In consideration of numerous systems of the ROS/RNS production, the cells contain a network of enzymatic and non-enzymatic antioxidant defense mechanisms maintaining redox homeostasis in cells.
Enzymatic and non-enzymatic antioxidant systems are strategically located between blood, cytoplasm, and mitochondria, working together to regulate ROS and RNS.
The antioxidant enzymatic system includes superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT). Other antioxidant enzymes such as thioredoxin (TRX), glutaredoxin (GRX), and peroxiredoxin (PRX) also contribute to cellular protection against oxidation.
In addition to the enzymatic antioxidant system, there is the nonenzymatic system: for example, the glutathione (GSH) conducts an antioxidant role in a various manner [Citation53,Citation54]. Uric acid is another important non-enzymatic antioxidant: produced through purine metabolism is an important cell tool to destroy of peroxyl radicals, hydroxyl radicals, and singlet oxygen [Citation55,Citation56].
Finally, α-lipoic acid and bilirubin are two additional non-enzymatic antioxidants:
α-lipoic acid, frequently found in numerous foods, provide antioxidant effects by recycling vitamin C [Citation57,Citation58];
bilirubin, derived by heme metabolism, acts against peroxyl radicals and hydrogen peroxide [Citation59–61], playing an important antioxidant role.
The physical activity influence on antioxidant systems
The first suggestion that physical exercise results in free radical-mediated damage to tissues appeared in 1978, and the past three decades have resulted in a large growth of knowledge regarding exercise and oxidative stress.
Skeletal muscle has been shown to generate a complex set of reactive oxygen and nitrogen species (ROS) both at rest and during contractile activity. The primary ROS generated are superoxide and nitric oxide and the pattern and magnitude of their generation is influenced by the nature of the contractile activity. It is increasingly clear that the ROS generated by skeletal muscle play an important role in influencing redox-regulated processes that control, at least some of, the adaptive responses to contractile activity. These processes are also recognized to be modified during aging and in some disease states, providing the potential that interventions affecting ROS activity may influence muscle function or viability in these situations [Citation62].
Strenuous exercise increases oxygen consumption and causes disturbance of intracellular pro-oxidant–antioxidant homeostasis. The mitochondrial electron transport chain, polymorphoneutrophil, and xanthine oxidase have been identified as major sources of intracellular free radical generation during exercise [Citation63,Citation64].
Indeed, free radicals generated during or after exercise may come from several sources: (1) the mitochondria, from which oxygen radicals that have escaped scavenging enzymes present in the mitochondria may leak into the sarcoplasm; (2) the capillary endothelium, where a hypoxia or reoxygenation process is created during exercise; and (3) an oxidative burst from inflammatory cells mobilized as a result of muscle or tissue damage [Citation23,Citation64–68].
Reactive oxygen species pose a serious threat to the cellular antioxidant defense system, such as diminished reserve of antioxidant vitamins and glutathione, and increased tissue susceptibility to oxidative damage [Citation69,Citation70].
An acute bout of exercise at similar relative workload and duration enhanced muscle oxidant production in both young and old skeletal muscle, accompanied by increased oxidative stress [Citation71–73]. This evidence has extended the relevance of the free radical theory of aging to senescent skeletal muscles that are especially vulnerable to oxidative damage caused by strenuous exercise [Citation69,Citation74].
Both a systemic inflammatory response as well as DNA damage has been observed following exhaustive endurance exercise. Hypothetically, exercise-induced DNA damage might either be a consequence of inflammatory processes or causally involved in inflammation and immunological alterations after strenuous prolonged exercise [Citation67].
Exercise greatly increases the production of oxygen radicals in humans. In untrained persons, older men and women, and those with an inadequate antioxidant system, the increased rates of lipid peroxidation resulting from oxygen radical production may cause skeletal damage. The overwhelming consensus of the literature is that long- or short-term supplementation with vitamins E or C has no ergogenic effect on submaximal exercise performance, aerobic capacity, or muscle strength. However, the effects of these antioxidant vitamins may be subtle, and previous studies may not have examined appropriate endpoints. The protection against the generation of oxygen radicals and lipid peroxidation observed in untrained persons performing exercise and the enhanced acute-phase response to eccentric exercise observed in untrained older subjects indicate that vitamin E may be of some benefit in the adaptive response to exercise. In addition, the positive health benefits of using vitamins E and C may suggest an additive or synergistic effect when combined with regular exercise. Since the extent of oxidation is dependent on the exercise mode, intensity, and duration, and is specifically related to the degree of oxidant production, the programs of exercise should be very precise to avoid an induction of important damages due to physical activity [Citation75–77].
Oxidative balance in aging
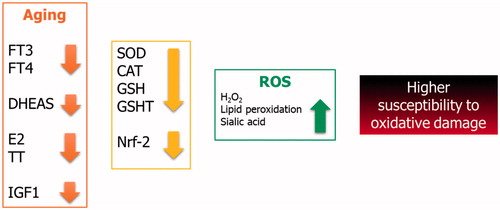
Older people have a different hormone profile than that of young people. Indeed, the former have lower serum concentrations of thyroid, adrenal and gonadal-derived hormones, and insulin like growth factor 1 (IGF1). Clinical and experimental evidences have shown that these hormones play a relevant role in the oxidant–antioxidant balance. Therefore, their decline throughout the life may expose aging people to oxidative damage.
Both free triiodothyronine (FT3) and, to a lesser extent, free thyroxine (FT4) decline throughout life [Citation77] and both of them influence the oxidant status [Citation78]. Indeed, SOD and CAT activities and GSH levels significantly decrease, whereas lipid peroxidation and sialic acid significantly increase in hypothyroid rats, suggesting a reduced antioxidant activity and the occurrence of a higher oxidative stress in hypothyroid animals [Citation79]. Accordingly, an increased production of ROS (by ∼42%) and a decreased GSH transferase activity (by ∼20%) have been described in post-thyroidectomy hypothyroid patients [Citation80]. Consistent with these findings, treatment with thyroxine decreases oxidative stress indexes in hypothyroid patients [Citation81]. Thus, these findings suggest that the decrease of thyroid hormones in aging people may contribute to a higher oxidative stress and predispose them to oxidative damage by dipping of enzymes with a scavenger activity.
Dehydroepiandrosterone sulfate (DHEAS) can alleviate oxidative stress through the ERK 1/2 and NF-kB signaling [Citation82]. It has been shown that DHEAS protects muscle cells from oxidative stress through the activation of the Nrf-2 pathway. In fact, it significantly decreases the loss of muscle cell death associated with H2O2-induced toxicity [Citation83]. Therefore, its decrease over the lifespan, probably due to the reduction of 17,20-lyase activity [Citation84], could be responsible for a higher susceptibility of muscle cells to oxidative stress in aging people.
Age-related hypogonadism negatively affect human health. In men, it impacts on physical performance, metabolic balance, cardiovascular function, and sexual sphere [Citation85–87]. Accordingly, testosterone replacement therapy has been shown to induce weight loss in obese hypogonadal men [Citation88]. Also, a worse metabolic profile influences gonadal status and sexuality [Citation89–94]. Hypogonadism has been shown to affect the oxidative balance. Indeed, estrogens seem to protect muscle cells from oxidative stress damage in mice [Citation95]. This protective effect of estrogens has also been shown in primary cultures of human muscle that are protected from lipid peroxidation through the AKT and p38-MAPK modulation [Citation96]. Furthermore, estrogen replacement therapy lowers oxidative stress indexes in Winstar rats [Citation97]. Similarly, castrated male rats showed higher levels of oxidative stress indexes [Citation98], thus leading to the hypothesis that hypotestosteronemia, often occurring in the aging male [Citation99,Citation100] may also increase oxidative stress. These findings suggest gonadal hormone decrease in menopause or in patients with the so called late-onset hypogonadism might lead to a higher susceptibility to the oxidative damage in aging people.
Finally, the decline of IGF1 serum levels with aging [Citation101] has been already shown to be responsible for the dysregulation of the nuclear factor (erythroid-derived 2-like) 2 (Nrf-2) dependent antioxidant response [Citation102]. In fact, Nrf-2 is known to orchestrate the cellular response to oxidative stress [Citation103]. IGF1-deficient mice show a decreased expression of Nrf-2 and its target genes and an increased oxidative stress (leading to vascular damage) compared to control animals [Citation102]. Endothelial apoptosis induced by oxidative stress is inhibited by IGF1 receptor (IGF1R) expression. Moreover, oxidative stress is able to decrease IGF1R expression in the endothelial tissue [Citation104], thus leading to endothelial dysfunction. Therefore, it may be hypothesized that a higher tissues susceptibility to oxidative stress may be related to the lower IGF1 serum levels in aging people.
In summary, the hormonal profile of aging people may contribute to increase the oxidative stress which the whole body and the muscle tissue are exposed to (). Therefore, older men and women should take care in undertaking all the activities leading to a high oxidative stress production, including high-intensity or prolonged exercise.
Conclusions
Even if a large number of studies analyzed the effects of PA on antioxidant systems, to date, no unambiguous theory was achieved. The scientific data are frequently in contrast, on the one hand, the PA was seen as a protector factor with an antioxidant capacity, and on the other hand, it was seen with negative effects on oxidant production. Mild or moderate exercise could determine modest elevations in ROS within skeletal muscle [Citation105,Citation106]. Furthermore, it could have a positive action on antioxidant systems.
In contrast, during high‐intensity or prolonged exercise, ROS accumulate and/or the antioxidant defense system may not be able to buffer the excessive exercise‐induced ROS, resulting in redox imbalance which has been shown to cause impair skeletal muscle contributing to peripheral fatigue [Citation107–109].
In conclusion, the muscle exercise can not only be positive, but also very negative for human wellness and aging processes. Aging people show a greater susceptibility to oxidative stress damage, in part due to the decline of their hormonal asset. The metabolic damages exerted by strenuous physical activity should more precisely be emphasized by the experts of muscle physiology. The identification of the optimal amount of physical activity remains one of the most important research fields of the scientific community.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210.
- Brown WJ, Pavey T, Bauman AE. Comparing population attributable risks for heart disease across the adult lifespan in women. Br J Sports Med. 2015;49:1069–1076.
- Warburton DE, Bredin SS. Reflections on physical activity and health: What should we recommend? Can J Cardiol. 2016;32:495–504.
- Warburton DE, Nicol C, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809.
- Warburton DE, Nicol C, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961–974.
- Warburton DER, Taunton J, Bredin SSD, et al. The risk-benefit paradox of exercise. BC Medical Assoc J. 2016;58:210–218.
- Warburton DE, Charlesworth S, Ivey A, et al. A systematic review of the evidence for Canada’s physical activity guidelines for adults. Int J Behav Nutr Phys Act. 2010;7:39.
- Deibert P, Solleder F, König D, et al. Soy protein based supplementation supports metabolic effects of resistance training in previously untrained middle aged males. Aging Male. 2011;14:273–279.
- Sealey RM, Twomey J, Pringle FA, et al. A 12-week lifestyle intervention for middle-aged, overweight men who are supporters of local sporting clubs. Aging Male. 2013;16:118–122.
- Rebar A, Stanton R, Geard D, et al. A metameta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9:366–378.
- Bertozzi G, Sessa F, Albano GD, et al. The role of anabolic androgenic steroids in disruption of the physiological function in discrete areas of the central nervous system. Mol Neurobiol. 2018;55:5548–5556.
- Reiner M, Niermann C, Jekauc D, et al. Long-term health benefits of physical activity – a systematic review of longitudinal studies. BMC Public Health. 2013;13:209.
- Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: A systematic review related to Canada’s physical activity guidelines. Int J Behav Nutr Phys Act. 2010;7:38.
- Cox EP, O’Dwyer N, Cook R, et al. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: a systematic review. J Sci Med Sport. 2016;19:616–628.
- Bramanti V, Grasso S, Tibullo D, et al. Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J Neurosci Res. 2016;94:90–98.
- Petito A, Altamura M, Iuso S, et al. The relationship between personality traits, the 5HTT polymorphisms, and the occurrence of anxiety and depressive symptoms in elite athletes. PLoS One. 2016;11:e0156601.
- Sousa LE, Figueiredo AA, Netto JMB. Correlation between pelvic floor strength and physical activity level in healthy men. Aging Male. 2018;21:1–5.
- Chieffi S, Iachini T, Iavarone A, et al. Flanker interference effects in a line bisection task. Exp Brain Res. 2014;232:1327–1334.
- Chieffi S, Iavarone A, La Marra M, et al. Vulnerability to distraction in schizophrenia. J Psychiatry. 2015;18:228.
- Di Bernardo G, Messina G, Capasso S, et al. Sera of overweight people promote in vitro adipocyte differentiation of bone marrow stromal cells. Stem Cell Res Ther. 2014;5:4.
- Lapi D, Vagnani S, Cardaci E, et al. Rat pial microvascular responses to melatonin during bilateral common carotid artery occlusion and reperfusion. J Pineal Res. 2011;51:136–144.
- Messina A, De Fusco C, Monda V, et al. Role of the orexin system on the hypothalamus-pituitary-thyroid axis. Front Neural Circ. 2016;10:66.
- Messina G, Viggiano A, Tafuri D, et al. Role of orexin in obese patients in the intensive care unit. J Anesth Clin Res. 2014;5:395.
- Chieffi S, Iavarone A, Iaccarino L, et al. Age-related differences in distractor interference on line bisection. Exp Brain Res. 2014;232:3659–3664.
- Messina G, Zannella C, Monda V, et al. The beneficial effects of coffee in human nutrition. Biol Med. 2015;7:240.
- Marra ML, Valenzano A, Ruberto M, et al. The effects of overweight and obesity on cognitive functions and psychological well-being. Acta Med Mediterr. 2017;33:1225–1231.
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29:345–350.
- He L, He T, Farrar S, et al. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553.
- Avola R, Di Tullio MA, Fisichella A, et al. Glial fibrillary acidic protein and vimentin expression is regulated by glucocorticoids and neurotrophic factors in primary rat astroglial cultures. Clin Exp Hypertens. 2004;26:323–333.
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276.
- Le Moal E, Pialoux V, Juban G, et al. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27:276–310.
- Cobley JN, Close GL, Bailey DM, et al. Exercise redox biochemistry: conceptual, methodological and technical recommendations. Redox Biol. 2017;12:540–548.
- Vidal K, Robinson N, Ives SJ. Exercise performance and physiological responses: the potential role of redox imbalance. Physiol Rep. 2017;5:e13225.
- Powers SK, Duarte J, Kavazis AN, et al. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9.
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241.
- Zima AV, Copello JA, Blatter LA. Effects of cytosolic NADH/NAD + levels on sarcoplasmic reticulum Ca2+ release in permeabilized rat ventricular myocytes. J Physiol. 2004;555:727–741.
- Scarlett D-JG, Herst PM, Berridge MV. Multiple proteins with single activities or a single protein with multiple activities: the conundrum of cell surface NADH oxidoreductases. Biochim Biophys Acta. 2005;1708:108–119.
- Sakellariou GK, Vasilaki A, Palomero J, et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013;18:603–621.
- Zuo L, Christofi FL, Wright VP, et al. Lipoxygenase-dependent superoxide release in skeletal muscle. J Appl Physiol. 2004;97:661–668.
- Angelova PR, Abramov AY. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. 2016;100:81–85.
- Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, et al. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta - General Subjects. 2013;1830:4650–4659.
- Gong MC, Arbogast S, Guo Z, et al. Calcium-independent phospholipase A2 modulates cytosolic oxidant activity and contractile function in murine skeletal muscle cells. J Appl Physiol. 2006;100:399–405.
- Battelli MG, Polito L, Bortolotti M, et al. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev. 2016;2016:3527579.
- Gomez-Cabrera MC, Close GL, Kayani A, et al. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol Endocrinol Metab. 2010;298:R2–R8.
- Judge AR, Dodd SL. Oxidative damage to skeletal muscle following an acute bout of contractile claudication. Atherosclerosis. 2003;171:219–224.
- Judge AR, Dodd SL. Xanthine oxidase and activated neutrophils cause oxidative damage to skeletal muscle after contractile claudication. Am J Physiol Heart Circ Physiol. 2004;286:H252–H256.
- Atalay M, Seene T, Hänninen O, et al. Skeletal muscle and heart antioxidant defences in response to sprint training. Acta Physiol Scand. 1996;158:129–134.
- Hellsten Y, Apple FS, Sjödin B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Applied Physiology. 1996;81:1484–1487.
- Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, et al. Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem Biophys Res Commun. 2013;435:501–505.
- Holloway TM, Bloemberg D, Da Silva ML, et al. High-intensity interval and endurance training are associated with divergent skeletal muscle adaptations in a rodent model of hypertension. Am J Physiology - Regulatory Integrative and Comparative Physiology. 2015;308:R927–R934.
- Hellsten Y, Svensson M, Sjödin B, et al. Allantoin formation and urate and glutathione exchange in human muscle during submaximal exercise. Free Radic Biol Med. 2001;31:1313–1322.
- Bramanti V, Tomassoni D, Grasso S, et al. Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res. 2012;37:2795–2804.
- Seminotti B, Amaral AU, Ribeiro RT, et al. Oxidative stress, disrupted energy metabolism, and altered signaling pathways in glutaryl-CoA dehydrogenase knockout mice: potential implications of quinolinic acid toxicity in the neuropathology of glutaric acidemia Type I. Mol Neurobiol. 2016;53:6459–6475.
- Lee C-T, Yu L-E, Wang J-Y. Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons. Nitric Oxide Biol Chem. 2016;54:38–50.
- Giallongo C, Tibullo D, La Cava P, et al. BRIT1/MCPH1 expression in chronic myeloid leukemia and its regulation of the G2/M checkpoint. Acta Haematol. 2011;126:205–210.
- Tasaki E, Sakurai H, Nitao M, et al. Uric acid, an important antioxidant contributing to survival in termites. PLoS One. 2017;12:e0179426.
- Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014;6:80.
- Padmalayam I, Hasham S, Saxena U, et al. Lipoic acid: synthase (LASY): a novel role in inflammation, mitochondrial function, and insulin resistance. Diabetes. 2009;58:600–608.
- Barañano DE, Rao M, Ferris CD, et al. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098.
- Bjelakovic G, Stojanovic I, Jevtovic-Stoimenov T, et al. Polyamine oxidase activity in peripheral blood of newborn infants with neonatal hyperbilirubinemia: Is bilirubin an antioxidant? J Basic Clin Physiol Pharmacol. 2008;19:103–118.
- Shcherbinina MB. Low blood bilirubin level: possible diagnostic and prognostic importance. Klinicheskaia Meditsina. 2007;85:10–14.
- Messina G, Monda V, Moscatelli F, et al. Role of orexin system in obesity. Biol Med. 2015;7:248.
- Messina G, Palmieri F, Monda V, et al. Exercise causes muscle GLUT4 translocation in an insulin-independent manner. Biol Med. 2015;S3:007. doi:10.4172/0974-8369.1000s3007
- Bonaventure A, Harewood R, Stiller CA, CONCORD Working Group, et al. Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4:e202–e217.
- Lapi D, Vagnani S, Pignataro G, et al. Rat pial microvascular responses to transient bilateral common carotid artery occlusion and reperfusion: quercetin’s mechanism of action. Front Physiol. 2012;3:99.
- Lapi F, Cipriani F, Caputi AP, et al. Assessing the risk of osteonecrosis of the jaw due to bisphosphonate therapy in the secondary prevention of osteoporotic fractures. Osteoporos Int. 2013;24:697–705.
- Messina G, Valenzano A, Moscatelli F, et al. Effects of emotional stress on neuroendocrine and autonomic functions in skydiving. J Psychiatry. 2015;18:280–282.
- Rinaldi B, Guida F, Furiano A, et al. Effect of prolonged moderate exercise on the changes of nonneuronal cells in early myocardial infarction. Neural Plasticity. 2015;2015:265967.
- Messina G, De Luca V, Viggiano A, et al. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013;2013:639280.
- Triggiani AI, Valenzano A, Ciliberti MAP, et al. Heart rate variability is reduced in underweight and overweight healthy adult women. Clin Physiol Funct Imag. 2017;37:162–167.
- Messina G, Di Bernardo G, Viggiano A, et al. Exercise increases the level of plasma orexin A in humans. J Basic Clin Physiol Pharmacol. 2016;27:611–616.
- Messina G, Vicidomini C, Viggiano A, et al. Enhanced parasympathetic activity of sportive women is paradoxically associated to enhanced resting energy expenditure. Autonomic Neurosci. 2012;169:102–106.
- Willems SM, Wright DJ, Day FR, et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat Comms. 2017;8:16015.
- Monda M, Messina G, Scognamiglio I, et al. Short-term diet and moderate exercise in young overweight men modulate cardiocyte and hepatocarcinoma survival by oxidative stress. Oxid Med Cell Longev. 2014;2014:131024.
- Valenzano A, Moscatelli F, Triggiani AI, et al. Heart-rate changes after an ultraendurance swim from italy to albania: a case report. Int J Sports Physiol Perform. 2016;11:407–409.
- Viggiano A, Chieffi S, Tafuri D, et al. Laterality of a second player position affects lateral deviation of basketball shooting. J Sports Sci. 2014;32:46–52.
- Strich D, Karavani G, Edri S, et al. FT3 is higher in males than in females and decreases over the lifespan. Endocr Pract. 2017;23:803–807.
- Ramli NSF, Mat Junit S, Leong NK, et al. Analyses of antioxidant status and nucleotide alterations in genes encoding antioxidant enzymes in patients with benign and malignant thyroid disorders. Peer J. 2017;5:e3365.
- Oktay S, Uslu L, Emekli N. Effects of altered thyroid states on oxidative stress parameters in rats. J Basic Clin Physiol Pharmacol. 2017;28:159–165.
- Baldissarelli J, Pillat MM, Schmatz R, et al. Post-thyroidectomy hypothyroidism increases the expression and activity of ectonucleotidases in platelets: possible involvement of reactive oxygen species. Platelets. 2017;1–10. doi:10.1080/09537104.2017.1361017
- Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674–678.
- Cheng AJ, Yamada T, Rassier DE, et al. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol. 2016;594:5149–5160.
- Jeon S, Hur J, Kim J. DHEA alleviates oxidative stress of muscle cells via activation of Nrf2 pathway. Appl Biochem Biotechnol. 2015;176:22–32.
- Baulieu EE. Androgens and aging men. Mol Cell Endocrinol. 2002;198:41–49.
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;30:1–12.
- Rezanezhad B, Borgquist R, Willenheimer R, et al. Association between serum levels of testosterone and biomarkers of subclinical atherosclerosis. Aging Male. 2017;22:1–5.
- Maggio M, Ceda GP, Lauretani F, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–47.
- Salman M, Yassin DJ, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20:45–48.
- Angelova P, Kamenov Z, Tsakova A, et al. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male. 2018;21:130–137.
- Dursun M, Besiroglu H, Cakir SS, et al. Increased visceral adiposity index associated with sexual dysfunction in men. Aging Male. 2017;22:1–6.
- Samipoor F, Pakseresht S, Rezasoltani P, et al. The association between hypogonadism symptoms with serum testosterone, FSH and LH in men. Aging Male. 2018;21:1–8.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Yu XH, Zhao J, Zhang SC, et al. The impact of age, BMI and sex hormone on aging males' symptoms and the international index of erectile function scores. Aging Male. 2017;20:235–240.
- Samipoor F, Pakseresht S, Rezasoltani P, et al. Awareness and experience of andropause symptoms in men referring to health centers: a cross-sectional study in Iran. Aging Male. 2017;20:153–160.
- Michelucci A, Boncompagni S, Canato M, et al. Estrogens protect calsequestrin-1 knockout mice from lethal hyperthermic episodes by reducing oxidative stress in muscle. Oxid Med Cell Longev. 2017;2017:6936897.
- Surico D, Ercoli A, Farruggio S, et al. Modulation of oxidative stress by 17 β-estradiol and genistein in human hepatic cell lines in vitro. Cell Physiol Biochem. 2017;42:1051–1062.
- Escalante CG, Mora SQ, Bolaños LN. Hormone replacement therapy reduces lipid oxidation directly at the arterial wall: a possible link to estrogens' cardioprotective effect through atherosclerosis prevention. J Mid-Life Health. 2017;8:11–16.
- Kataoka T, Hotta Y, Maeda Y, et al. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14:1540–1548.
- Golan R, Scovell JM, Ramasamy R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. Aging Male. 2015;18:201–204.
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12:5–12.
- D'Costa AP, Ingram RL, Lenham JE, et al. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil Suppl. 1993;46:87–98.
- Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329.
- Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Nat Acad Sci. 2008;105:2325–2330.
- Kavurma MM, Figg N, Bennett MR, et al. Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J. 2007;407:79–87.
- Powers SK, Ji LL, Kavazis AN, et al. Reactive oxygen species: impact on skeletal muscle. Comprehens Physiol. 2011;1:941–969.
- Chen J, Xu L, Huang C. DHEA inhibits vascular remodeling following arterial injury: a possible role in suppression of inflammation and oxidative stress derived from vascular smooth muscle cells. Mol Cell Biochem. 2014;388:75–84.
- Reid MB. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic Biol Med. 2008;44:169–179.
- Salomone F, Li Volti G, Vitaglione P, et al. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Translational Res. 2014;163:593–602.
- Reid MB. Redox interventions to increase exercise performance. J Physiol (Lond). 2016;594:5125–5133.