Abstract
Objective
To evaluate the impacts of statin treatment on the risk of benign prostatic enlargement (BPE) progression in hyperlipidemia patients.
Methods
Newly diagnosed hyperlipidemia patients (n = 7961), identified from Taiwan’s National Health Insurance Research Database, were divided into four statin cohorts (statin use >365 days, n = 1604; statin use 181–365 days, n = 813; statin use 91–180 days, n = 739; and statin use 31–90 days, n = 713) and one control cohort (cohort that used no statins, n = 4092). Study endpoint was occurrence of BPE progression (BPE diagnosis plus receiving BPE-related medications or surgery). Relative risks of BPE progression in the statin cohorts compared to the control cohort were analyzed.
Results
Multivariable Cox proportional hazards regression analyses demonstrated that BPE progression risk in the cohort used statins for >365 days was significantly lower than the control cohort (adjusted hazard ratio: 0.70, 95% confidence interval: 0.58 ∼ 0.85, p < .001). However, BPE progression risks of the other three statin cohorts did not significantly differ from the control cohort. Trend analysis revealed that the effects of statin treatment on decreasing BPE progression risk were significantly related to statin treatment duration (p = .001).
Conclusions
Hyperlipidemia patients with long-term statin treatment (more than 365 days) are associated with a reduced risk of BPE progression.
Introduction
Benign prostatic enlargement (BPE) is common in aging males, and the prevalence of BPE with lower urinary tract symptoms (LUTSs) ranges 50%–75% in males older than 50 years of age [Citation1]. Previous studies indicated that BPE is a progressive condition [Citation2,Citation3]. The BPE progression may result in prostate size enlargement and worsening of LUTSs (especially acute urine retention) [Citation4]. Patients with BPE progression may need to receive BPE-related medications and/or surgery [Citation5]. Preventing the occurrence of BPE progression is important for patients with BPE.
The definitive pathogenesis of BPE progression remains to be determined. Nevertheless, existing evidence supports the concept that the crucial factors in mediating BPE progression may involve genetic, homeostatic, hormonal, inflammatory, and metabolic issues. For instance, patients with BPE progression are noted to have alterations in genetic expression of insulin receptor, insulin-like growth factor, and insulin-like growth factor-binding protein-3 [Citation6]. Loss of prostatic glandular homeostasis between cell renewal and apoptosis, loss of hormonal balance between estrogen and androgen, and alteration in prostatic blood circulation have also been suggested to contribute to the development of BPE progression [Citation7–9].
Of note, inflammation and metabolic factors (e.g. hyperlipidemia) have also been shown to be crucial factors in mediating BPE progression [Citation6–16]. For instance, prostatic inflammation was noted to be associated with prostate enlargement [Citation10]. Dysregulation of the immune response in prostate tissues with T-cell activation and cytokine upregulation was shown to induce prostate growth [Citation17]. In addition, hyperlipidemia can cause prostatic inflammation and was proposed to be an important factor in BPE progression [Citation18]. This concept was confirmed by our recent population-based cohort study, as our data demonstrated that hyperlipidemia patients were associated with a higher risk of BPE progression compared to nonhyperlipidemic patients [Citation19].
As mentioned above, hormone, inflammation, and hyperlipidemia are crucial factors in BPE progression. In line with this notion, it is thus likely that agents with hormone manipulation, lipid-lowering, or anti-inflammatory effects may have the potential to reduce the risk of BPE progression. Statins are potent inhibitors of cholesterol biosynthesis and are widely used in clinical settings as lipid-lowering drugs [Citation20]. Statins also possess testosterone-lowering and potent anti-inflammatory effects [Citation20,Citation21]. Based on these characteristics, we speculated that statins may have the potential to reduce the risk of BPE progression in hyperlipidemia patients. This concept was partially confirmed by previous data. For instance, in a population-based cohort study, statin use was noted to be associated with a reduced risk of BPE and LUTSs [Citation22]. Data from a randomized prospective study with a total of 124 patients demonstrated that statin use could reduce the prostatic volume and improve LUTSs in patients with metabolic syndrome [Citation23]. However, large-scale data in this regard have not been evaluated to date. To further elucidate this issue, we conducted this population-based longitudinal cohort study with the hypothesis that statin use could reduce the risk of BPE progression in hyperlipidemia patients.
Materials
Ethics
This study complies with the Personal Information Protection Act in Taiwan and was approved by the Institutional Review Board of Taipei Tzu Chi Hospital (protocol no.: 06-W02-018). Because the National Health Insurance (NHI) Research Database (NHIRD) consists of anonymous data, no patient consent is acquired.
Database
This study was conducted using data from the NHIRD, an insurance claims database released by the National Health Research Institutes for research purposes in Taiwan. The NHIRD is a database containing comprehensive longitudinal computerized medical records of all people in the NHI program. The NHI program covers approximately 99% of the Taiwanese population (approximate 23.5 million). Data on one million randomly selected individuals from all beneficiaries of the NHIRD were used to create the Longitudinal Health Insurance Database 2010 (LHID2010). There were no significant differences in the age, sex, or average insured payroll-related amount between individuals in the NHIRD and those in the LHID2010. LHID2010 contains patient demographics, diagnostic data, medical services received, and drug codes. The diagnostic accuracy of the NHI claims data with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes has been validated [Citation24]. This study employed the ICD-9-CM codes to identify subject’s medical diagnosis (). This study also employed the anatomical therapeutic chemical (ATC) classification system codes and the Taiwan NHI reimbursement procedure codes to identify subject’s exposure to medications and procedures, respectively ().
Table 1. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, the Taiwan National Health Insurance reimbursement procedure codes, and the anatomical therapeutic chemical (ATC) classification system codes used in the study.
Study design and study endpoint
Hyperlipidemia patients were identified with at least two separate in- or outpatient medical records between January 2000 and December 2012, indicating a new diagnosis of hyperlipidemia. Hyperlipidemia patients with statin use were identified with the ATC classification system code. Physicians in Taiwan adhere to the diagnostic criteria of hyperlipidemia and prescribing guidelines for statins provided by the NHI Administration, Ministry of Health and Welfare, Taiwan [Citation25].
Hyperlipidemia patients were divided into four statin cohorts (i.e. statin use for >365 days, for 181–365 days, for 91–180 days, and for 31–90 days) and one control cohort (i.e. cohort that used no statins). The first prescription date for statins was used as the index date for hyperlipidemia patients with statin use (i.e. the statin cohorts). The statin prescription duration after the index date was defined as the statin treatment duration. The date of the hyperlipidemia diagnosis was used as the index date for hyperlipidemia patients who did not use statins (i.e. the control cohort). The study endpoint was the occurrence of BPE progression (i.e. a BPE diagnosis plus use of alpha-blockers or 5-alpha reductase inhibitors [5ARIs] or receiving transurethral resection of the prostate [TURP]).
Patients of a female gender, who were aged younger than 40 years or older than 100 years, had a diagnosis of hyperlipidemia on only one occasion, or had a diagnosis of BPE before the diagnosis of hyperlipidemia or a diagnosis of prostate cancer, were excluded. Patients who received statins before the diagnosis of hyperlipidemia received statins during admission or received statins for less than 31 days were also excluded. The confounders considered in this study were identified within 1 year before the index date. Comorbidities related to BPE progression considered in this study were diabetes mellitus (DM) [Citation26], hypertension [Citation27], coronary heart disease (CHD) [Citation28], obesity [Citation29], and liver cirrhosis [Citation30]. Medications related to BPE progression were nonsteroidal anti-inflammatory drugs (NSAIDs) [Citation31], metformin [Citation32], aspirin [Citation31], and lipid-lowering drugs except statins [Citation33]. Potential surveillance bias can occur due to the frequency of health checkups among the five cohorts, and so urologist visits were recorded and included as a confounding factor.
Statistical analysis
The SPSS statistical package (version 21.0, SPSS, IBM Corporation, Somers, NY) was used to analyze all data. Continuous data were presented as mean ± standard deviation. Differences among the five cohorts were measured by the chi-squared test or the Kruskal–Wallis H-test. A p value of < .05 was considered statistically significant. The number needed to treat (NNT) was calculated according to previously published model [Citation34]. The relative risks of having BPE progression in the statin cohorts compared to the control cohort were estimated by Cox proportional hazard regressions. The trend analysis regarding the effects of duration of statin use on the risk of having BPE progression was also performed using Cox proportional hazards regression. Multivariable Cox models were used to adjust for possible confounders that might influence the risk of BPE progression. In addition, a multiple logistic regression was conducted to estimate a propensity score, in which statin use was the dependent variable and confounders were independent variables. The propensity score was further adjusted in the Cox regression analyses.
Results
Characteristics of study subjects
In total, 7961 patients with newly diagnosed hyperlipidemia were identified. As mentioned above, hyperlipidemia patients were divided into four statin cohorts (i.e. with statins use for >365 days, n = 1604; for 181–365 days, n = 813; for 91–180 days, n = 739; and for 31–90 days, n = 713) and the control cohort (n = 4092) (). The five cohorts significantly differed in age (p < .001; ). The five cohorts also significantly differed in the incidences of DM, hypertension, CHD, metformin use, aspirin use, other lipid-lowering drug use, and urologist visits (all p < .05; ). In contrast, the incidences of obesity, liver cirrhosis, and NSAID use did not significantly differ among the five cohorts ().
Figure 1. Flowchart of the study design. LHID: Longitudinal Health Insurance Database; BPE: benign prostate enlargement.
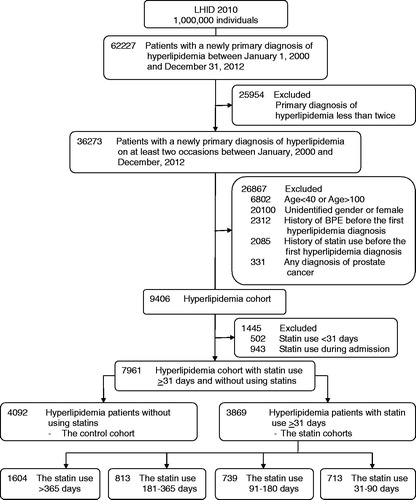
Table 2. Distribution of sample characteristics according to the duration of statin use in patients with hyperlipidemia.
Risk of BPE progression in hyperlipidemia patients
The incidences of BPE progression in the cohort with statin use for >365 days (8.3%), the cohort with statin use for 181–365 days (8.6%), the cohort with statin use for 91–180 days (7.7%), the cohort with statin use for 31–90 days (7.2%), and the control cohort (11.3%) differed significantly (p < .001). In addition, the mean time for the occurrence of BPE progression in the cohort with statin use for >365 days (4.67 ± 3.00 years), the cohort with statin use for 181–365 days (3.30 ± 2.38 years), the cohort with statin use for 91–180 days (3.04 ± 2.56 years), the cohort with statin use for 31–90 days (2.32 ± 2.44 years), and the control cohort (3.83 ± 3.00 years) also significantly differed (p < .001).
Our data further revealed that the risk of having BPE progression in the cohort with statin use for >365 days was significantly lower than the control cohort (hazard ratio [HR] = 0.70, 95% confidence interval [CI]: 0.58–0.85, p < .001; ). The risk of having BPE progression in the cohort with statin use for >365 days remained significantly lower than the control cohort after adjusting for potential confounders (adjusted HR =0.66, 95%CI: 0.54–0.8, p < .001; ) and propensity score (adjusted HR =0.68, 95%CI: 0.56–0.82, p < .001; ). Comparing to the control cohort, the NNT for preventing one BPE progression in the cohort with statin use for >365 days was 32.82. To test the robustness of our results, we further stratified our analyses according to the baseline confounders. The results indicated that, compared with the control cohort, the cohort with statin use >365 days was associated with a lower risk of BPE progression in all subgroups stratified by confounding factors (). As the case number of obesity and liver cirrhosis was small, we thus did not include these two factors into stratified analysis.
Figure 2. The risk of having benign prostate hyperplasia progression in the cohort with statin use >365 days compared to the cohort that used no statins stratified by confounders. DM: diabetes mellitus; CHD: coronary heart disease; NSAIDs: nonsteroidal anti-inflammatory drugs; HR: hazard ratio; CI: confidence interval.
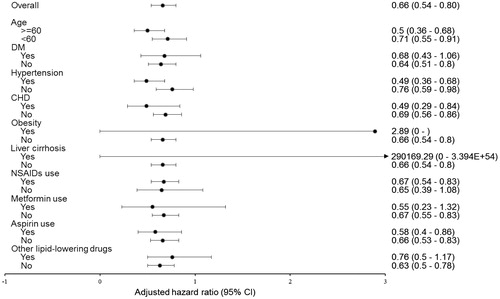
Table 3. Statin use duration in predicting benign prostate enlargement progression in hyperlipidemia patients.
Of note, the risks of having BPE progression in the cohorts with statin use for 181–365 days, with statin use for 91–180 days, and with statin use for 31–90 days did not significantly differ from the control cohort (). In addition, the trend analysis revealed that the effects of statin use on decreasing the risk of BPE progression in the study cohorts were significantly related to the duration of statin use (p = .001; ). The trends remained the same after adjusting for potential confounders (p < .001; ) and propensity score (p < .001; ).
Discussion
Inflammatory, metabolic, and hormonal factors are important factors for BPE progression [Citation6,Citation8,Citation12,Citation13,Citation16,Citation17]. Hyperlipidemia induces prostatic inflammation, so it is reasonable to observe an association between hyperlipidemia and BPE progression [Citation19,Citation33]. As statins possess potent lipid-lowering and anti-inflammation capacities, we thus hypothesized that statin use might decrease the risk of BPE progression in hyperlipidemia patients. Findings from this study confirmed our hypothesis and demonstrated for the first time that hyperlipidemia patients treated with statins for more than 365 days had a 30%–34% decrease in the risk of experiencing BPE progression (i.e. receiving BPE-related medications or TURP) compared to those hyperlipidemia patients who used no statins.
As mentioned above, several factors can influence BPE progression. For instance, previous reports indicated that patients of an advanced age [Citation28], and with DM [Citation26], hypertension [Citation27], CHD [Citation28], and/or obesity [Citation35] are at an increased risk of BPE progression. Liver cirrhosis patients, on the contrary, were reported to have a lower rate of BPE compared to the general population [Citation30]. In addition, NSAIDs [Citation31], aspirin [Citation31], metformin [Citation32], and lipid-lowering drugs [Citation33] were reported to decrease the risk of prostate enlargement. Data from this study revealed that some of these confounders significantly differed in these five cohorts. Possible impacts of these confounding factors were thus controlled for using a multivariable Cox proportional hazard regression model and propensity score analysis in this study. After controlling for these potential confounders, our data revealed the same trend and demonstrated a reduced risk of having BPE progression in hyperlipidemia patients treated with statins for more than 365 days. The NNT in association with one patient free of BPE progression was 32.82 for patients used statins more than 365 days. Our data also demonstrated that the protective effect of statins was related to the treatment duration. Collectively, these data highlight the beneficial potential of long-term statin use (more than 365 days) on decreasing the risk of BPE progression in hyperlipidemia patients.
Data from this study revealed that more than half of the included hyperlipidemia subjects (52.2%) did not receive statin treatment. In addition, among statin-treated hyperlipidemia patients, only 39.9% of them received long-term statin treatment (>365 days). The reason why physicians did not prescribe statins or did prescribe statins but for only a short period of time (e.g. less than 365 days) for the included hyperlipidemia patients remains to be elucidated. Of note, findings from this study demonstrate that the protective effect of statins is related to the treatment duration. Data from previous reports seem to support this notion [Citation36,Citation37]. Previous data demonstrated that the therapeutic effects of statins on reducing the risk of myocardial infarction and stroke in hyperlipidemia patients were only seen in those who received statin treatment for a treatment duration of at least 1 year [Citation36,Citation37]. As both the formation of atherosclerotic lesions and BPE progression in hyperlipidemia patients were associated with chronic inflammation [Citation33], it is thus reasonable to observe that the beneficial effects of statins were only seen in those hyperlipidemia patients with long-term statin use (e.g. >365 days).
In addition to the treatment duration, treatment adherence also affects the therapeutic effects of treatment. For instance, 5ARIs are effective drugs for decreasing the risk of urine retention and receiving a BPE-related operation in patients with a large prostate [Citation38,Citation39]. Persistent use of 5ARIs is a key factor in preventing BPE progression, as an increased prostatic volume and exacerbation of voiding symptoms will occur if treatment with 5ARIs is discontinued [Citation40,Citation41]. One previous population-based study reported that the incidence of patients with good statin adherence was approximately 40.8% in Taiwan [Citation42]. This study did not investigate statin adherence. However, judging from our data that hyperlipidemia patients who received long-term statin treatment (i.e. >1 year) accounted for approximately 39.9%, these data seem to suggest that these patients may have better statin adherence than those who received statin treatment for less than 1 year. If so, then it is reasonable to observe beneficial effects of long-term statin treatment on reducing the risk of BPE progression in hyperlipidemia patients.
Although this study shows the beneficial effects of long-term statin use on reducing BPE progression in hyperlipidemia patients, there are some important limitations of our study. First, nutrition and dietary factors are important for prostatic disease progression [Citation43–45], information about personal behaviors (e.g. alcohol consumption, smoking, dietary habits, and physical exercise), lifestyle, and family history were lacking in the NHIRD. Second, direct evidence to delineate a cause–effect relationship between statin use and BPE progression is still lacking. Large-scale prospective randomized clinical studies are required before further conclusions can be drawn. Third, different types of statins may have different effects on preventing BPE progression. However, the individual effects of different types of statins were not analyzed in this study. Fourth, detailed information about clinical symptoms, the International Prostate Symptom Score, prostate-specific antigen level, and image findings are important indicators for TURP [Citation46]. These indicators are unavailable in the NHIRD. Fifth, adverse events may occur in some patients undergoing statin therapy. A risk–benefit assessment is suggested if further interpretation of our data is intended.
Conclusions
Data from this population-based longitudinal cohort study show the potential of long-term statin use (more than 365 days) to reduce the risk of BPE progression in hyperlipidemia patients. The protective effect of statins is related to the treatment duration.
Acknowledgements
Part of the study results had been presented at the 33rd European Association of Urology Annual Congress, March 16–20 2018, Copenhagen, Denmark.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43:289–297.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol. 1999;162:1301–1306.
- Meigs JB, Barry MJ, Giovannucci E, et al. Incidence rates and risk factors for acute urinary retention: the health professionals followup study. J Urol. 1999;162:376–382.
- Fitzpatrick JM. The natural history of benign prostatic hyperplasia. BJU Int. 2006;97:3–6.
- Emberton M, Marberger M, de la Rosette J. Understanding patient and physician perceptions of benign prostatic hyperplasia in Europe: the Prostate Research on Behaviour and Education (PROBE) Survey. Int J Clin Pract. 2008;62:18–26.
- Sreenivasulu K, Nandeesha H, Dorairajan LN, et al. Gene expression of insulin receptor, insulin-like growth factor increases and insulin-like growth factor-binding protein-3 reduces with increase in prostate size in benign prostatic hyperplasia. Aging Male. 2018;21:138–144.
- Torrealba N, Rodriguez-Berriguete G, Vera R, et al. Homeostasis: apoptosis and cell cycle in normal and pathological prostate. Aging Male. 2018;1–11. DOI: 10.1080/13685538.2018.1470233
- Asiedu B, Anang Y, Nyarko A, et al. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male. 2017;20:17–22.
- Allen S, Aghajanyan I. Use of thermobalancing therapy in ageing male with benign prostatic hyperplasia with a focus on etiology and pathophysiology. Aging Male. 2017;20:28–32.
- Bushman W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am. 2009;36:403–415.
- Calogero AE, Burgio G, Condorelli RA, et al. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2018;1–8. DOI:10.1080/13685538.2018.1434772
- Arivazhagan J, Nandeesha H, Dorairajan LN, et al. Association of elevated interleukin-17 and angiopoietin-2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118.
- Besiroglu H, Dursun M, Otunctemur A, et al. The association between triglyceride high density lipoprotein cholesterol ratio and benign prostate hyperplasia in non-diabetic patients: a cross-sectional study. Aging Male. 2017;20:198–204.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Teoh JY, Chiu PK, Chan SY, et al. Androgen deprivation therapy, diabetes and poor physical performance status increase fracture risk in Chinese men treated for prostate cancer. Aging Male. 2015;18:180–185.
- Kaplan SA, Lin J, Johnson-Levonas AO, et al. Increased occurrence of marked elevations of lipoprotein(a) in ageing, hypercholesterolaemic men with low testosterone. Aging Male. 2010;13:40–43.
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216.
- Gacci M, Corona G, Vignozzi L, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. 2015;115:24–31.
- Shih HJ, Huang CJ, Lin JA, et al. Hyperlipidemia is associated with an increased risk of clinical benign prostatic hyperplasia. Prostate. 2018;78:113–120.
- Calabro P, Yeh ET. The pleiotropic effects of statins. Curr Opin Cardiol. 2005;20:541–546.
- Schooling CM, Au Yeung SL, Freeman G, et al. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57.
- St Sauver JL, Jacobsen SJ, Jacobson DJ, et al. Statin use and decreased risk of benign prostatic enlargement and lower urinary tract symptoms. BJU Int. 2011;107:443–450.
- Zhang X, Zeng X, Dong L, et al. The effects of statins on benign prostatic hyperplasia in elderly patients with metabolic syndrome. World J Urol. 2015;33:2071–2077.
- Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidem Drug Saf. 2011;20:236–242.
- Li YH, Ueng KC, Jeng JS, et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116:217–248.
- Qu X, Huang Z, Meng X, et al. Prostate volume correlates with diabetes in elderly benign prostatic hyperplasia patients. Int Urol Nephrol. 2014;46:499–504.
- Hwang EC, Kim SO, Nam DH, et al. Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. Low Urin Tract Symptoms. 2015;7:32–36.
- Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5:212–218.
- Jung JH, Ahn SV, Song JM, et al. Obesity as a risk factor for prostatic enlargement: a retrospective cohort study in Korea. Int Neurourol J. 2016;20:321–328.
- Frea B, Annoscia S, Stanta G, et al. Correlation between liver cirrhosis and benign prostatic hyperplasia: a morphological study. Urol Res. 1987;15:311–314.
- St Sauver JL, Jacobson DJ, McGree ME, et al. Protective association between nonsteroidal antiinflammatory drug use and measures of benign prostatic hyperplasia. Am J Epidemiol. 2006;164:760–768.
- Wang Z, Xiao X, Ge R, et al. Metformin inhibits the proliferation of benign prostatic epithelial cells. PLoS One. 2017;12:e0173335.
- Freeman MR, Solomon KR. Cholesterol and benign prostate disease. Differentiation. 2011;82:244–252.
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Ross SD, Allen IE, Connelly JE, et al. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Intern Med. 1999;159:1793–1802.
- Deshpande S, Quek RG, Forbes CA, et al. A systematic review to assess adherence and persistence with statins. Curr Med Res Opin. 2017;33:769–778.
- McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563.
- Debruyne F, Barkin J, van Erps P, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46:488–494.
- Kim W, Jung JH, Kang TW, et al. Clinical effects of discontinuing 5-alpha reductase inhibitor in patients with benign prostatic hyperplasia. Korean J Urol. 2014;55:52–56.
- Cindolo L, Pirozzi L, Sountoulides P, et al. Patient’s adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol. 2015;15:96.
- Li YC, Huang WL. Effects of adherence to statin therapy on health care outcomes and utilizations in Taiwan: a population-based study. BioMed Res Int. 2015;2015:149573.
- Lopez-Guarnido O, Urquiza-Salvat N, Saiz M, et al. Bioactive compounds of the Mediterranean diet and prostate cancer. Aging Male. 2018;1–10. DOI:10.1080/13685538.2018.1430129
- Pascual-Geler M, Urquiza-Salvat N, Cozar JM, et al. The influence of nutritional factors on prostate cancer incidence and aggressiveness. Aging Male. 2018;21:31–39.
- Russo GI, Di Mauro M, Regis F, et al. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male. 2018;21:48–54.
- Guzelsoy M, Aydos MM, Coban S, et al. Comparison of the effectiveness of IPSS and VPSS without any help in LUTS patients: a prospective study. Aging Male. 2017;1–7. DOI:10.1080/13685538.2017.1414178
