?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, the antioxidant enzyme activities such as (SOD, GSH, and CAT) and malondialdehyde (MDA) level which is the end product of lipid peroxidation, were determined from the serum samples taken from patients diagnosed with prostate cancer Van Yuzuncu Yıl University Medical Faculty of Educational Research and Training Hospital and İstanbul Bagcilar Education Research Hospital. The SOD, GSH, and CAT activity of patient groups was found significantly lower than the healthy control group in patients with prostate cancer (p < .05). Serum MDA level is found significantly high when compared to control groups. MDA levels increased in patients that suffer prostate cancer disorder. Whereas, firstly antioxidant enzymes activity of SOD, GSH and CAT have been decreased in control groups. Thus, we concluded that the cause of development of prostate cancer may be the result of an imbalance between the antioxidants and oxidative stress. As a result, SOD, CAT, GSH, and MDA may play an important role in the etiopathogenesis of prostate cancer.
Keywords:
Introduction
Prostate cancer (PCa) is the most common malignancy and the second leading cause of cancer-related mortality in men in the Western world including Australia and the USA [Citation1]. PCA is a multifocal neoplasm, which forms solid tumors of glandular origin. Prostate diseases such as hyperplasia and cancer lead to the loss of homeostasis as a result of glandular aging [Citation2].
The risk of PCa increases with age but the etiology and pathogenesis are poorly understood [Citation3]. Meta-analysis suggests that HPV-16 infection could represent a risk factor for PCa whereas found no such association for HPV-18 [Citation4]. Clinically localized PCa is managed with observation, surgery, or radiation treatment; the latter may be combined with androgen-deprivation therapy (ADT). Men with metastatic disease are managed almost exclusively with ADT and chemotherapy [Citation5]. The natural history of untreated PCa is still poorly understood, with a wide variation in outcomes in patients with apparently similar cancers based on standard staging and pathological grading [Citation6]. Being able to risk stratify individuals with regard to clinical risk is, therefore, necessary in order to personalize therapeutic strategies, with the aim of minimizing harm to those at low risk, and maximizing the therapeutic armament to those at highest risk.
Reactive oxygen species (ROS) comprehend free radical and nonfree radical oxygen-containing molecules such as hydrogen peroxide (H2O2), superoxide (O2•−), singlet oxygen (1/2 O2), and the hydroxyl radical (•OH). There are also reactive nitrogen species [Citation7].
RNS are derived from nitric oxide through the reaction with O2-• to form ONOO-•. RSS is easily formed from thiols by reaction with ROS [Citation8]. RSS can ascribe formation of high levels of ROS and oxidative stress and lessen the redox balance [Citation7]. The two faced role of ROS has demonstrated that ROS within cells behave as secondary messengers in intracellular outstanding overflow that generates and provide the oncogenic phenotype of cancer cells in which ROS can also generate cellular senescence and apoptosis and therefore can act as anti-tumorigenic species [Citation9]. Reactive oxygen and nitrogen species (ROS/RNS) that appear as a result of a respirative cycle of the oxidative phosphorylation that possibly pushes biological macromolecules (e.g. cellular DNA), giving rise to single-strand and double-strand splits that finally causes cell aging, mutagenic changes, cardiovascular diseases, and cancerous tumor growth [Citation10].
Free radicals are generated when our cells obtain energy from food and oxygen and microbial infections, pollutants/toxins such as cigarette smoke, ionizing and UV radiation, alcohol, pesticides, and ozone [Citation7]. All cells contain detailed antioxidant defense systems including low and high molecular weight components to protect against ROS attack. Several clinical studies have demonstrated that increased OS is related to PCa and that antioxidants have the potential to protect men from PCa [Citation11,Citation12]. Multiple in vitro and in vivo studies have attempted to elucidate the mechanisms of initiation and progression of PCa in relation to OS [Citation13]. Oxidative free radicals caused by multiple factors such as modulation of androgens, inflammation, vitamin D, tumor suppressor gene (p53), antioxidants, and age related OS may initiate PCa. Relations between Mediterranean dietary patterns and PCa are still inconclusive [Citation14]. The high content of bioactive phytochemicals in the Mediterranean diet is very important in the prevention of PCA [Citation15]. The results suggested that a diet with higher intakes of these foods as Mediterranean diet may lower the risk of PCa in the studied population [Citation16]. In the other literature, it found that an inverse association between dietary isoflavone intake and PCa, while a positive association was found with lignans intake [Citation17].
More specifically, in men with PCa, it has been suggested that serum androgens promote ROS production and accumulation in PCa cells [Citation18]. Androgen-associated redox homeostasis is involved in the signal transduction network of multimeric redox sensitive transcription factors, enzymes, and epigenetic modifications. Androgens have been shown to promote cancer by increasing reactive oxygen derivatives in tissue [Citation19]. Androgen-induced ROS levels in prostate epithelial cells play a critical role in PCa development, progression, and recurrence. It results suggest a role for prostatic expression of PI3K has prognostic markers for PC. Also, PI3K/AKT/mTOR and Bcl-2 family are becoming an important therapeutic target and predictive biomarkers of onset and progression of PCa [Citation20]. The literature study has revealed a set of critical genes, which can provide etiologic clues as to enzalutamide-resistant prostate cancer and guide novel therapeutic approaches [Citation21]. Further, studies demonstrate that castration or estrogen therapy can lead to the regression of cancer in patients with metastatic PCa [Citation22]. Hence, it may be proposed that ADT combined with antioxidant agents may inhibit the progression of PCa [Citation23].
The aim of this study was to investigate serum oxidative stress levels and some antioxidant enzyme activities in prostate cancer.
Materials and methods
Material
The total population of the study that diagnosed and monitored was ranging from 45–70 year(s) male of age which included 25 patients who had been diagnosed with prostate cancer and 25 healthy individuals. Biochemical parameters were determined by serum samples. Before the collection of blood samples for this study, local ethical committee approval was obtained from Medical Faculty, Clinic and Laboratory Research Center, Van Yuzuncu Yıl University. Collection of blood samples for this study was obtained from in Department of Urology Medical Faculty, Van Yuzuncu Yıl University and Istanbul Educational Research and Training Hospital Department of Urology. From selected healthy and sick individuals, 4 ml blood was taken from venous conveniently as the study subject and centrifuged with 5000 r/min for 10 min and then the serums were separated from plasma. The separated serums were used to determine the superoxide dismutase (SOD), reduced glutathione (GSH), catalase (CAT), and malondialdehyde (MDA) levels.
Determination of superoxide dismutase (SOD) activity
SOD activity was determined by using the proposed method of Popov et al. SOD accelerates the dismutation of hydrogen peroxide and molecular oxygen of superoxide radicals (O2-•) formed during the oxidative energy production. This method is based on the reading of optic density resulted from using of xanthine and xanthine oxidase in which superoxide radicals that generated from the blue colored formazan dye of the nitro blue tetrazolium (N.B.T) in the optical density wavelength of 560 nm. The SOD that exists in the sample serum inhibits the formazan reaction by excluding superoxide radicals from the environment. Under the experimental conditions, 1 unit of SOD is the % 50 inhibition of N.B.T reduction rate.
Determination of reduced glutathione (GSH) activity
In the hemolysis of EDTA blood which prepared with distilled water, all the proteins that don't carry sulfhydryl (SH) group are precipitated with precipitation solution. The reduced glutathione (GSH) was measured as the final product of the reaction was achieved. That was the formation of the yellow color, of obtained clear liquid of sulfhydryl groups and DTNB (5′, 5′ - (dithiobis 2-nitrobenzoic acid). Measurement of there is deduced glutathione level in the EDTA blood was done in 412 nm wavelength in the spectrophotometer within 24 h.
OD1 = First absorbance before addition of DTNB at 412 nm.
OD2 = Second absorbance after addition of DTNB at 412 nm.
E1 = 1 in the calculations.
13600 is the molar extinction coefficient of the yellow color that formed during the interaction of GSH and DTNB.
Determination of catalase (CAT) activity
The concentration of the catalase enzyme is determined by measuring absorbance by placing 1.4 ml of H2O2 at 30% in the test tube and then added 0.1 ml phosphate buffer and 1.4 ml of the sample. The cuvette solution is then measured every 30 s on a three phase wavelength of 240 nm [Citation26]. E.U = (2.3/Δx) x (log A1/logA2) activity = U/L
Determination of malondialdehyde (MDA) level
The reaction of fatty acids with free radicals result in malondialdehyde, which is the final product of lipid peroxidation, is measured with thiobarbituric acid that gives a colored form.
200 ml from the blood is taken and put into 1 tube. 800 ml phosphate buffer, 25 ml BHT solution, and 500 ml of % 30 TCA were added. The tubes were stirred with vortex and kept on ice for 2 h. Then, the tubes were centrifuged at 2000 rpm for 15 min. 1 ml from the supernatant was taken and transferred to other tubes. Then, 75 ml of EDTA and 250 ml of TBA were added. Tubes were mixed in the vortex and kept in a hot water bath for 15 min. Then, they were brought to room temperature and their absorbance was read in UV/V as spectrophotometer at 532 nm. C = F × 6.41 × A
Statistical analysis
Defined statistics were expressed as mean and standard deviation. Binary group comparisons in cases t-test was provided the condition normal distribution, normal distribution, Mann–Whitney U-test cases that are not provided the condition statistic. It is the significance level 5% has been taken as the statistics calculations and statistical package SPSS program was used for calculations. All data were analyzed using SPSS Windows version 13.0 (SPSS Inc., Chicago, IL).
Results
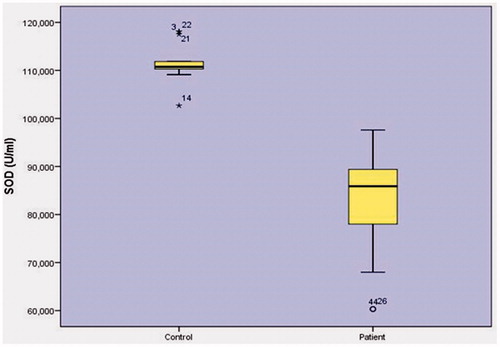
When SOD (superoxide dismutase) enzyme activity was inspected (), the relationship between control group levels (111.2793 ± 3.1127U/L) and patient group activities (83.1228 ± 9.8823 U/L) was found to have a statistically significant relationship (p < .001).
Table 1. Comparison according to the control group and patients with prostate cancer.
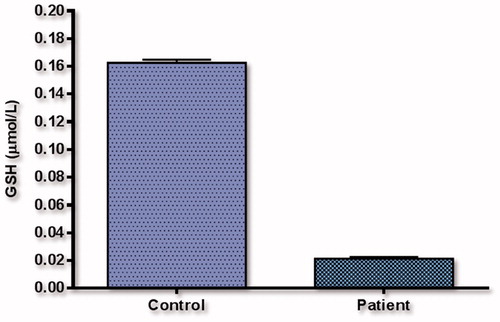
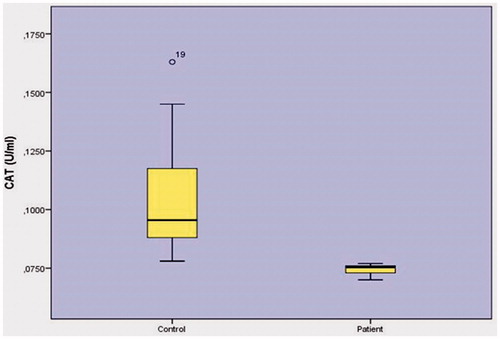
When GSH (reduced glutathione) activity was inspected (), the relationship between control group levels (0.1625 ± 0.0022 mmol/gpr) and patient group levels (0.0212 ± 0.0013 mmol/gpr) was found to have a statistically insignificant relationship (p < .001). When CAT catalase enzyme activity was inspected (), the relationship between control group levels (0.1040 ± 0.0221U/L) and patient group levels (0.0748 ± 0.0019 U/L) was found to have a statistically insignificant relationship (p < .001).
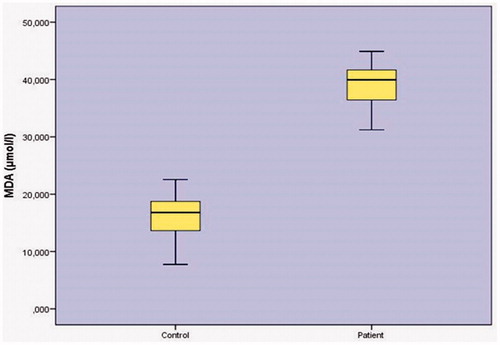
When MDA (malondialdehyde) level was determined, as shown in . The relationship between control group levels (16.1330 ± 4.0390 µmol/L) and patient group levels (38.9103 ± 3.7050 µmol/L) was found to have a statistically significant relationship (P < .001) ().
GSH, CAT, MDA, and SOD for descriptive statistics and comparing results are given in . Analysis of GSH, CAT, MDA and SOD the difference between patients and control group averages for statistics has been found to be significant (p < .001).
Results while we examined GSH (reduced glutathione) activity as in . As control group levels (0.1625 ± 0.0022 mmol/gpr) and patient group levels (0.0212 ± 0.0013 mmol/gpr) were compared (p < .001) statistically insignificant relationship was found.
CAT catalase enzyme activity as in . As control group levels (0.1040 ± 0.0221 U/L) and patient group levels (0.0748 ± 0.0019U/L) were compared (p < .001) statistically insignificant relationship was found.
MDA (malondialdehyde) level was inspected as shown in (), a control groups (16.1330 ± 4.0390 µmol/L) and patient group levels (38.9103 ± 3.7050 µmol/L) was obtained. As we observed from the (), patient groups ‘MDA level was found to be relatively high as compared to statistically significant relationship (p < .001) between control group levels.
SOD (superoxide dismutase) enzyme activity was inspected (), the relationship between the control group levels (111.2793 ± 3.1127 U/L) and patient group levels (83.1228 ± 9.8823U/L) was found to have a statistically significant relationship (p < .001) as can be seen in patient groups.
Discussion
Cancer is multifaceted, and several factors play a role in cancer development, such as bacteria, viruses, radiation, genetics, environmental factors, and dietary habits [Citation24]. Prostate cancer sometimes might be dangerous around three million men who got diagnosed with prostate cancer are still living today in the United Stated and the world [Citation25]. The management of prostate cancer in the elderly is a major public health concern in most countries [Citation26]. In prostate cancer, hormone therapy raises Alzheimer's disease risk [Citation27]. Sexual activity and sexual function have investigated via the Expanded Prostate Cancer Index Composite (EPIC) and an original self-reported questionnaire. In the same survey showed that more than quarter of preoperative middle-aged Japanese prostate cancer patients surveyed had actual sexual activity, though not within the preceding 4 weeks [Citation28]. Benign prostatic hyperplasia (BPH) is a common age for men. Inflammation and angiogenesis are known to play a role in the development prostate tumors [Citation29]. Some nucleotide polymorphisms are significantly associated with the clinical characteristics of benign prostatic hyperplasia and the efficacy of benign prostatic hyperplasia treatment [Citation30]. Diabetes may be an important predictor of the presence of high-risk prostate cancer in men with BPH (benign prostatic hyperplasia) who have undergone Holmium laser enucleation of the prostate (HoLEP) [Citation31].
Androgen deprivation therapy, diabetes and poor physical performance status increase fracture risk in Chinese men treated for prostate cancer [Citation32]. Androgen deprivation therapy (ADT) for the treatment of prostate cancer (PCa) causes an increase in total body fat, leading to a net gain in body weight. Also, the serum adiponectin and aP2 levels have independent implications in ADT for PCa [Citation33]. Androgen deprivation therapy is the standard and locally most effective onset systematic therapy is advanced or metastatic prostate cancer. In a study conducted in the literature, the prostate-specific antigen fall pattern in advanced prostate cancer is associated with androgen deprivation therapy and prostate-specific antigen progression [Citation34]. It results suggest that TRT might have a protective effect against high-grade PCa [Citation35]. In literature study demonstrated that testosterone replacement therapy (TRT) did not affect serum PSA level, prostate volume and maximal urinary flow rate. It study also suggests that TRT does not cause the risk for prostate cancer development [Citation36]. It has observed a coexistence of an altered hormone profile with increased sex hormones and leptin in PCa patients, in accordance with the new perspective of PCa pathogenesis [Citation37].
Some randomized studies have compared intermittent hormone therapy (IHT) with continuous hormone therapy (CHT) for the treatment of locally advanced prostate cancer (PCa) [Citation38].
Genetic contribution to prostate cancer risk has been proven and there is a wealth of information about the molecular genetics of the disease. Genetic variants in ATP6 and ND3 mitochondrial genes have been reported not to be associated with aggressive prostate cancer in overweight or obese Mexican Mestizo men [Citation39]. The relationship between long-term erectile dysfunction and biochemical recurrence has been investigated after permanent seed I (125) implant brachytherapy for prostate cancer [Citation40].
Reactive oxygen species (ROS) that incorporate nitrogen dioxide, hydroxyl radicals, nitric oxide, peroxynitrite and superoxide play an essential part in crucial procedures, for example, blood pressure regulation, neurotransmission, cell movement, insusceptible control, smooth muscle relaxation and microorganism protection the enzymes that control ROS are catalase and superoxide dismutase (SOD) as well as coenzyme Q10, ascorbic acid, glutathione, vitamin E and A. The ROS over-production prompts an oxidative anxiety condition, and at lifted fixations, ROS promptly make oxidative harm biomolecules including catalysts, proteins, lipids and DNA. Levels of the more oxidative stress can be let cell harm such as stroke, Alzheimer's illness, Parkinson's illness, rheumatoid joint inflammation, cardiovascular brokenness and cancer [Citation41].
Oxidative stress could be alleviated and regulate to some level by regular antioxidants, they have a small impact on a great degree raised levels of ROS, and this is because of their particular instruments of activity. For instance, SOD changes into hydrogen peroxide and oxygen over two atoms of superoxide, yet it needs another catalase, enzyme, to finish the reactant cycle. Furthermore, as with SOD, numerous common antioxidants could just extinguish maybe a couple receptive animal groups per atom before requiring recovery. In this way, these characteristic antioxidants can be effortlessly overpowered by the ROS hoisted levels traumatic wound or illness, and the impacts are exacerbated when the injury is joined by hemorrhagic stun [Citation42]. The advantage of antioxidant treatment is generally evident in models where the antioxidant is managed before the damage happens, and few have been appeared to have any huge influence post damage.
There is consequently a particular essential to create manufactured antioxidant medications that renormalize lifted oxidative anxiety levels, and particularly treatments that can be managed after damage has occurred.
Epidemiological, experimental, and clinical studies have demonstrated that markers of OS are associated with the development and progression of cancer. The present study seeks to evaluate the association between PCa and OS and antioxidants. Overall, results of our review confirm that markers of OS are increased in PCa patients compared with healthy controls, with the strongest and most consistent circulating biomarker being MDA.
The oxidation of lipid or lipid peroxidation is one of the most commonly reported indices of OS which is recognized as a pathological factor contributing to chronic disease including cancer and ageing [Citation43]. The most frequently studied markers of lipid peroxidation are MDA and isoprostanes [Citation44]. In literature, some studies have measured OS with MDA and have reported OS to be associated with PCa. In those studies, high levels of OS in PCa were consistent, although, MDA was measured using different methods such as thiobarbituric acid test, thiobarbituric acid-reactive substances test, and chromatographic assays (high-performance liquid chromatography-diode array detection-fluoro and liquid chromatography–mass spectrometry – diode array detection). One study reported that MDA levels correlated with the Gleason score and progression of disease [Citation45]. However, another study [Citation46] has observed an association between OS and advanced PCa but not localized PCa. To confirm this result will require further studies with an adequate sample size, in our research, we used the spectrophotometer to measure absorption on the principle of color change. Four studies have measured lipid peroxidation with isoprostanes, which are prostaglandin-like compounds formed from the free radical catalyzed peroxidation of arachidonic acid [Citation47–50]. These two studies were likely hampered by the limitation of small sample sizes. Further, differences in study populations may also be a cause of divergent findings.
The MDA levels were obtained 3.15 ± 0.58 (µmol/ml) and 4.55 ± 1.483 (µmol/ml) in control and patient groups respectively from blood samples which were significant p < .05, also in saliva samples the MDA levels were 0.19 ± 0.02 (µmol/ml) and 0.5433 ± 0.258 (µmol/ml) in control and patient groups, respectively.
Lipid peroxidation and antioxidant status in OSCC patients were estimated and compared the sensitivity and specificity of circulating biomarkers (MDA, sialic acid, catalase, SOD, GSH and neuraminidase) with β-2 microglobulin (β-2MG) at different thresholds in blood and saliva using receiver operating characteristics (ROC) curve design [Citation51]. To measure outcomes of protein oxidation, DNA damage with 8-OHdG and with or
are commonly used in cancer research [Citation44]. Three studies have reported a difference of 8-OHdG between two groups while one study did not observe a difference. The latter study suggested that there has a need to improve the validated analytical procedure of measuring 8-OHdG using plasma or serum samples. The results of these three studies are similar to previous studies which demonstrated that higher DNA damage correlated with the risk of PCa the H2O2 induced DNA damage level has significantly higher in incident cases (mean ± SD; 6.61 ± 4.43, n = 102) than controls (5.30 ± 3.60, n = 128) or prevalent cases (4.47 ± 3.19; n = 56). Incident cases with a positive smoking history had significantly higher H2O2 -induced DNA damage than never-smokers (7.57 ± 4.82 versus 4.52 ± 2.40; p < .001) [Citation52]. Three studies have measured protein oxidation with
or
that showed OS to be consistently higher in PCa. These findings are in agreement with previous studies which suggested that ROS, including oxygen and nitrogen-free radicals, may cause specific oxidative DNA damage and play a leading role in initiation and promotion of carcinogenesis [Citation53]. When the range (malondialdehyde), as shown in (), result control group levels (16.1330 ± 4.0390 µmol/L) and the group of patients levels (38.9103 ± 3.7050 µmol/L) were found to be statistically significant (p < .001). We have not had the chance to compete with any oxidative stress and oxidant enzymes on the prostate since they have not been tested on enzymes. That is why we are going to work with in the original and you are going to be with CAT, SOD, and GSH-related enzymes are considered primary endogenous antioxidants while vitamins C, E, and A (converted from beta-carotene) are considered exogenous antioxidants as they are directly involved in elimination of ROS [Citation54]. This is both endogenous and exogenous [Citation9,Citation51].
For example, GSH-Px removes both H2O2 and lipid peroxides using GSH. SOD metabolizes and protects the cells against , mediated by lipid peroxidation, and CAT acts on H2O2 and decomposes it to H2O and OH−. The exogenous antioxidants (vitamins A, C, and E) at the molecular and cellular level are also considered to be effective in eliminating free radicals and prevent chronic diseases including cancer [Citation9]. Thus, our review further evaluated the relationship between antioxidants and men with PCa, in addition to OS.
Further, catalase levels were obtained 4.29 ± 0.83 (µmol/mol of protein) and 0.75 ± 0.63 (µmol/mol of protein) in control and patient groups respectively from blood samples which were significant p < .05. In saliva samples, the catalase levels were 1.14 ± 0.16 (µmol/mol of protein) and 1.17 ± 0.70 (µmol/mol of protein) in control and patient groups respectively [Citation51].
In the present study, when the catalase activity was examined () result levels of control group (0.1040 ± 0.0221U/L) and group of patients activities (0.0748 ± 0.0019 U/L) were found to be statistically significant (p < .001). In the prostate cancer, the enzyme catalyzed by the oxidase enzyme catalase has not been studied in the literature. It is the first work done in the literature. In the meantime, the catalase enzyme can be detected in the prostate cancer.
Fifteen studies have measured antioxidant indicators. SOD was measured in eight studies with serums. Five studies have reported low SOD levels in patients [Citation54,Citation55]. it has reported contrasting results, and one study found no differences in Sixty men with PCa that were subjected to combined two-fraction treatment with HDR (tot. 20 Gy) and EBRT (46 Gy). Blood samples were taken before treatment, immediately afterwards, after 1.5–3 months, and approximate two years and after that control group consisted of 30 healthy men. Erythrocyte glutathione peroxidase activity in the patients was As low as is in the results than in healthy subjects by 34% (p < .001), 50% (p < .001), 30% (p < .05), and 61% (p < .001), respectively [Citation57]. In the present study, when (superoxide dismutase) enzyme activity was examined () (111.2793 ± 3.1127U/L) and the patient group activities (83.1228 ± 9.8823 U/L) were found to be statistically significant (p < .001). In the prostate cancer, the antioxidant enzyme, SOD enzyme, is firstly introduced in the literature to lighten the studies that are to be carried out. Furthermore, in this case, the SOD enzyme may significantly increase in prostatic activity.
CAT has measured on erythrocytes and whole blood samples in six studies. Four studies have reported lower CAT levels in patients [Citation55,Citation57] and two studies that found no differences [Citation56]. The main reason for the inconsistency in SOD and CAT values could be attributed to the progression of disease. This hypothesis is consistent with who observed that an alteration in CAT and SOD values existed between patients with localized disease and those with bone metastases [Citation55].
In literature, four studies have reported lower GSH levels in patients whereas two studies that reported conflicting results. Investigation of the GSH-dependent system has revealed that the amount of the GSH in erythrocytes of the patients with prostate tumors, sharply increased in both, BPH (∼1.8 times, p < .01) and PCafxrzs (∼4 times, p < .0001) groups, as compared with the control group [Citation55,Citation57].
In literature, GSH-Px values have measured to be lower in patients. In contrast, GST values have founded higher in men with PCa. The GSH-dependent enzyme levels have either decreased or increased or unchanged. The reason for the inconsistency of GSH-dependent enzyme activities could be influenced by the prostate-specific antigen values, as suggested by several studies [Citation58]. Furthermore, GSH and GSH-dependent enzymes have been known to be of central importance in the detoxification of peroxides, hydro peroxides, xenobiotics, and drugs [Citation59]. Hence, modification of GSH-dependent enzyme activities can be explained by the interdependence and dynamics of the GSH enzyme family pathway. GSH is turned to glutathione disulfide by GSH-Px. Glutathione disulfide is reduced again by GR using nicotinamide adenine dinucleotide phosphate as a cofactor. GSH has the ability to directly scavenge cellular ROS nonenzymatically as well as serving as a cofactor for GSH-Px in the reduction of H2O2 and other peroxide species [Citation60]. GSH-Px is also responsible for detoxifying other lipid peroxides to the corresponding alcohol [Citation36]. GST catalyzes the conjugation of GSH to a wide variety of endogenous and exogenous electrophonic compounds. GSH conjugation is the first step in the mercapturic acids pathway that leads to the elimination of toxic compounds [Citation61].
In the present study, when cheating (reduce glutathione) activity of the enzyme (), was examined result levels control range (0.1625 ± 0.0022 mmol/gpr) and patient group levels (0.02124 ± 0.0013 mmol/gpr) found a statistically significant relationship (p < .001). Since we did not work on the GSH before the prostate cancer, we could not have any chance of meeting them.
In our study, these results support the results of previous studies. Free radicals that accumulate over time and decrease in antioxidants can be lead to DNA damage to the prostate gland and eventually to the occurrence or development of prostate cancer. We were found low levels of enzymatic antioxidants such as CAT, SOD. We were found high MDA which indicate high rates of oxidation in patients with prostate cancer.
This opens doors for new research in which enzymatic antioxidants are used as a means of reducing oxidative processes and accumulation of free radicals, which is a primary cause of prostate cancer. Feeding on a diet rich on account of antioxidants may be able to prevent prostate cancer.
Conclusions
With an increase in age, the oxidative anxiety increments and among pathological circumstance, it might exasperates attributable to harm in tissues initiating extra intricacy. The current examination demonstrates expanded the level of MDA, marker of lipid peroxidation and diminished catalase, SOD and GSH, pointers of cell antioxidants in the patient’s prostate cancer blood. The plasma cell antioxidants diminished level shows that prostate cancer is a sickness of expanded oxidative anxiety. There are inadequate examinations demonstrating the expanded level of MDA in prostate cancer proposing oxidative anxiety. Enzymes of antioxidant, such as GPx and SOD, have significantly decreased in group patients. The antioxidant enzymes circulating can be worked to try to counter improved lipid, results of our review suggest that dysregulation of redox balance occurs in patients with PCa.
As a result, in proteometricity, SOD, CAT, GSH, and MDA can be important in the pathogenesis of prostate cancer by controlling and reducing oxidation levels.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aebi HE, Bergmeyer H. (Ed.). 1980. In: Oxidoreductases, transferases (Methods of enzymatic analysis Volume 3: Enzymes). Verlag Chemie, Deerfield Beach, FL, pp. 273–282.
- Torrealba N, Rodríguez-Berriguete G, Vera R, et al. Homeostasis: apoptosis and cell cycle in normal and pathological prostate. Aging Male. 2018;1–11. DOI:10.1080/13685538.2018.1470233
- Clair DKS, Oberley TD, Ho YS. Overproduction of human Mn-superoxide dismutase modulates paraquat-mediated toxicity in mammalian cells. FEBS Lett. 1991;293:199–203.
- Russo GI, Calogero AE, Condorelli RA, et al. Human papillomavirus and risk of prostate cancer: a systematic review and meta-analysis. Aging Male. 2018;23:7.
- Plastaras JP, Guengerich FP, Nebert DW, et al. Xenobiotic metabolizing cytochromes P450 convert prostaglandin endoperoxide to hydroxyheptadecatrienoic acid and the mutagen, malondialdehyde. J Biol Chem. 2000;275:11784–11790.
- Lykkesfeldt J, Viscovich M, Poulsen HE. Plasma malondialdehyde is induced by smoking: a study with balanced antioxidant profiles. BJN. 2004;92:203–206.
- Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative Med Cell long. 2013;2013:1.
- Lü JM, Lin PH, Yao Q, et al. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860.
- Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56.
- Çekiç SD, Çetinkaya A, Avan AN, et al. Correlation of total antioxidant capacity with reactive oxygen species (ROS) consumption measured by oxidative conversion. J Agric Food Chem. 2013;61:5260–5270.
- Sandalio LM, Del Río LA. Localization of superoxide dismutase in glyoxysomes from Citrullus vulgaris. Functional implications in cellular metabolism. J PlantPhysiol. 1987;127:395–409.
- Leone M, Cupane A, Militello V, et al. Fourier transform infrared analysis of the interaction of azide with the active site of oxidized and reduced bovine Cu, Zn superoxide dismutase. Biochemistry. 1998;37:4459–4464.
- Teixeira HD, Schumacher RI, Meneghini R. Lower intracellular hydrogen peroxide levels in cells over expressing Cu Zn-superoxide dismutase. Proc Natl Acad Sci. 1998;95:7872–7875.
- Urquiza-Salvat N, Pascual-Geler M, Lopez-Guarnido O, et al. Adherence to Mediterranean diet and risk of prostate cancer. Aging Male. 2018;1–7. DOI:10.1080/13685538.2018.1450854
- López-Guarnido O, Urquiza-Salvat N, Saiz M, et al. Bioactive compounds of the Mediterranean diet and prostate cancer. Aging Male. 2018.27:10.
- Pascual-Geler M, Urquiza-Salvat N, Cozar JM, et al. The influence of nutritional factors on prostate cancer incidence and aggressiveness. Aging Male. 2018;21:31–39.
- Russo GI, Di Mauro M, Regis F, et al. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male. 2018;21:48–54.
- Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–L173.
- Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci. 1999;658:666.
- Torrealba N, Rodriguez-Berriguete G, Fraile B, et al. PI3K pathway and Bcl-2 family. Clinicopathological features in prostate cancer. Aging Male. 2018;1–12. DOI:10.1080/13685538.2018.1424130
- Qian S, Xia J, Liu H, et al. Integrative transcriptome analysis identifies genes and pathways associated with enzalutamide resistance of prostate cancer. Aging Male. 2018;9:1–7.
- Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate‐specific antigen recurrence after radical prostatectomy: long term follow up. BJUinternational. 2012;109:32–39.
- Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149.
- Williams GM. DNA reactive and epigenetic carcinogens. Exp Toxicol Pathol. 1992;44:457–464.
- American Cancer Society. Cancer Facts & Figures 2016. Atlanta, Ga: American Cancer Society; 2016). Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009; 3601320–3601328.
- Molina-Garrido MJ, Guillén-Ponce C. Use of geriatric assessment and screening tools of frailty in elderly patients with prostate cancer. Review. Aging Male. 2017;20:102–109.
- Jhan JH, Yang YH, Chang YH, et al. Hormone therapy for prostate cancer increases the risk of Alzheimer's disease: a nationwide 4-year longitudinal cohort study. Aging Male. 2017;20:33–38.
- Taniguchi H, Kinoshita H, Koito Y, et al. Preoperative sexual status of Japanese localized prostate cancer patients: comparison of sexual activity and EPIC scores. Aging Male. 2017;20:261–265.
- Arivazhagan J, Nandeesha H, Dorairajan LN, et al. Association of elevated interleukin-17 and angiopoietin-2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118.
- Qian X, Xu D, Liu H, et al. Genetic variants in 5p13.2 and 7q21.1 are associated with treatment for benign prostatic hyperplasia with the α-adrenergic receptor antagonist. Aging Male. 2017;20:250–256.
- Ohwaki K, Endo F, Shimbo M, et al. Comorbidities as predictors of incidental prostate cancer after Holmium laser enucleation of the prostate: diabetes and high-risk cancer. Aging Male. 2017;20:257–260.
- Teoh JY, Chiu PK, Chan SY, et al. Androgen deprivation therapy, diabetes and poor physical performance status increase fracture risk in Chinese men treated for prostate cancer. Aging Male. 2015;18:180–185.
- Urushima H, Inomata-Kurashiki Y, Nishimura K, et al. The effects of androgen deprivation therapy with weight management on serum aP2 and adiponectin levels in prostate cancer patients. Aging Male. 2015;18:72–76.
- Akbay E, Bozlu M, Çayan S, et al. Prostate-specific antigen decline pattern in advanced prostate cancer receiving androgen deprivation therapy and relationship with prostate-specific antigen progression. Aging Male. 2017;20:173–175.
- Yassin A, Salman M, Talib RA, et al. Is there a protective role of testosterone against high-grade prostate cancer? Incidence and severity of prostate cancer in 553 patients who underwent prostate biopsy: a prospective data register. Aging Male. 2017;20:125–133.
- Efesoy O, Apa D, Tek M, et al. The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male. 2016;19:79–84.
- Grosman H, Fabre B, Lopez M, et al. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male. 2016;19:40–45.
- Dong Z, Wang H, Xu M, et al. Intermittent hormone therapy versus continuous hormone therapy for locally advanced prostate cancer: a meta-analysis. Aging Male. 2015;18:233–237.
- Canto P, Benítez Granados J, MartínezRamírez MA, et al. Genetic variants in ATP6 and ND3 mitochondrial genes are not associated with aggressive prostate cancer in Mexican-Mestizo men with overweight or obesity. Aging Male. 2016;19:187–191.
- Morgia G, Castelli T, Privitera S, et al. Association between long-term erectile dysfunction and biochemical recurrence after permanent seed I(125) implant brachytherapy for prostate cancer. A longitudinal study of a single-institution. Aging Male. 2016;19:15–19.
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
- Slemmer JE, Shacka JJ, Sweeney MI, et al. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–414.
- National Cancer Institute. 2011. Treatment Choices for Men With Early-Stage Prostate Cancer. Retrieved 8–19, 2017 [cited 2017 Aug 19], Available from: https://www.cancer.gov/publications/patient-education/prostate-cancer-treatment-choices.pdf
- Karimi Galougahi K, Antoniades C, Nicholls SJ, et al. Redox biomarkers in cardiovascular medicine. Eur Heart J. 2015;36:1576–1582.
- Yilmaz MI, Saglam K, Sonmez A, et al. Antioxidant system activation in prostate cancer. Bter. 2004;98:13.
- Yossepowitch O, Pinchuk I, Gur U, et al. Advanced but not localized prostate cancer is associated with increased oxidative stress. J Urol. 2007;178:1238–1244.
- Camphausen K, Ménard C, Sproull M, et al. Isoprostane levels in the urine of patients with prostate cancer receiving radiotherapy are not elevated. Int J Radiat Oncol Biol Phys. 2004;58:1536–1539.
- Barocas DA, Motley S, Cookson MS, et al. Oxidative stress measured by urine F2-isoprostane level is associated with prostate cancer. J Urol. 2011;185:2102–2107.
- Brys M, Morel A, Forma E, et al. Relationship of urinary isoprostanes to prostate cancer occurence. Mol Cell Biochem. 2013;372:149–153.
- Yang S, Pinney S.M, Mallick P, et al. Impact of oxidative stress biomarkers and carboxymethyllysine (an advanced glycation end product) on prostate cancer: a prospective study. Clin Genitourinary Cancer. 2015;13:e347–e351.
- Rasool M, Khan SR, Malik A, et al. Comparative studies of salivary and blood sialic acid, lipid peroxidation and antioxidative status in Oral Squamous Cell Carcinoma (OSCC). Pak J Med Sci. 1969;30:466.
- Lockett KL, Hall MC, Clark PE, et al. DNA damage levels in prostate cancer cases and controls. Carcinogenesis. 2006;27:1187–1193.
- Sci JE, Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C. 2009;27:120–139s.
- Surapaneni KM, Venkata GR. Lipid peroxidation and antioxidant status in patients with carcinoma of prostate. Indian J Physiol Pharmacol. 2006;50:350–354.
- Battisti V, Maders LD, Bagatini MD, et al. Oxidative stress and antioxidant status in prostate cancer patients: relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother. 2011;65:516–524.
- Woźniak A, Masiak R, Szpinda M, et al. Oxidative stress markers in prostate cancer patients after HDR brachytherapy combined with external beam radiation. Oxidative Medicine and Cellularlongevity. 2012;2012:1.
- Kotrikadze N, Alibegashvili M, Zibzibadze M, et al. Activity and content of antioxidant enzymes in prostate tumors. Exp Oncol. 2008;30:244–247.
- Pande D, Negi R, Karki K, et al. Simultaneous progression of oxidative stress, angiogenesis, and cell proliferation in prostate carcinoma. In: Urologic oncology: seminars and original investigations. Vol. 31, No. 8, pp. 1561–1566. Elsevier.
- Saydam N, Kirb A, Demir Ö, et al. Determination of glutathione, glutathione reductase, glutathione peroxidase and glutathione S-transferase levels in human lung cancer tissues. Cancer Lett. 1997;119:13–19.
- Backos DS, Franklin CC, Reigan P. The role of glutathione in brain tumor drug resistance. Biochem Pharmacol. 2012;83:1005–1012.
- Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369.