Abstract
Purpose: To evaluate the pharmacological effects of goji berry (Lycium chinense P. Mill) in an animal model of late-onset hypogonadism (LOH).
Materials and methods: Thirty 18-month-old male Sprague–Dawley (SD) rats were used as the LOH aged rat model. Rats were divided into five groups: a control group (n = 6), low concentration goji berry extract group (150 mg/kg/day) (n = 6), high concentration goji berry extract group (300 mg/kg/day) (n = 6), low concentration goji berry complex extract group (150 mg/kg/day) (n = 6), and high goji berry complex concentration extract group (300 mg/kg/day) (n = 6). After six weeks of treatment, sperm counts and motility, serum testosterone level, androgen receptor (AR) expression, oxidative stress marker, and apoptotic factors were examined.
Results: Goji berry extracts increased testosterone level to 2.07 ± 0.06 pmol/L in the goji berry 150 mg/kg group, 2.39 ± 0.08 pmol/L in the goji berry 300 mg/kg group, 2.97 ± 0.03 pmol/L in the goji berry complex 150 mg/kg group, and 3.34 ± 0.04 pmol/L in the goji berry complex 300 mg/kg group compared to 1.86 ± 0.03 pmol/L in the control group, respectively (p < .05). AR expressions were increased in testis tissue significantly but were not significant in prostate tissue.
Conclusions: Goji berry might improve LOH by reversing testicular dysfunction via an anti-oxidative stress mechanism without inducing prostate disease.
Introduction
Late-onset hypogonadism (LOH) is a clinical syndrome found in older men that is associated with decreased serum testosterone levels [Citation1]. This condition is also associated with muscle mass loss, bone mineral density decrease, depression, insomnia, fatigue, and sexual dysfunction [Citation2]. Because LOH is associated with testosterone deficiency, androgen replacement therapy (ART) is one of the important treatments. ART can improve LOH-related symptoms by enhancing libido and sexual function, and maintaining muscle and bone mass [Citation3–7]. However, the long-term effects of ART are uncertain, and prostate and breast cancer are absolute contraindications for ART [Citation8]. Furthermore, exogenous androgen can affect fertility, so ART therapy is not appropriate for patients who plan to father children in the near future.
Goji berry, a herbal medicine widely consumed by oriental people, has been shown to exhibit anti-cancer and immuno-enhancing activity [Citation9]. Goji berry has become more popular over the last decade because of public acceptance of goji berry as a “super food” with highly advantageous nutritive and antioxidant properties [Citation10]. It contains various compounds such as betaine, beta-sitosterol, scopoletin, β-carotenes, phenolic compounds, and polysaccharides [Citation11,Citation12]. Outstandingly among them, betaine, which is an alkaloid, is well known as a beneficial compound to prevent chronic disease including diabetes, and it is also positively effective on the endurance and resistance for exercise [Citation13,Citation14]. In addition, the anti-aging property of goji berry has been attributed to polysaccharides isolated from the red-colored fruits and has been investigated in various animal models. However, to our knowledge, the effects of goji berry on LOH have not previously been investigated.
In this study, we, therefore, evaluated the effects of goji berry on the improvement of testosterone level in an LOH rat model and investigated the underlying mechanisms of its protective effect on hypogonadism.
Materials and methods
Preparation of goji berry (Lycium chinense P. Mill) and goji berry complex extracts
Goji berry (Lycium chinense P. Mill) was obtained from Cheongyang-gun, Chungnam, Republic of Korea. Plant material was decocted with 30% EtOH for 8 h and named as BMG-30. After filtration and drying, the extract was administrated orally to mice at 150 mg/kg/day or 300 mg/kg/day. Goji berry complex, which is also abbreviated as BMGC-30, is a mixture of 40% goji berry, 30% Schisandra chinensis P. Baillon, and 30% Acanthopanax sessiliflorus. This mixture was decocted and extracted using the same method as described for goji berry.
Animal groups and study design
This study was performed in accordance with the Guide for Care and Use of Laboratory Animals. The treatment protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Catholic University of Korea, Seoul St Mary's Hospital (IRB approval number: CUMC-2015–0035-02). Experimental animals were obtained from Samtaco Bio Co. (Osan, Korea). Eighteen- month-old SD male rats were housed three animals per cage in polysulfone cages.
Rats were randomly divided into five groups of six animals each as follows:
Control (normal saline)
Low concentration goji berry extract for 6 weeks (150 mg/kg/day)
High concentration goji berry extract for 6 weeks (300 mg/kg/day)
Low concentration goji berry complex for 6 weeks (150 mg/kg/day)
High concentration goji berry complex for 6 weeks (300 mg/kg/day).
The plant combination dissolved in distilled water was administered orally through an 8 F red Rob-Nel catheter once a day and this was also applied to the control group in the same way.
After six weeks, all of the rats were sacrificed, the blood was sampled by puncturing of the inferior vena cava, and the prostates were excised and weighed. The oxidative stress and apoptosis were analyzed in the serum and prostates from all of the rats.
Measurement of sperm counts and motility
To perform sperm counts and evaluate the motility of sperm in the cauda epididymis, we resected the tail of the left epididymis and measured the weight. After resection of the left vas deferens, the tissue was placed in a petri dish containing 3 ml modified Tyrode's medium, and cultured in a 5% CO2 incubator for 10 min at 37 °C. After removal of the tissue in the culture medium, 0.1 ml of culture solution was mixed with 2% eosin and 3% citric acid mixture at a 1:1 ratio to make smear samples. We counted 100 motile sperm per tissue under a microscope at 200× magnification and expressed this value as a percentage of total sperm.
Serum total testosterone level
Blood from each male mouse was collected from the inferior vena cava. Serum testosterone concentration was quantified by enzyme immunoassay using a testosterone kit (BioVendor, Brno, Czech Republic) according to the manufacturer’s instructions. Absorbance at 570 nm was read with a PowerWave HT Microplate Spectrophotometer (BioTek Instruments Inc., Winooski, VT USA).
Measurement of androgen receptor (AR) level
Testis and prostate proteins transferred to membranes and then probed with an anti-AR antibody (1:1000, Santa Cruz Biotechnology, Dallas, TX, USA), and anti-β-actin antibody (1:10000, Santa Cruz Biotechnology, Dallas, TX, USA) as an internal control.
Densitometric analysis of band intensity was detected by Luminescent Image Analysis System (LAS-3000; Fujifilm, Tokyo, Japan).
Measurement of oxidative stress
Total DNA was extracted from the tissue using a DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA). A DNA oxidation kit (Highly Sensitive 8-OHdG Check ELISA; Japan Institute for the Control of Aging, Fukuroi, Japan) was used to measure levels of 8-OHdG. SOD AssayKit-WST (Dojindo Laboratories, Kumamoto, Japan) was used to measure SOD activity (CuZnSOD and MnSOD) in serum according to the manufacturer’s instructions.
Immunohistochemistry
We immunostained testis to analyze the expression of the apoptosis-related factors; Bcl-2 and BAX. We excised the testis and embedded it in paraffin to prepare thick sections (3 μm-thick). Sections were stained with the primary antibodies Bcl-2 and BAX at 1: 300 dilutions at 4° overnight. After the addition of secondary antibody, tissue sections were incubated for 1 h at room temperature with Alexa Fluor 488 (Life Technologies, Carlsbad, CA, USA).
Phosphorylations of akt, ERK
Testicular protein bands were transferred to PVDF membranes (Millipore, Billerica, USA) at 70 V for 2 h. The membranes were incubated in a 1:500 dilution of rabbit AKT, phospho-AKT, ERK, or phospho-ERK (Abcam, Cambridge, UK) in TBST overnight at 4 °C, followed by 30 min of washing with TBST. Bound antibody was detected with goat anti-rabbit horseradish peroxidase conjugate (diluted 1:5,000 in TBST; Abcam) for 1 h at room temperature. The ECL method (Amersham, Arlington Heights, IL, USA) was used for development of protein bands.
Statistical analysis
Statistical analyses were performed using SPSS software (version 22.0, Chicago, IL, USA). All data are presented as means ± SDs. The significance of differences among groups was analyzed by Scheffe’s test, and p values <.05 were considered statistically significant.
Results
Testis, epididymis, and body weight
As shown in , there were no significant differences in testis, epididymis, and body weight among the control group, goji berry groups (150 mg/kg and 300 mg/kg), and goji berry complex groups (150 mg/kg and 300 mg/kg) (p > .05).
Table 1. Comparison of testis, epididymal and body weight among groups.
Sperm counts, motility
There were no differences in sperm counts among treatment groups and the control group.
The goji berry complex group treated with goji berry complex groups had significantly higher sperm motility than the control group (); but there were no significant differences in sperm motility between any of the other treatment groups and the control group.
Table 2. Comparison of sperm counts and motility among groups.
Testosterone
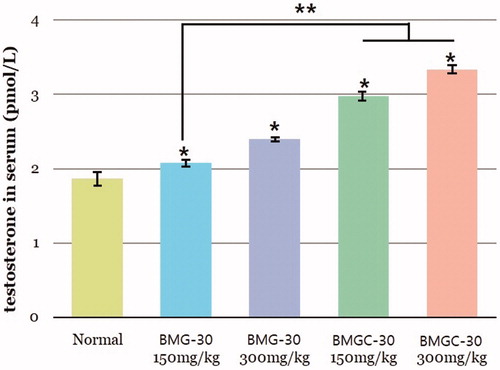
Serum testosterone levels in the treatment groups increased in a dose-dependent manner (). Mean serum testosterone level was 1.86 ± 0.03 pmol/L in the control group, 2.07 ± 0.06 pmol/L in the goji berry 150 mg/kg group, 2.39 ± 0.08 pmol/L in the goji berry 300 mg/kg group, 2.97 ± 0.03 pmol/L in the goji berry complex 150 mg/kg group, and 3.34 ± 0.04 pmol/L in the goji berry complex 300 mg/kg group. These differences in testosterone concentrations among goji berry groups versus the control group were statistically significant (p < .05). On comparison of the single and complex group, goji berry complex 150, 300 mg groups were higher than a single 150 mg group statistically significant (p < .05). Other goji berry group results were not different statistically.
AR
Expression of AR was measured in tissues of the testis and prostate. Mean AR/β-actin relative intensity of testis was 1.55 ± 0.02 in the control group, 1.79 ± 0.05 in the goji berry 150 mg/kg group, 1.86 ± 0.08 in the goji berry 300 mg/kg group, 2.12 ± 0.03 in the goji berry complex 150 mg/kg group, and 2.23 ± 0.07 in the goji berry complex 300 mg/kg group (. These increases in AR expression among goji berry groups versus the control group were statistically significant (p < .01). On comparison of the single and complex group, results were not different statistically respectively.
Figure 2. Expression of androgen receptor of goji berry and control groups in both testis and prostate tissue. (A) testis, (B) prostate. Data are expressed as means. *Statistical significance in comparison with control group. (BMG-30 = goji berry, BMGC-30 = goji berry complex).
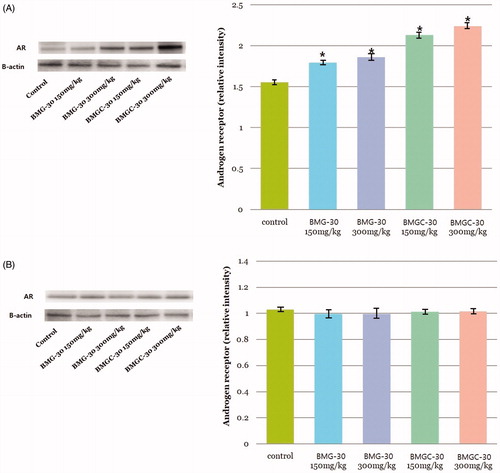
On the prostate tissue, mean AR/β-actin relative intensity was 1.02 ± 0.02 in the control group, 0.99 ±0.08 in the goji berry 150 mg/kg group, 0.99 ± 0.07 in the goji berry 300 mg/kg group, 1.01 ± 0.02 in the goji berry complex 150 mg/kg group, and 1.01 ± 0.03 in the goji berry complex 300 mg/kg group (. Unlike the results of testis tissue, there were no statistical differences between control and goji berry groups.
Oxidative stress level
Oxidative stress in testicular tissue was evaluated quantitatively by measuring 8-OHdG by ELISA. Mean 8-OHdG level was 9.89 ± 0.07 pmol/L in the control group, 8.79 ± 0.17 pmol/L in the goji berry 150 mg/kg group, 7.90 ± 0.12 pmol/L in the goji berry 300 mg/kg group, 7.78 ± 0.17 pmol/L in the goji berry complex 150 mg/kg group, and 7.61 ± 0.46 pmol/L in the goji berry complex 300 mg/kg group, respectively (. This decrease in oxidative stress according to goji berry dose was statistically significant versus the control group. On comparison ofthe single and complex group, goji berry complex 300 mg group was higher than a single 150 mg group statistically significant (p < .05). Other goji berry group results were not different statistically.
Figure 3. Protective effect of goji berry against oxidative stress. (A) 8-OHdG (oxidative stress marker) (B) SOD (superoxidase). Data are expressed as means. *Statistical significance in comparison with control group. **Statistical significance in comparison with goji berry 150 mg/kg group. (BMG-30 = goji berry, BMGC-30 = goji berry complex).
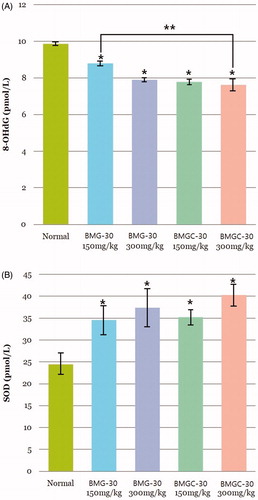
SOD activity was significantly higher in all treatment groups than the control group, with higher SOD activity in the complex extract treatment groups than the goji berry extract-only treatment groups. Mean SOD level was 24.45 ± 3.19 pmol/L in the control group, 34.58 ± 5.62 pmol/L in the goji berry 150 mg/kg group, 37.32 ± 8.37 pmol/L in the goji berry 300 mg/kg group, 35.18 ± 2.10 pmol/L in the goji berry complex 150 mg/kg group, and 40.27 ± 6.51 pmol/L in the goji berry complex 300 mg/kg group (. On comparison of the single and complex group, results were not different statistically, respectively.
Levels of Bcl-2 and BAX
Levels of Bcl-2, which is known to inhibit apoptosis, were significantly higher in the treatment groups than the control group (p < .05) (. Mean Bcl-2 mean intensity was 43.50 ± 15.49 pmol/L in the control group, 54.36 ± 16.15 pmol/L in the goji berry 150 mg/kg group, 74.43 ± 23.54 pmol/L in the goji berry 300 mg/kg group, 103.07 ± 27.54 pmol/L in the goji berry complex 150 mg/kg group, and 159.48 ± 36.56 pmol/L in the goji berry complex 300 mg/kg group (. On comparison of the single and complex group, goji berry complex 150, 300 mg groups were higher than a single 150 mg group statistically significant (p < .05). Other goji berry group results were not different statistically.
Figure 4. Comparison of anti-apoptotic effects of goji berry groups based on immunohistochemical staining for the apoptosis-related markers (A) Bcl-2 and (B) BAX. Data are expressed as means. *Statistical significance in comparison with control group. **Statistical significance in comparison with goji berry 150 mg/kg group. (BMG-30 = goji berry, BMGC-30 = goji berry complex).
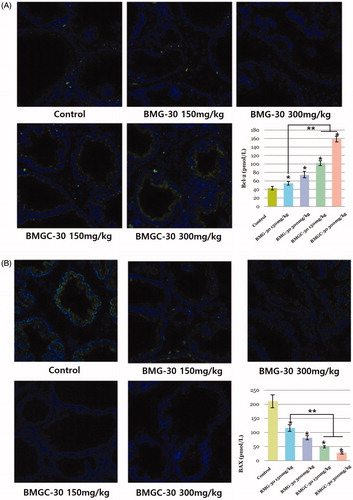
Expression of BAX, which is an apoptotic activator, was lower in all treatment groups than the control group. In addition, BAX levels were significantly lower in the complex extract treatment groups than the goji berry extract-only treatment groups, and also decreased according to extract dose. Mean BAX intensity was 220.23 ± 58.81 pmol/L in the control group, 149.31 ± 15.99 pmol/L in the goji berry 150 mg/kg group, 88.30 ± 35.12 pmol/L in the goji berry 300 mg/kg group, 57.15 ± 21.74 pmol/L in the goji berry complex 150 mg/kg group, and 39.30 ± 27.88 pmol/L in the goji berry complex 300 mg/kg group (. On comparison of single and mix group, goji berry complex 150, 300 mg groups were higher than a single 150 mg group statistically significant (p < .05). Other goji berry group results were not different statistically.
Phosphorylated Akt and ERK
Phosphorylation of Akt and ERK kinase in human cells is essential for cellular defense against various stresses. Levels of phosphorylated Akt and ERK were significantly higher in the treatment groups than the control group and showed a dose-dependent relationship. Mean AKT relative intensity was 0.02 ± 0.00 pmol/L in the control group, 0.13 ± 0.01 pmol/L in the goji berry 150 mg/kg group, 0.37 ± 0.05 pmol/L in the goji berry 300 mg/kg group, 0.85 ± 0.05 pmol/L in the goji berry complex 150 mg/kg group, and 0.84 ± 0.11 pmol/L in the goji berry complex 300 mg/kg group (. Ont comparison of the single and complex group, goji berry complex 150, 300 mg groups were higher than a single 150 mg group statistically significant (p < .05). Other goji berry group results were not different statistically.
Figure 5. Enhanced phosphorylation of kinases in response to goji berry treatment: (A) Akt, (B) ERK. Data are expressed as means. *Statistical significance in comparison with control group. **Statistical significance in comparison with goji berry 150 mg/kg group. (BMG-30 = goji berry, BMGC-30 = goji berry complex).
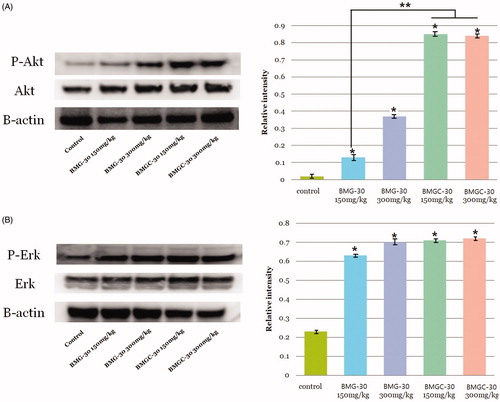
Mean ERK relative intensity was 0.23 ± 0.02 pmol/L in the control group, 0.63 ± 0.01 pmol/L in the goji berry 150 mg/kg group, 0.70 ± 0.03 pmol/L in the goji berry 300 mg/kg group, 0.71 ± 0.07 pmol/L in the goji berry complex 150 mg/kg group, and 0.72 ± 0.05 pmol/L in the goji berry complex 300 mg/kg group (. On comparison of the single and complex group, results were not different statistically, respectively.
Discussion
Advances in medicine have prolonged the human lifespan, and the proportion of the world’s population aged 60 years and older is growing faster than any other age group. In addition, obesity, which is known to provide an important cause of LOH, is still growing nowadays [Citation15]. Treatment of male LOH is important because the associated reduction in testosterone is accompanied by symptoms such as loss of muscle mass and strength, decreasing libido, erectile dysfunction, and emotional changes [Citation3–7]. Hormone replacement therapy is widely used to treat LOH. Injections, patches, and a gel formulation are mainly used, but these hormone replacements induce higher androgen levels than physiologically normal, their action time is unstable, and they have side effects on the skin. There is, therefore, the demand for products that have minimal side-effects but that are effective. Among traditional herbal products, goji berry (Lycium chinense P. Mill) has drawn attention due to its antioxidant effects [Citation9,Citation10]. We established an animal model of LOH to evaluate the effects of goji berry on LOH.
Aging involves progressive degeneration of physiological function that impairs the ability of an organism to maintain homeostasis and eventually increases the organism’s susceptibility to disease and death [Citation16,Citation17]. It is widely accepted that oxidative stress and apoptosis are the two major factors involved in the aging process [Citation18–20]. Apoptosis is defined as the set of biochemical and morphological changes at different cellular levels that result in the elimination of unwanted cells. It is an important physiological process but is also involved in many age-related degenerative processes [Citation21,Citation22]. However, apoptosis has a negative effect on aging because it often destroys vital cells that prevent aging.
In our animal experiments, we observed that goji berry increased testosterone levels, so we found the possibility that it could relieve hypogonadal symptoms in clinical practice. This result was shown with the greatest increase in the goji berry complex 300 mg group and there was a similar increase in the other parameters as well. Recently, one of the dilemmas in the LOH treatment is whether testosterone and hypogonadal symptom match together [Citation23]. The cause of this dilemma has not yet been clarified, one of the reasons is thought to be due to the difference in AR expression at the same testosterone level [Citation24]. Therefore, we measured the expression of AR in the testis. AR is known to affect the development of benign prostatic hyperplasia (BPH) and prostate cancer, but its mechanism is still unclear [Citation25]. Because both diseases are affected by testosterone, changes in AR may affect the risk of them. So, we measured AR in the prostate tissues additionally to confirm whether goji affects the variation of AR or not in the prostate. Wu et al. reported a study that some herbal materials could inhibit the AR and proliferation of prostate by protecting it from oxidative stress in BPH animal model [Citation26]. In contrast, there are other studies that suggest some materials may increase AR expression in the testis tissue in the hypogonadal animal model [Citation27]. Oxidative stress is known to be associated with homeostasis in many organs as well as in the prostate and the testis. The effects of this protection from oxidative stress in some organs have not been fully elucidated, but depending on the disease model, the effect may vary in different organs [Citation28–31]. In our study, AR expression was only significant in the testis tissue. We found the possibility of improving hypogonadal symptom regardless of testosterone level and without prostatic disease problems in goji berry treatment.
To confirm the mechanism of this action, we examined the effects of oxidative stress and apoptosis. We found that levels of 8-OHdG decreased, SOD activity increased, and markers of apoptosis decreased in rats treated with goji berry extracts compared to the control group. These results suggest that goji berry may protect against testicular dysfunction in aged rats by suppressing oxidative stress and apoptosis. These findings also suggest that the observed increase in serum testosterone levels may be due to the protective effects of goji berry on Leydig cells because these are the cells that produce testosterone and induce spermatogenesis in the testis; this hypothesis, however, requires evaluation in future studies.
Our study had several limitations. First, we evaluated levels of oxidative and apoptotic markers just in testis tissue, not in Leydig cells. Second, we used the control group as just old rats, we did not make a hypogonadal model using anti-androgen medication, so we could not make the positive control. However, we used an old age rat to reproduce a more natural and suitable LOH model. Third, prolonged exposure to herbal medicine that elevates testosterone could down-regulate AR, so long-term study is needed. Finally, we could not measure gonadotropins, which may provide important information about the effect on testosterone.
We investigated the effects of goji berry as an alternative treatment to ART to reverse testicular dysfunction in LOH. In addition, goji berry showed the possibility that the expression of AR could be increased in testis, so it could improve symptoms regardless of testosterone level. However, since free radicals are implicated in many diseases and age-related conditions, the antioxidant dependent actions of goji berry may have a wide range of anti-aging effects. These therapeutic effects are at least, in part, attributable to a decrease in oxidative stress and apoptosis in testis. These findings suggest that goji berry extracts may be an alternative medicine for the treatment of LOH without increasing prostate cancer or BPH risk.
Conclusions
We found that goji berry extracts had positive effects on testosterone replacement and AR expression in a rat model of LOH.
Goji berry extracts may protect against LOH by increasing serum testosterone levels through decreasing apoptosis and minimizing oxidative damage. Furthermore, goji berry may have the potential to improve LOH-related symptoms in eugonadal patients by a different mechanism to ART potentially involving protection of Leydig cells via anti-oxidation and anti-apoptosis mechanisms without inducing prostate disease.
Disclosure statement
The authors report no conflict of interest in this work.
Additional information
Funding
References
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA, and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2009;12:5–12.
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135.
- Comhaire FH. Andropause: hormone replacement therapy in the ageing male. Eur Urol. 2000;38:655–662.
- Morales A, Johnston B, Heaton JP, et al. Testosterone supplementation for hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol. 1997;157:849–854.
- Morley JE. Testosterone replacement and the physiologic aspects of aging in men. Mayo Clin Proc. 2000; 75 Suppl: S83–S87.
- Morley JE. Testosterone replacement in older men and women. J Gend Specif Med. 2001;4:49–53.
- Morley JE, Perry HM. 3rd. Androgen treatment of male hypogonadism in older males. J Steroid Biochem Mol Biol. 2003;85:367–373.
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559.
- Gan L, Hua Zhang S, Liang Yang X, et al. Immunomodulation and antitumor activity by a polysaccharide-protein complex from Lycium barbarum. Int Immunopharmacol. 2004;4:563–569.
- Luo Q, Cai Y, Yan J, et al. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149.
- Zhou X, Xu G, Wang Q. Chemical constituents in the roots of Lycium chinense Mill. Zhongguo zhong yao za zhi = China J Chinese Materia Medica. 1996;21:675–676. 704.
- Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–549.
- Song Y, Xu B. Diffusion profiles of health beneficial components from goji berry (lyceum barbarum) marinated in alcohol and their antioxidant capacities as affected by alcohol concentration and steeping time. Foods (Basel, Switzerland). 2013;2:32–42.
- Cholewa JM, Guimaraes-Ferreira L, Zanchi NE. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids. 2014;46:1785–1793.
- Corona G, Vignozzi L, Sforza A, et al. Obesity and late-onset hypogonadism. Mol Cell Endocrinol. 2015;418: 120–133.
- Levy S, Robaire B. Segment-specific changes with age in the expression of junctional proteins and the permeability of the blood-epididymis barrier in rats. Biol Reprod. 1999;60:1392–1401.
- Zirkin BR, Chen H. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–981.
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605.
- Harman D. The aging process. Proc Natl Acad Sci USA. 1981;78:7124–7128.
- Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol. 2006;68:253–278.
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–132.
- Warner HR, Hodes RJ, Pocinki K. What does cell death have to do with aging? J Am Geriatr Soc. 1997;45:1140–1146.
- Scovell JM, Ramasamy R, Wilken N, et al. Hypogonadal symptoms in young men are associated with a serum total testosterone threshold of 400 ng/dL. BJU Int. 2015;116:142–146.
- Zitzmann M, Nieschlag E. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int J Androl. 2003;26:76–83.
- Chatterjee B. The role of the androgen receptor in the development of prostatic hyperplasia and prostate cancer. Mol Cell Biochem. 2003;253:89–101.
- Wu X, Gu Y, Li L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of Qing Ye Dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol Lett. 2017;265:9–16.
- Zang ZJ, Ji SY, Dong W, et al. A herbal medicine, saikokaryukotsuboreito, improves serum testosterone levels and affects sexual behavior in old male mice. Aging Male. 2015;18:106–111.
- Vital P, Castro P, Ittmann M. Oxidative stress promotes benign prostatic hyperplasia. Prostate. 2016;76:58–67.
- Duan T, Fan K, Chen S, et al. Role of peroxiredoxin 2 in H2O2induced oxidative stress of primary Leydig cells. Mol Med Rep. 2016;13:4807–4813.
- Morales CR, Pedrozo Z, Lavandero S, et al. Oxidative stress and autophagy in cardiovascular homeostasis. Antioxid Redox Signal. 2014;20:507–518.
- Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354.