Abstract
Objective: We investigated the association between the triglycerides to high-density lipoprotein cholesterol (TG/HDL) ratio and low testosterone and sex hormone-binding globulin (SHBG) levels in middle-aged and elderly men.
Methods: This cross-sectional study included 1055 men aged ≥45 years who underwent a medical examination. The cutoff points for low testosterone and SHBG levels were determined as 12.2 and 29.4 nmol/L, which corresponded to the 25th percentile of the current sample. The odds ratios (ORs) and 95% confidence intervals (95% CIs) for low testosterone and SHBG levels were calculated after adjusting for confounding variables across TG/HDL ratio quartiles (Q1: ≤ 1.70, Q2: 1.71–2.54, Q3: 2.55–3.77, Q4: ≥3.78) using multiple logistic regression analysis.
Results: The proportion of subjects with low testosterone and SHBG levels significantly increased in accordance with TG/HDL ratio quartile. The ORs (95% CIs) of the lowest TG/HDL ratio quartile compared to the highest TG/HDL ratio quartile for low levels of testosterone and SHBG were 1.96 (1.13–2.75) and 3.90 (2.38–6.38), respectively, after adjusting for age, body mass index, smoking status, alcohol drinking, regular exercise, diastolic blood pressure, fasting plasma glucose, and total cholesterol level.
Conclusions: The TG/HDL ratio was inversely associated with testosterone and SHBG levels in middle-aged and elderly Korean men.
Introduction
Insulin resistance promotes atherogenic dyslipidemia, as manifested by increased triglycerides (TG) and decreased levels of high-density lipoprotein (HDL) cholesterol [Citation1]. Serum lipid profiles are frequently evaluated in general clinical practice. Among lipid profile parameters, the TG to HDL cholesterol (TG/HDL) ratio has emerged as a strong predictor of insulin resistance. Although the homeostasis model assessment of insulin resistance (HOMA-IR) is used to quantify insulin resistance, it is not routinely evaluated in standard clinical practice. Epidemiological studies have reported that high TG/HDL ratio has a strong positive association with HOMA-IR in both adult and adolescent populations [Citation2,Citation3]. Therefore, the TG/HDL ratio, an easy and economical parameter in clinical settings, has become a surrogate marker for insulin resistance.
Testosterone is the dominant male sex hormone and is produced and synthesized by the Leydig cells of the testes. Sex hormone-binding globulin (SHBG), the specific sex steroid-binding protein, is produced by the liver and controls the availability of free testosterone in the bloodstream [Citation4]. Traditionally, reduced blood concentrations of testosterone and SHBG have been used to evaluate male hypogonadism, but recent cross-sectional and longitudinal studies have highlighted the association of low testosterone and SHBG levels with insulin resistance-related disorders such as type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease [Citation5–7].
Although the pathophysiologic mechanisms underlying the relationships of low testosterone and SHBG levels with cardiometabolic diseases are not fully understood, insulin resistance plays a central role, and previous studies have found inverse associations between testosterone and SHBG levels and HOMA-IR [Citation6,Citation8]. However, there has been very little research into the association between TG/HDL ratio, an alternative marker of insulin resistance, and testosterone and SHBG levels in a general male population. Therefore, we examined the associations of TG/HDL ratio with testosterone and SHBG levels in middle-aged and elderly Korean men.
Materials and methods
Study participants
We retrospectively reviewed the medical records of 1284 male participants aged ≥45 years who underwent a medical examination at the Health Promotion Center of Gangnam Severance Hospital in Seoul, Korea, between November 2011 and July 2013. The subjects voluntarily visited the center to regularly check their health condition. Informed consent was obtained from each participant. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University College of Medicine, Seoul, Korea. We also excluded participants who met at least one of the following criteria: a history of exogenous testosterone therapy; a history of ischemic heart disease, stroke, cancer, thyroid, respiratory, renal, hepatobiliary, or rheumatologic disease; a positive test for hepatitis B antigens or hepatitis C antibodies; leukocyte count ≥10,000 cells/μL or C-reactive protein (CRP) ≥ 10.0 mg/L; and missing data or failure to fast for 12-h prior to testing. After these exclusions, 1055 participants were included in the final analysis.
Data collection
Each participant completed a questionnaire about his lifestyle and medical history. Self-reported cigarette smoking, alcohol consumption, and physical activity characteristics were gleaned from the questionnaires. Smoking status was categorized as nonsmoker, ex-smoker, and current smoker. Alcohol drinking was defined as alcohol consumption ≥ two times per week. Participants were also asked about the type and frequency of leisure-time physical activity on a weekly basis. Regular exercise was defined as exercise ≥ three times per week. Body mass and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, in light indoor clothing without shoes. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2). Systolic blood pressure and diastolic blood pressure (DBP) were measured using the patient’s right arm with a standard mercury sphygmomanometer (Baumanometer, W.A. Baum Co Inc., Copiague, NY). All blood samples were obtained from the antecubital vein after a 12-h overnight fast. Fasting plasma glucose, total cholesterol, triglyceride, and HDL-cholesterol levels were measured by enzymatic methods using a Hitachi 7600 automated chemistry analyzer (Hitachi Co., Tokyo, Japan). Testosterone and SHBG concentrations were measured using an electrochemiluminescence assay (ELISA) with a Modular Analytics E170 system (Roche Diagnostic Systems, Basel, Switzerland). Additionally, leukocyte counts were quantified using an automated blood cell counter (ADVIA 120, Bayer, NY), and high-sensitivity CRP concentrations were measured using the Roche/Hitachi 912 System (Roche Diagnostics, Indianapolis, IN), a latex-enhanced immunoturbidimetric method with a lower limit of detection of 0.02 mg/L.
Statistical analysis
Normal distribution was evaluated by determining skewness using a Kolmogorov-Smirnov test; total testosterone, SHBG, and CRP levels had skewed distributions. TG/HDL ratio quartiles were categorized as follows: Q1: ≤ 1.70, Q2: 1.71–2.54, Q3: 2.55–3.77, Q4: ≥ 3.78. The clinical characteristics of the study population according to TG/HDL ratio quartiles were compared using one-way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables according to the normality of distributions and chi-square test for categorical variables. Continuous data are presented as mean (standard deviation, SD) or median (interquartile range, IQR), and categorical data are presented as frequencies. The cutoff points for low testosterone and SHBG levels were determined as 12.2 and 29.4 nmol/L, respectively, which corresponded to the 25th percentile of the current sample. The odds ratios (ORs) and 95% confidence intervals (95% CIs) for low testosterone and SHBG level were calculated after adjusting for confounding variables across TG/HDL ratio quartiles using multiple logistic regression analysis. All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and statistical significance was determined at p < .05.
Results
shows the distribution of clinical characteristics across TG/HDL ratio quartiles. The fourth TG/HDL ratio quartile had the highest mean values of BMI, fasting plasma glucose, total cholesterol, and leukocyte count; the highest median value of CRP level; and the lowest median values of testosterone and SHBG levels. The highest rates of current smoking and alcohol drinking also occurred in the fourth TG/HDL ratio quartile.
Table 1. Clinical characteristics of the study population according to TG/HDL ratio quartiles.
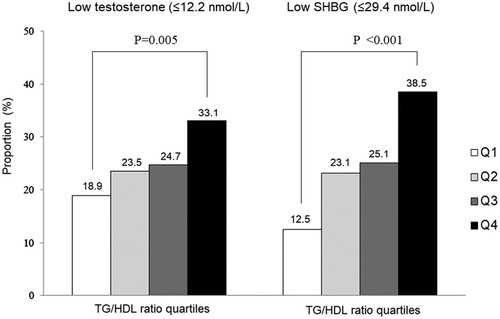
shows the proportion of subjects with low testosterone and SHBG in each TG/HDL ratio quartile. The proportion of low testosterone and SHBG significantly increased in accordance with TG/HDL ratio quartile.
Figure 1. Proportion of subjects with low testosterone and SHBG by TG/HDL ratio quartile (p values were calculated using ANOVA test).
describes the ORs for low testosterone and SHBG according to the TG/HDL ratio quartile. After adjusting for age, BMI, smoking status, alcohol drinking, regular exercise, DBP, fasting plasma glucose, and total cholesterol level, the adjusted ORs (95% CIs) of the highest quartile vs. the lowest quartile for low testosterone and SHBG were 1.96 (1.13–2.75) and 3.90 (2.38–6.38), respectively.
Table 2. Odds ratios and 95% confidence intervals for low testosterone and low SHBG according to TG/HDL ratio quartiles.
Discussion
In this cross-sectional study, we found that the TG/HDL ratio was inversely and independently associated with testosterone and SHBG levels in middle-aged and elderly Korean men. Our findings are consistent with the results of previous studies that demonstrated that low testosterone and SHBG levels were closely associated with insulin resistance disorders such as central obesity, type 2 diabetes, and metabolic syndrome [Citation9–11]. In a study of Taiwanese men, low testosterone and SHBG levels were significantly inverse related with metabolic component such as obesity, blood pressure, glucose, and triglyceride [Citation12]. The HOMA-IR is recognized as a traditional marker of insulin resistance [Citation13]; however, it is not routinely evaluated in a standard clinical setting. In the present study, we selected the TG/HDL ratio as an alternative marker of insulin resistance. As part of the lipid profile, TG/HDL ratio is a rapid, economical parameter to test, and high TG/HDL ratio has emerged as a reliable indicator of insulin resistance in clinical practice [Citation2,Citation14]. We confirmed that insulin resistance, characterized by a higher TG/HDL ratio, is significantly associated with low testosterone and SHBG levels in middle-aged and elderly Korean men.
There are plausible mechanisms for the significant association between TG/HDL ratio and low testosterone and SHBG levels in men. A low testosterone or SHBG level could be considered a consequence of insulin resistance. Males with low testosterone and SHBG levels had a higher incidence risk of insulin resistance and metabolic syndrome in prospective studies [Citation6,Citation15]. A significant decrease of testosterone leads to an increase of abdominal visceral fat mass and a decrease of skeletal muscle mass, which stimulates an influx of free fatty acid to the liver, induces hepatic insulin resistance [Citation16], and decreases synthesis of SHBG from the liver [Citation17]. Low testosterone levels along with low muscle and high fat mass inversely correlate with oxidative phosphorylation gene expression in mitochondria [Citation18], which also plays a pivotal role in the development of insulin resistance [Citation19]. A decreased SHBG may not play a suppressive role in hepatic lipogenesis, which in turn may aggravate insulin resistance [Citation20]. Moreover, visceral adipose tissue, associated with low testosterone and SHBG levels, secretes a number of adipocytokines such as tumor necrosis factor-α and interleukin-6, which cause a chronic low-grade inflammatory state, which is another core feature of insulin resistance and metabolic syndrome [Citation21,Citation22]. Notably, in the present study, low testosterone and SHBG levels were positively correlated with inflammatory markers including leukocyte count and CRP level.
Some limitations should be considered in the interpretation of this study. First, because of the cross-sectional design, caution should be used in causal and temporal interpretations. Although a significant inverse relationship between the TG/HDL ratio and testosterone and SHBG levels existed in this study, it cannot be concluded whether TG/HDL ratio is a risk factor actively involved in the development of low testosterone and SHBG levels or just an epiphenomenon. Further prospective research is warranted to elucidate these inverse correlations between TG/HDL ratio and testosterone and SHBG levels. Second, because the study participants were limited to middle-aged and elderly Korean males who visited a single hospital for health promotion screenings and appeared to be slightly healthier than most community-based cohorts, the study population may not be representative of the general population. Third, only one measurement of testosterone level from a baseline examination was included in the analyses. To minimize the diurnal variation in testosterone level, all blood samples were drawn early in the morning. Despite these potential limitations, we believe this is the first study to report the inversely graded associations of TG/HDL ratio and low testosterone and SHBG levels.
In summary, the TG/HDL ratio was inversely and independently associated with testosterone and SHBG levels in middle-aged and elderly Korean men. Our findings suggest that a higher TG/HDL ratio may be interpreted as a state of lower testosterone and SHBG levels.
Acknowledgments
The authors would like to thank all those who underwent a medical examination at the Health Promotion Center of Gangnam Severance Hospital in Seoul, Korea between November 2011 and July 2013.
Disclosure statement
The authors declare no conflicts of interest.
References
- Laws A, Reaven GM. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992;231:25–30.
- Kim JS, Kang HT, Shim JY. The association between the triglyceride to high-density lipoprotein cholesterol ratio with insulin resistance (HOMA-IR) in the general Korean population: based on the National Health and Nutrition Examination Survey in 2007–2009. Diabetes Res Clin Pract. 2012;97:132–138.
- Park JM, Lee JY, Dong JJ, et al. Association between the triglyceride to high-density lipoprotein cholesterol ratio and insulin resistance in Korean adolescents: a nationwide population-based study. J Pediatr Endocrinol Metab. 2016;29:1259–1265.
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53:58–68.
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male. 2014;17:161–165.
- Li C, Ford ES, Li B, et al. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care. 2010;33:1618–1624.
- Mody A, White D, Kanwal F, et al. Relevance of low testosterone to non-alcoholic fatty liver disease. Cardiovasc Endocrinol. 2015;4:83–89.
- Brand JS, Rovers MM, Yeap BB, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One. 2014;9:e100409.
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041.
- MacDonald AA, Herbison GP, Showell M, et al. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311.
- Haring R, Volzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010;31:1494–1501.
- Liao CH, Huang CY, Li HY, et al. Testosterone and sex hormone-binding globulin have significant association with metabolic syndrome in Taiwanese men. Aging Male. 2012;15:1–6.
- Mojiminiyi OA, Abdella NA. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin Chem Lab Med. 2010;48:1629–1634.
- González-Chávez A, Simental-Mendía LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79:126–131.
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494.
- Frayn KN. Visceral fat and insulin resistance-causative or correlative? Br J Nutr. 2000;83(Suppl 1):S71–S77.
- Plymate SR, Jones RE, Matej LA, et al. Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids. 1988;52:339–340.
- Pitteloud N, Mootha VK, Dwyer AA, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–1642.
- Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273.
- Peter A, Kantartzis K, Machann J, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173.
- Festa A, D’Agostino R, Jr., Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42–47.
- Leal Vde O, Mafra D. Adipokines in obesity. Clin Chim Acta. 2013;419:87–89.
