Abstract
In the current aging society, the occurrence of the locomotive syndrome, a condition in which the locomotive organs are impaired, is increasing. The locomotive system includes support (bones), mobility and impact absorption (joints and intervertebral disks), drive and control (muscles, nerves), and network (blood vessels). The impairment of any of those systems can lead to a major decrease in quality of life. In recent years, several studies on methods to improve and prevent conditions impairing the locomotive syndrome have been conducted. Almost in parallel with the structure supporting mobility and body functions, testosterone levels decrease with age. Testosterone is a hormone-regulating several pathways affecting each aspect of the locomotive syndrome. Testosterone is regulated by the pituitary gland triggering several processes in the body through genomic and non-genomic pathways, affecting muscles, bones, nerves, joints, intervertebral discs, and blood vessels. The purpose of this review is to investigate the role of testosterone in each of the systems involved in the locomotive syndrome.
Introduction
Due to progress in medical field, the average expectance of life has improved considerably over the last several decades. However, the locomotive functions of the body do often not keep up with that longevity, leading to several impairments in mobility in the elderly. Even though the aging body is relatively healthy, the bones, joints, intervertebral disks, muscles, and nerves regress which cause difficulties in movement, leading to an impaired quality of life. In 2007, the Japanese Orthopaedic Association proposed the name “Locomotive Syndrome” as an inclusive term to describe this condition [Citation1]. This syndrome can ultimately lead to increased morbidity and mortality in the elderly. Indeed, hip fracture due weakened bones or severe sarcopenia can lead to an increased risk in this population becoming bedridden, and the diseases associated with such a state.
For women, osteoporosis occurs in the postmenopausal state or with aging in males. Additionally, secondary osteoporosis is associated with by several endocrine disorders (Hypogonadism, pituitary disorders, diabetes mellitus, thyrotoxicosis, and pregnancy-associated osteoporosis), autoimmune and chronic diseases (Rheumatic disorders, chronic renal disease, chronic pulmonary disease, gastrointestinal diseases, transplantation, granulomatous diseases, and systemic mastocytosis), bone and malignant diseases (Multiple myeloma, lymphomas and leukemias, metastatic bone disease, anemia, and Gaucher’s disease), and smoking and excessive alcohol intake [Citation2]. Currently, several methods of treatment including anti-resorptive treatments (Oestrogen, selective estrogen-receptor modulators, calcitonin, bisphosphonates, and RANKL antibody) and anabolic treatments (Parathyroid hormone or parathyroid hormone-related peptide analogus) [Citation3].
Joint diseases include inflammation of a joint (Arthitis), inflammation of a fluid-filled sac cushioning the joint (Bursitis), and injuries that dislocate the ends of the bones (Dislocations). Joint inflammation is caused by chemicals from the body entering the blood or tissues, increasing the blood flow and causing fluid leaks resulting in swelling, ultimately affecting the nerves and cause pain. Drug therapies for joint pain include Tylenol (acetaminophen), non-steroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, anti-anxiety drugs, antidepressants, and strong pain killers.
Connecting vertebral bodies in the spine, the intervertebral discs regulate load transfers and movements [Citation4]. Degeneration of these discs progresses with aging through a shift in the cellular microenvironment, often leading to lower back pain [Citation4]. Treatments of this condition are often limited to physiotherapy and anti-inflammatory medications [Citation4].
Skeletal muscle is essential for daily activities and movements. However, muscle mass decreases with aging, a phenomenon called sarcopenia [Citation5]. Causes of sarcopenia might be a cascade of steps like (1) decrease of physical activity, (2) impaired protein metabolism, (3) decreases in anabolic hormones (Growth hormone, testosterone, (4) impaired neuromuscular function, (5) altered gene expression, and (6) apoptosis [Citation6]. Treatments for sarcopenia include drug-based pharmaceutical treatments (testosterone, growth hormone, dehydroepiandrosterone, estrogen, ghrelin, vitamin D, angiotensin converting enzyme inhibitor, and eicosapentaenoic acid) and non-drug based treatments (resistance training, protein and amino acid supplementation, and abstinence from smoking) [Citation7].
Decreased lean body mass and increased adipose tissue lead to the metabolic syndrome (MetS) that is associated with higher prevalence of cardiovascular risks. Low testosterone levels have been demonstrated to be associated with MetS diagnosis in non-diabetic men older than 40 y [Citation8]. MetS has been observed in many patients with erectile dysfunction, suggesting an aggravating effect of MetS in combination with hypogonadism on male sexual functions [Citation9]. Furthermore, high prevalence of low testosterone levels has been observed in obese men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia [Citation10], emphasizing the negative relationship between adipose tissue and testosterone levels.
As we can see from the above, several medications are independently used to treat each disease of the locomotive syndrome. On the other hand, it has been recently shown that testosterone administration improves bone mineral density in hypogonadal men with osteoporosis or osteopenia [Citation11]; mitigate joint pain [Citation12,Citation13] enhances chondrogenesis in intervertebral discs [Citation14] and reverses sarcopenia in the elderly [Citation15]. Testosterone replacement therapy has also been shown to positively affect the cardiovascular system in hypogonadal men [Citation16]. Indeed, testosterone improves glucose uptake and metabolism, and therefore, contributes to the prevention of diabetes which is correlated with cardiovascular diseases [Citation16]. For example, a recent study showed that 24 w of testosterone replacement therapy improve insulin-signaling genes (IR-β, IRS-1, AKT-2, and GLUT4) expression in adipose tissue [Citation17]. Anti-inflammatory properties of testosterone have been widely studied in recent years. Pro-inflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor-α, are major causes of the onset of conditions related to the locomo syndrome (osteoporosis and arthritis) [Citation18]. Testosterone has been shown to be negatively associated with pro-inflammatory markers [Citation19–21]. It has been proposed that testosterone reduces the enzyme phospholipase D activity [Citation22] and therefore, inhibits inflammation. Indeed, a recent study showed that 24 w of testosterone replacements therapy decrease C-reactive protein, IL-β, and TNFα [Citation17]. These results suggest that healthy testosterone levels are important to prevent an increase in pro-inflammatory cytokines and the following diseases.
A recent study investigating testosterone levels, comorbidities, and aging males’ symptoms (AMS) found several correlations among testosterone levels, psychiatric disorders, dyslipidemia, and diabetes mellitus [Citation23], showing again the multiple repercussions of testosterone levels. Another recent study showed that age, AMS score, International Prostate Symptom Score (IPSS), and highly sensitive C-reactive protein (hs-CRP) levels are significantly correlated with the Sexual Health Inventory for Men (SHIM), being an independent factor affecting cardiovascular risk [Citation24]. Low testosterone has also been associated with higher prevalence of depression [Citation25], emphasizing neural effects of testosterone.
The aim of this manuscript is to review the possible application of testosterone replacement therapy as a holistic treatment for several symptoms of the locomotive syndrome.
Aging and decline in testosterone
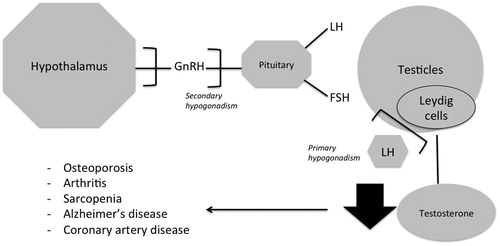
For males, the decline in testosterone caused by ageing is known as andropause; that is, a partial androgen deficiency of the aging male, and therefore a late-onset hypogonadism (LOH) [Citation26]. Hypogonadism can be divided into primary hypogonadism due to testicular failure and secondary hypogonadism caused by impaired hypothalamic-pituitary-gonadal axis (HPGA). One study showed that 9% of hypogonadal men had primary, 50% had secondary, and 41% had compensated hypogonadism (normal testosterone levels with higher levels of luteinizing hormone (LH) [Citation27].
Aging and primary hypogonadism
In response to pituitary-secreted LH stimulation, testosterone is synthesized by Leydig cells within the testes. In the Leydig cells, LH binds to its receptors the steroid activating receptor is synthesized and cholesterol is carried across the mitochondrial membrane leading to conversion of cholesterol to pregnenolone [Citation28] which travels from the mitochondria to the smooth endoplasmic reticulum where testosterone is synthesized [Citation26]. However, the response of Leydig cells to LH decreases with age [Citation29]. Furthermore, it has been demonstrated that older males have less Leydig cells in their testes as compared with young males [Citation30].
Aging and secondary hypogonadism
Impairments in gonadotropin-releasing hormones (GnRH) which stimulate LH and follicle stimulating hormone (FSH), may lead to a malfunctioning HPGA. GnRH secretion is pulsatile and is produced in the hypothalamus and has been shown to decrease with aging [Citation31].
Joints and testosterone
Cohort studies showed that individuals with arthritis have lower testosterone levels as compared with healthy individuals [Citation32,Citation33]. Another clinical trial showed that men with rheumatoid arthritis have lower bioavailable testosterone, including many hypogonadal individuals [Citation34]. Testosterone replacement therapy has been shown to have protective effects on the progression of arthritis, probably through improved hypothalamo-pituitary-adrenal (HPA) axis response, especially through decreased pro-opiomelancortin mRNA in the anterior pituitary and plasma corticosterone [Citation35]. In osteoarthritis and rheumatoid arthritis testosterone showed favorable anti-inflammatory properties by decreasing aromatization and increase anti-inflammatory 5α-reduced androgens [Citation36]. Indeed, higher levels of estrogen relative to androgens and aromatase conversion products (estrone) have been observed in patients with rheumatoid arthritis [Citation37]. Estrogens have a complex role in inflammation with both pro- and anti-inflammatory actions [Citation38]. As estrogens can be pro-inflammatory and androgens anti-inflammatory, production of 5α-reduced androgens from testosterone might lead to decreased inflammation [Citation39].
Intervertebral discs and testosterone
Intervertebral discs are major parts of the vertebral column, and the mobility of the spine largely depends on the health of disc tissue. Estrogen with its ability to regenerate bone collagen is important to maintain tissues rich in bone and collagen [Citation40]. Recent research showed that testosterone also enhances chondrogenesis of male human intervertebral disc cells [Citation14]. The expressions of aggrecan, collagen type I and II, increased following treatment with testosterone [Citation14]. However, no beneficial effects were observed in female cells [Citation14]. Besides the addition of an aromatase inhibitor (anastrazole) counteracted the effects of testosterone, suggesting that testosterone enhances chondrogenesis of male intervertebral disc cells in part through conversion to estradiol [Citation14].
Muscle and testosterone
Testosterone, through genomic and non-genomic pathways, increases the number of satellite cells and myonuclei, enhances protein synthesis, decreases protein breakdown, improves nitrogen retention and intracellular calcium, and increases the number of red blood cells leading to increased oxygen delivery to the muscles [Citation41], leading to improved muscle mass and quality. Low testosterone levels lead to sarcopenia through increases in oxidative stress, activation of c-Jun NH2-terminal kinase, and cyclin-dependent kinase inhibitor p21; however, these impairments in muscle mass can be reversed through testosterone supplementation [Citation15]. The role of satellite cells regulated in part by testosterone in muscle repair seems to be very important and might prevent sarcopenia [Citation42]. Indeed, an increase in the number of myonuclei is necessary for muscle repair and or growth; however, the number of myonuclei depends on the number of myogenic precursor cells (satellite cells) located between the basal and plasma membrane of the myofibers [Citation42]. Quiescent satellite cells are activated in response to such exercise and injury but testosterone seems to increase the number of active satellite cells. With increasing age, blood testosterone levels significantly drop and trigger sarcopenia. The loss of muscle mass leads to severe impairments in every day life activities, therefore healthy serum testosterone levels are very important to prevent sarcopenia. Indeed, testosterone replacement therapy is advantageos toward reversing sarcopenia in aging [Citation15].
Erectile dysfunction is related to the aging process and is caused by impaired cavernal smooth muscle cell relaxation, which can worsen if not treated with androgens [Citation43]. The severity of erectile dysfunction seems to be related to mean weight, waist circumference, triglycerides, total cholesterol, HbA1c, fasting glucose, and Aging Males Symptoms (AMS) score [Citation44]. However, testosterone replacement therapy combined with phosphodiesterase-5 inhibitors has shown to improve this condition [Citation45].
Nerves and testosterone
Neuroprotective properties of testosterone have been widely recognized, especially protecting against neurodegenerative disorders such as Alzheimer’s disease, mild cognitive impairment, or depression [Citation46]. Testosterone is also believed to have neurotrophic effects such as neuronal differentiation and enhanced neurite outgrowth following stimulation of androgen pathways in cultured neural cells [Citation47,Citation48]. Protective functions of testosterone have not only been observed in the brain but also in motor neurons. Motor neurons diseases lead to weak skeletal muscles. Motor neurons have been shown to contain androgen receptors, which regulate genomic effects of testosterone [Citation49,Citation50], suggesting a strong influence of testosterone on motor skills. Indeed, a recent study demonstrated that androgen-treated cells grow larger cell bodies and wider neurotic processes. Furthermore, androgen enhance the survival of androgen receptor expressing cells but not control cells, suggesting a trophic role of androgens on motor neurons through androgen receptors [Citation51].
Testosterone and blood vessels
High cholesterol is associated with impairments of the blood vessels. High levels of low density lipoprotein (LDL) can cause a buildup of plaque in the arteries which can lead to narrowing of the vessels and ultimately trigger coronary artery disease. However, high density (HDL) lipoprotein cleans the arteries from LDL and sends it back to the liver. HDL also helps to decrease inflammation and protect the heart. Long-term testosterone replacement treatment results in increased HDL and decreased LDL in hypogonadal men [Citation52], suggesting protective effects of testosterone on arteries.
Natural ways to keep healthy testosterone levels
Each impairment of these building blocks of the locomotive system can be treated separately. However, testosterone replacement therapy might possibly be a holistic treatment for all the symptoms of the locomotive syndrome. Most certainly pharmaceutical means of elevating testosterone in men exist. But, such approaches can have drawbacks and barriers to success. Testosterone can, however, be manipulated in non-pharmaceutical, nature ways too. Specifically, testosterone can be increased through nutrition [Citation53], sleep [Citation54], and resistance training [Citation55].
Dietary high-fat consumption can lead to obesity, which is associated with decreased testosterone levels. One theory proposes that with increasing adipose tissue mass, the expression of aromatase increases, leading to higher conversion rates of testosterone to estradiol, stimulating a negative feedback to the pituitary gonadotropin secretion, and resulting in impaired testosterone levels [Citation56]. A recent study showed that high fat diet causes low testosterone levels through suppression of the testicular leptin and JAK-STAT pathway [Citation57]. Besides, obesity can cause pathological damage of the Leydig cells, oxidative stress in testis tissue, and high leptin levels, ultimately causing secondary hypogonadism [Citation58]. It has been recently suggested that increased inflammation through the interleukin-1-receptor pathway triggers obesity-related hypogonadism [Citation59]. Furthermore, some aspects of testosterone production are regulated by insulin and the development of insulin resistance due to obesity disruption that regulatory loop [Citation60].
Sleep duration is positively correlated to testosterone levels up to ∼9.9 h, after which it decreases [Citation61]. Sleep quality can be improved in individuals with low testosterone, but androgen replacement, but large doses of exogenous testosterone can cause impairments in sleep quality [Citation62].
Resistance training is widely recognized as a very effective tool for increasing muscle mass and strength, those benefits may partly be due to the rise in testosterone they induce even though a direct relationship between resistance training-induced acute raises in testosterone and muscle hypertrophy is still under debate [Citation41]. Nevertheless, some studies observed significant raises in testosterone levels after resistance training [Citation63], and even significant correlations with basal testosterone levels and muscle cross-sectional area after 21 w of resistance training [Citation64]. Interestingly, moderate intensity aerobic has been shown to increase testosterone marginally [Citation65], but large volumes of aerobic (i.e. endurance) training are also associated with suppressed testosterone in men [Citation66].
Put together, the management of risk factors and health can prevent decreases of testosterone [Citation67]. It seems that lifestyle adaptations regarding inclusion of resistance training exercise, nutrition, and sleep can increase muscle mass and testosterone leading to possible preventive and, mitigation of the effects of the locomotive syndrome.
Testosterone replacement therapy as preventive treatment for the locomotive syndrome?
Testosterone levels decrease with aging, leading to several complications including muscle dystrophy, arthritis, osteoporosis, impairment of the spine mobility, diabetes, and cardiovascular diseases. By keeping a healthy testosterone level in elderly, many of these complications might be avoided. Individuals suffering from symptoms above might benefit in consulting a physician and get their testosterone levels checked. Healthy males produce between 2.1 and 11.0 mg of T per day [Citation68]. Testosterone replacement therapy often recommends a weekly administration of 75–100 mg of T or 150–200 mg every 2 w [Citation69] to maintain testosterone levels within a healthy range (400–700 ng/dL) [Citation70]. Testosterone replacement therapy can, therefore, be a powerful treatment to prevent diseases associated with the locomotive syndrome if natural ways to maintain healthy testosterone levels do not function anymore. Indeed, the 2015 European Association of Urology guidelines and several studies suggest that after lifestyle improvements, testosterone replacement therapy may provide several benefits to hypogonadal men including sustained weight loss [Citation71] enhanced body composition, decreased BMI, enhanced sexual function [Citation72] glycemic control, and lipid profile [Citation73] improved. A recent cohort study including more than 800 patients showed that testosterone replacement therapy is safe and well-tolerated in hypogonadic males [Citation74]. However, to avoid relapse, lifelong testosterone therapy might be necessary [Citation73,Citation75]. Several methods of application such as injection, gels, or creams have been proven to be safe and even improve cardiovascular risk factors [Citation76]. Even though numerous methods of treatment are readily available nowadays, a large population of the untreated or not diagnosed men who could benefit from testosterone replacement therapy exists [Citation77].
Conclusion
The progress of modern medicine has led to a major extension in life expectation. However, the quality of life in the elderly largely depends on the extent of their mobility and the ability to live without external help. However, even though organs are healthy, the supporting structure composed of bones, muscles, nerves, joints, intervertebral disks, and blood vessels tend to weaken with increasing age. If one of the structures above does not function properly, the locomotive abilities can be severely impaired, resulting in an increase of morbidity and mortality in the aging population. Despite the existence of pharmaceutical medications for each of those conditions, they often do not cure the disease but only temporarily treat it. On the other hand, testosterone declines with age leading to the downregulation of pathways involved in many aspects of the locomotive syndrome. Therefore, testosterone replacement therapy might be a holistic treatment for each of the mentioned locomotive symptoms.
Declaration of interest statement
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13:1–2.
- Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clin Cornerstone. 2000;2:11–19.
- Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diab Endocrinol. 2017;5:898–907.
- Smith LJ, Nerurkar NL, Choi K-S, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis report of the European working group on Sarcopenia in older people. Age and Ageing. 2010;39:412–423.
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:M911–M916.
- Wakabayashi H, Sakuma K. Comprehensive approach to sarcopenia treatment. Curr Clin Pharmacol. 2014;9:171–180.
- Blaya R, Thomaz LDGR, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. The Aging Male. 2016;19:85–89.
- Aslan Y, Guzel O, Balci M, et al. The impact of metabolic syndrome on serum total testosterone level in patients with erectile dysfunction. The Aging Male. 2014;17:76–80.
- Kaplan SA, Lee JY, O’Neill EA, et al. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. The Aging Male. 2013;16:169–172.
- Shigehara K, Konaka H, Koh E, et al. Effects of testosterone replacement therapy on hypogonadal men with osteopenia or osteoporosis: a subanalysis of a prospective randomized controlled study in Japan (EARTH study). The Aging Male. 2017;20:1–145.
- Fischer L, Clemente JT, Tambeli CH. The protective role of testosterone in the development of temporomandibular joint pain. J Pain. 2007;8:437–442.
- Flake NM, Hermanstyne TO, Gold MS. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. Am J Physiol Regul Integr Comp Physiol. 2006;291:R343–R348.
- Bertolo A, Baur M, Aebli N, et al. Physiological testosterone levels enhance chondrogenic extracellular matrix synthesis by male intervertebral disc cells in vitro, but not by mesenchymal stem cells. Spine J. 2014;14:455–468.
- Kovacheva EL, Sinha Hikim AP, Shen R, et al. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010;151:628–638.
- Traish AM, Haider A, Haider KS, et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–433.
- Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Dia Care. 2016;39:82–91.
- Mundy GR. Osteoporosis and inflammation. Nutrition Reviews. 2008;65:S147–S151.
- Maggio M, Basaria S, Ceda G, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:711–119.
- Maggio M, Ceda G, Milaneschi Y, et al. Effects of testosterone treatment on inflammatory markers in older men. CLINICAL-Male Reproductive Endocrinology II & Case Reports: The Endocrine Society 2011; P3-209–P3-209.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318.
- Pergola C, Rogge A, Dodt G, et al. Testosterone suppresses phospholipase D, causing sex differences in leukotriene biosynthesis in human monocytes. The FASEB Journal. 2011;25:3377–3387.
- Panach-Navarrete J, Martínez-Jabaloyas JM, group D-S. The influence of comorbidities on the aging males’ symptoms scale in patients with erectile dysfunction. The Aging Male. 2017;20:1–152.
- Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. The Aging Male. 2016;19:239–243.
- Kulej-Lyko K, Majda J, von Haehling S, et al. Could gonadal and adrenal androgen deficiencies contribute to the depressive symptoms in men with systolic heart failure? The Aging Male. 2016;19:221–230.
- Golan R, Scovell JM, Ramasamy R. Age-related testosterone decline is due to waning of both testicular and hypothalamic-pituitary function. The Aging Male. 2015;18:201–204.
- Tajar A, Forti G, O'neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European male ageing study.J Clin Endocrinol Metab. 2010;95:1810–1818.
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925.
- Ector H, Lemmens A, De Geest H. Further studies on Leydig cell function in old-age. Sort 1974;100:250.
- NEAVES WB, Johnson L, Porter JC, et al. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59:756–763.
- Takahashi PY, Liu PY, Roebuck PD, et al. Graded inhibition of pulsatile luteinizing hormone secretion by a selective gonadotropin-releasing hormone (GnRH)-receptor antagonist in healthy men: evidence that age attenuates hypothalamic GnRH outflow. J Clin Endocrinol Metab. 2005;90:2768–2774.
- Spector T, Ollier W, Perry L, et al. Free and serum testosterone levels in 276 males: a comparative study of rheumatoid arthritis, ankylosing spondylitis and healthy controls. Clin Rheumatol. 1989;8:37–41.
- Lashkari M, Noori A, Oveisi S, et al. Association of serum testosterone and dehydroepiandrosterone sulfate with rheumatoid arthritis: a case control study. Electron Physician. 2018;10:6500.
- Tengstrand B, Carlström K, Hafström I. Bioavailable testosterone in men with rheumatoid arthritis-high frequency of hypogonadism. Rheumatology (Oxford). 2002;41:285–289.
- Harbuz M, Perveen-Gill Z, Lightman S, et al. A protective role for testosterone in adjuvant-induced arthritis. Br J Rheumatol. 1995;34:1117–1122.
- Schmidt M, Weidler C, Naumann H, et al. Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes–androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5α-reduced androgens. Arthritis Res Ther. 2005;7:R938.
- Castagnetta LA, Carruba G, Granata OM, et al. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003;30:2597–2605.
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574.
- Schmidt M, Naumann H, Weidler C, et al. Inflammation and sex hormone metabolism. Ann N Y Acad Sci. 2006;1069:236–246.
- Calleja-Agius J, Muscat-Baron Y, Brincat M. Estrogens and the intervertebral disc. Menopause Int. 2009;15:127–130.
- Fink J, Schoenfeld BJ, Nakazato K. The role of hormones in muscle hypertrophy. Phys Sportsmed. 2018;46:129–134.
- La Colla A, Pronsato L, Milanesi L, et al. 17β-Estradiol and testosterone in sarcopenia: role of satellite cells.Ageing Res Rev. 2015;24:166–177.
- La Vignera S, Condorelli R, Vicari E, et al. Original immunophenotype of blood endothelial progenitor cells and microparticles in patients with isolated arterial erectile dysfunction and late onset hypogonadism: effects of androgen replacement therapy. The Aging Male. 2011;14:183–189.
- Almehmadi Y, Yassin D-J, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. The Aging Male. 2015;18:186–194.
- Kaya E, Sikka SC, Kadowitz PJ, et al. Aging and sexual health: getting to the problem. Aging Male. 2017;20:65–80.
- Bia³ek M, Zaremba P, Borowicz KK, et al. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004;56:509–518.
- Beyer C, Green SJ, Hutchison JB. Androgens influence sexual differentiation of embryonic mouse hypothalamic aromatase neurons in vitro. Endocrinology 1994;135:1220–1226.
- Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Brain Res Dev Brain Res. 1997;98:74–81.
- Sar M, Stumpf WE. Androgen concentration in motor neurons of cranial nerves and spinal cord. Science. 1977;197:77–79.
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895.
- Brooks BP, Merry DE, Paulson HL, et al. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70:1054–1060.
- Yassin A, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study . Andrologia 2016;48:793–799.
- Moran LJ, Brinkworth GD, Martin S, et al. Long-term effects of a randomised controlled trial comparing high protein or high carbohydrate weight loss diets on testosterone, SHBG, erectile and urinary function in overweight and obese men. PloS One. 2016;11:e0161297
- Patel P, Kohn T, Ramasamy R. pd09-10 impaired sleep is associated with low testosterone in us adult males: results from the national health and nutrition survey. J Urol . 2018;199:e226.
- Ahtiainen JP, Nyman K, Huhtaniemi I, et al. Effects of resistance training on testosterone metabolism in younger and older men.Exp Gerontol. 2015;69:148–158.
- Fui MNT, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16:223.
- Yi X, Gao H, Chen D, et al. Effects of obesity and exercise on testicular leptin signal transduction and testosterone biosynthesis in male mice. Am J Physiol Regul Integr Comp Physiol. 2017;312:R501–R510.
- Zhao J, Zhai L, Liu Z, et al. Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue.Oxid Med Cell Longev. 2014;2014:190945.
- Ebrahimi F, Schuetz P, Mueller B, et al. Effects of IL-1 [beta] on the hypothalamic-pituitary-gonadal axis in men with obesity and metabolic syndrome-A randomized, double-blind, placebo-controlled trial. 2017;49:EP687. doi:10.1530/endoabs.49.EP687
- Pasquali R, Casimirri F, De Iasio R, et al. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metab. 1995;80:654–658.
- Auyeung TW, Kwok T, Leung J, et al. Sleep duration and disturbances were associated with testosterone level, muscle mass, and muscle strength—a cross-sectional study in 1274 older men.J Am Med Dir Assoc. 2015;16:630. e1–630. e6.
- Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl. 2014;16:262–265.
- Cadore EL, Lhullier FLR, Brentano MA, et al. Hormonal responses to resistance exercise in long-term trained and untrained middle-aged men. J Strength Cond Res. 2008;22:1617–1624.
- Ahtiainen JP, Pakarinen A, Alen M, et al. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol. 2003;89:555–563.
- Hayes LD, Sculthorpe N, Herbert P, et al. Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men. Aging Male. 2015;18:195–200.
- Hackney A. Effects of endurance exercise on the reproductive system of men: the “exercise-hypogonadal male condition”. J Endocrinol Invest. 2008;31:932–938.
- Haring R, Ittermann T, Völzke H, et al. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. The Aging Male. 2010;13:247–257.
- de Souza GL, Hallak J. Anabolic steroids and male infertility: a comprehensive review. BJU Int. 2011;108:1860–1865.
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559.
- Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Transl Androl Urol. 2016;5:834–843.
- Salman M, Yassin D-J, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. The Aging Male. 2017;20:45–48.
- Dohle G, Arver S, Bettocchi C, et al. Guidelines on male hypogonadism 2015. European Association of Urology [cited 2016 July]. Available from: http://uroweborg/wp-content/uploads/EAU-Guidelines-Male-Hypogonadism-2015 pdf.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus–a series of case reports. The Aging Male. 2015;18:164–168.
- Wolf J, Keipert D, Motazedi H, et al. Effectiveness and tolerability of parenteral testosterone undecanoate: a post-marketing surveillance study. The Aging Male. 2017;20:225–234.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. The Aging Male. 2016;19:64–69.
- Carruthers M, Cathcart P, Feneley MR. Evolution of testosterone treatment over 25 years: symptom responses, endocrine profiles and cardiovascular changes. The Aging Male. 2015;18:217–227.
- Trinick TR, Feneley MR, Welford H, et al. International web survey shows high prevalence of symptomatic testosterone deficiency in men. The Aging Male. 2011;14:10–15.