Abstract
Objective
Our study aimed to investigate the age-related growth in prostatic morphological parameters in Chinese benign prostatic hyperplasia (BPH) patients, and to find out the regularity of how these parameters change with aging.
Methods
Medical records of 1038 BPH patients were obtained from a retrospective database of first-visit men with BPH. Change regularity of prostatic anatomical factors with aging was analyzed.
Results
Patients were classified into four groups according to different age decades. All prostatic anatomical factors assessed in this research increased with age growth (p < .0001). However, these anatomical factors sustained stably when older than 70 years. By analyzing the detailed correlation between age and prostatic morphological parameters, transitional zone index (TZI) (Pearson r = 0.358, r2 = 0.128, p < .0001) and transitional zone width (TZW) (Pearson r = 0.344, r 2= 0.118, p < .0001) showed the best correlation coefficient with age. After adjusted the influence of cardiovascular disease (CVD) and diabetes mellitus (DM), the result remained still similarly.
Conclusion
Prostatic morphological parameters increase progressively with age growth when patients were younger than 70 years, indicating reasonable interventions to be provided to BPH patients before 70 years. In addition, TZI and TZW are two practical, easy-to-measure prostatic parameters that are significantly associated with the growth of age compared to others.
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common conditions among advance-aged men [Citation1]. In UK, BPH affects 3.2 million men and surgery for lower urinary tract symptoms (LUTS) is one of the 10 most common surgeries performed by the National Health Service [Citation2]. In the US, ∼25% of males have undergone surgery for BPH [Citation3]. For over a century, there have been two main etiologic factors for the pathogenesis of BPH: aging and androgens [Citation4]. Unlike other organs that atrophy with age, prostate volume (PV) usually increases. Autopsy studies revealed the presence of BPH in 42% of men aged 51–60 years and 85% among men older than 80 years [Citation5]. About half the number of men with BPH will develop an enlarged prostate gland, which is called benign prostatic enlargement (BPE).
Various methods to assess BPE have been reported, including digital rectal examination (DRE), magnetic resonance imaging (MRI), total prostate-specific antigen (PSA), and other novel indicators like visceral adiposity index (VAI) [Citation6], triglyceride (TG)/high density lipoprotein (HDL) ratio [Citation7], dihydrotestosterone (DHT)/serum total testosterone (T) ratio [Citation8] and so on. But transrectal ultrasound (TRUS) is still the most commonly used and standard detection method to evaluate whether BPE exists. TRUS of the prostate has a diagnostic accuracy >80% [Citation9]. PV, transitional zone volume (TZV), transitional zone index (TZI) and intravesical prostatic protrusion (IPP) are the most frequently-used prostatic anatomical factors to assess the size of the prostate. BPH can lead to the development of prostatic bladder outlet obstruction (BOO) and LUTS. Reports had verified that parameters including TZV, TZI, IPP etc. are more significantly associated with urinary symptom scores, maximum urinary flow rate (Qmax), and urodynamically confirmed obstruction compared to PV [Citation10,Citation11]. But the detailed correlation between age and prostatic anatomical factors and how these parameters change with the growth of age are still ambiguous.
In addition, the sixth national census of China in 2010 showed that 13.26% of the Chinese population were older than 60 years [Citation12]. The predicted increase in the proportion of elderly people in future years means that the prevalence of BPH and costs of treatment will continue to increase. From these viewpoints and in order to help clinicians to provide more reasonable and timely interventions to BPH patients, this research was investigated in a large cohort of first-visit men with BPH.
Patients and methods
This was a retrospective study conducted on patients diagnosed with BPH at their first outpatient visiting at Xinhua Hospital. From April 2013 to November 2017, a total of 1038 patients were analyzed. Detailed information of age, serum total PSA, prostatic anatomical factors including PV (transverse width, cephalocaudal height, anteroposterior length), TZV (transitional zone width (TZW), transitional zone length (TZL), transitional zone height (TZH)), TZI, and IPP were collected. Because of the positive association between BPH/LUTS and cardiovascular disease (CVD), diabetes mellitus (DM) [Citation13–15], these two comorbidities were also recruited. A systematic prostate biopsy was applied to patients with tPSA of over 4.0 ng/mL, and no malignant components were found. Patients with other comorbidities, such as bladder or prostate malignancy, a history of lower urinary tract surgery, urinary tract infection (UTI), or any other neurological disease (like Parkinson’s disease, a history of spinal operation) that could affect patients’ voiding function when diagnosed, were excluded.
The study was performed in accordance with applicable laws and regulations, and ethical principles as described in the Declaration of Helsinki. The Ethics Committee of Xinhua Hospital approved the study protocol and informed consent was exempted.
Assessment of prostatic anatomical factors
The ultrasound machine made by Siemens Sequoia 512 (EV8C4-S, frequency 3 ∼ 8 MHz) was used to estimate PV (mL), TZV (mL) and IPP (mm). IPP was measured by the vertical distance from the tip of the protruding prostate to the base of the urinary bladder, seen in the midsagittal plane of the TRUS [Citation16–18]. PV was also assessed by TRUS by placing the patient in the left decubitus position. The width (cm), length (cm) and height (cm) of the total prostate as well as those of the transition zone (TZ) were measured from both the transaxial and midsagittal planes. Measurements of the PV and TZV were calculated using the prostate ellipsoid formula (height × width × length × π/6). TZI was calculated by dividing the TZV by the PV [Citation19]. All measurements were performed by experienced sonographers.
Furthermore, based on the assumption that the PV growth rate is linear over time and using a PV of 20 mL at the age of 40 [Citation20], an age-adjusted PV growth rate was calculated [Citation21–23]. As a result, the annual prostate growth rate was calculated as (prostate volume-20 mL)/(age-40 years) [Citation12].
Statistical analysis
Statistical comparisons were performed using the statistical software GraphPad Prism 5. Continuous variables were expressed as mean ± standard deviation (SD). The intergroup differences were tested using the Student’s t-test and analysis of variance (ANOVA). Categorical variables were expressed as frequency (percentage). The intergroup differences were tested using Chi-square test. Pearson correlation analysis was used to analyze the interdependency between variables. Statistical significance was defined as a p < .05.
Results
Demographic data
A total of 1038 patients were reviewed for analyses. The patients were classified into four age decades: those between 40–59 years (Group 1), 60–69 years (Group 2), 70–79 years (Group 3), and those above 80 years (Group 4). All demographic characteristics of the 1038 patients were listed in .
Table 1. Comparison of patients’ characteristics according to age distribution (mean (SD) or n (%)).
Relationship between prostatic anatomical factors and age growth
There were significant differences in all prostatic anatomical factors detected in different age decades, with all the p < .0001 (). Post-test showed all prostatic anatomical factors increased progressively with the growth of age. But when patients were older than 70 years, the prostatic anatomical factors did not change that much and sustained stable (except for IPP, which didn’t increase obviously when patients were older than 60 years of age) ().
Relationship between PV growth rate and age decades
In the overall study population, PV increased at a mean (SD) rate of 1.63 (1.35) mL per year (range –3.00 mL to 15.86 mL), which corresponded to a mean (SD) 9.58 (7.95)% annual change (range –17.65% to 93.29%). Detailed, 735 (70.81%) men had prostate growth, and 303 (29.19%) had a stable (increased less than 5%) or decreased PV. Furthermore, the PV growth rate was calculated according to the age subgroup. As age increased, the PV growth rate was diminishing (). As in consistent with the previous results, the PV growth rate sustained stable when older than 70 years of age.
Table 2. Annual PV growth rate in different age decades (mean (SD)).
Correlation analysis between age growth and prostatic anatomical factors variation
Pearson correlation analysis was used to analyze the relationship between age and all prostatic anatomical factors assessed in this research. Results showed that PV (including width, length, and height), TZV (including TZW, TZL, and TZH), TZI and IPP all increased when patients’ age grew. But among those parameters, TZI (Pearson r = 0.358, r2 = 0.128, p < .0001) and TZW (Pearson r = 0.344, r2 = 0.118, p < .0001) showed the highest correlation coefficient (). Unfortunately, the correlation coefficient was still too low to make any clinical significance.
Figure 1. Pearson correlation analysis of age and prostatic anatomical factors (PV, PW, PL, PH, TZV, TZW, TZL, TZH, TZI and IPP). IPP: intravesical prostatic protrusion; PH: prostate height; PL: prostate length; PV: prostate volume; PW: prostate width; TZH: transitional zone length; TZI: transitional zone index; TZL: transitional zone length; TZV: transitional zone volume; TZW: transitional zone width. Pearson r of PV (A), PW (B), PL (C), PH (D), TZV (E), TZW (F), TZL (G), TZH (H), TZI (I), IPP (J) and age were 0.257, 0.179, 0.277, 0.286, 0.284, 0.344, 0.278, 0.320, 0.358, 0.201, respectively, with all the p < .0001***.
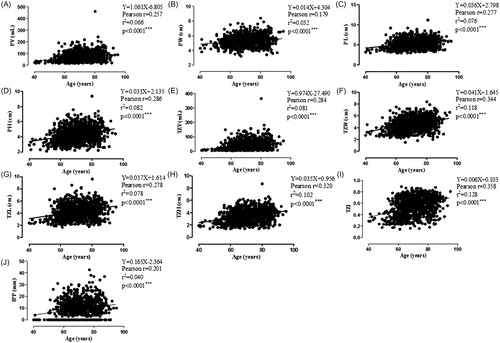
Furthermore, subgroup analysis according to the degree of comorbidity was applied, in order to get a more explicit conclusion. Pearson correlation analysis was used to analyze the relationship between age and all the prostatic anatomical factors. Similar to the total demography, TZI and TZW showed the highest correlation coefficients with age ().
Table 3. Subgroup pearson correlation analysis of age and prostatic anatomical factors according to the degree of comorbidity.
Discussion
BPH in the aging male will increase in socioeconomic and medical importance at a time of increased life expectancy and aging of the baby boomer generation [Citation24]. Prevalence of BPH in Asian men over age 50 years is reported to be as high as that of western countries [Citation25]. The precise molecular etiology of BPH is complicated and still unknown, but several theories have been proposed. These include aging, androgens, estrogens, oxidoreductase, embryonic reawakening, and inflammation theories [Citation26]. Autopsy studies have revealed that the prevalence of pathologic BPH increases apparently with the growth of age [Citation1]. The high prevalence of BPH in advance-aged men has led some to consider prostatic hyperplasia to be a ubiquitous result of aging [Citation27]. Thus, age is an important associated factor for the development of both histologic and clinically significant BPH. Although nearly all men will develop histologic BPH with age growth, it is thought that BPH initiation occurs under 30 years of age [Citation1]. Furthermore, younger men develop BPH but at much lower rates compared to aged men [Citation1]. This is in line with our discoveries. In our research, the mean age was 70.61 years and we found the youngest patient bothered with LUTS/BPH was 40 years. And among all the 1038 patients, those age ≥60 years occupied 90.27% (937/1,038) of the total population.
Sex hormone is another significant etiology of the pathologic BPH [Citation4,Citation26]. Previous studies have shown a positive correlation between BPH and changes in free T, DHT, estriol, and estradiol levels [Citation8,Citation28–30]. While the precise molecular etiology remains ambiguous, sex steroids have been implicated in the development and maintenance of BPH [Citation26,Citation27]. However, the circulating levels of many sex steroids show a marked decrease with age growth. In men, age-related steroid hormonal changes are less dramatic, but any of serum total T, free T and dehydroepiandrosterone sulfate (DHEAS) do show a significant age-related decrease in the circulation [Citation30–34]. That may partially explain the correlation between age and prostatic morphology discovered in our research. But controversies still exist, because some studies showed low testosterone occurred in more than one out of every five BPH patients at the mean (SD) age of 63.4 (6.8) years [Citation34], and androgen replacement therapy for hypogonadal men with mild BPH could contribute to the improvement of LUTS [Citation32]. Whereas, BPH is a multi-etiological disease, the precise etiology is still needed to be explored.
The prostate is pathologically subdivided into three anatomical regions. The anterior prostate makes up ∼20% of the volume of the prostate, the peripheral portion of the gland (forms the posterior and lateral surfaces of the prostate) represents ∼70% of the total glandular volume, and the central gland makes up the remainder of the prostate. Transitional zone (TZ) is the periurethral tissue located in the central. McNeal et al noted that BPH nodules arose from TZ [Citation35]. TZ hypertrophies with age and results in BPH [Citation36]. BPH is defined by proliferation of smooth muscle and epithelial cells in the TZ of the prostate [Citation37], and TZ is the leading cause of gland enlargement and intraprostatic pressure [Citation10,Citation38]. By analyzing the relationship between age and prostatic anatomical factors in our research, we found all parameters increased progressively with age. But among those, the measurements of TZ (especially TZI and TZW) had the best correlation with age, meaning TZ was the main risk factor resulting in the prostate gland enlargement and causing BPH. Furthermore, TZW was the main contributor to the TZ growth. And from the viewpoint of prostatic morphology with the finding of our research, we can speculate that TZI and TZW should be more significantly associated with symptom severity compared to other prostatic anatomical factors. This was in line with the results of the literature [Citation19,Citation39,Citation40].
Metabolic syndrome (MetS) is a cluster of metabolic disorders increasing the risk of CVD and type 2 DM, associated with obesity, dyslipidemia, hyperglycemia, elevated blood pressure and insulin resistance [Citation41–43]. There is also an evidence that metabolic disorders play a role in promoting the development of pathological and clinical BPH [Citation21,Citation44–48]. Moreover, family history, race/ethnicity, have also been reported to be risk factors of BPH [Citation42]. However all of the proposed etiologies above, require the presence of androgens [Citation27], and age-related changes in androgens are the main factors involved in the pathogenesis of BPH [Citation1,Citation28,Citation49]. As in consistent with those theories, our research found that CVD and DM would influence the correlation between age and prostatic anatomical factors, but the influence was not that much. Age and androgens are still the dominant influence factors of prostatic morphology.
However, our study has its limitations. First, it was conducted in a single institution and all TURS were performed by different sonographers, which may have been subject to selection bias. Further large-scale and well-designed studies conducted in multicenter will be mandatory. Second, it included only Chinese men. The population within each country differs culturally and socio demographically. And we suggest that our Chinese-oriented results need to be validated in other ethnic groups. Finally, the study did not include all components of MetS. We just analyzed the influence of CVD and DM. As a result, additional studies including these variables in the future are needed.
In a nutshell, prostatic anatomical factors increase with the growth of age when younger than 70 years. From this viewpoint, our present findings suggest reasonable interventions to be provided to BPH patients before 70 years of age. In addition, TZ growth, especially the TZW growth is the main contributor to the total prostate growth. TZI and TZW are two practical, easy-to-measure prostate parameters that are significantly associated with the growth of age than other parameters. However, the correlation coefficient was too low to make any clinical significance. Thus, the clinical usefulness of TZI and TZW should be further validated for managing men with BPH.
Acknowledgements
The authors thank Craig R. Brooks (Vanderbilt University Medical Center), and Xinyan Li (School of Pharmacy, Shanghai Jiao Tong University) for their statistical/writing assistance. The authors also wish to acknowledge the contributions of Weiming Wang, Liang Zhang and other colleagues in Xinhua Hospital (Department of Urology, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University) for their data collecting.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Yu Wu, upon reasonable request.
Additional information
Notes on contributors
Subo Qian
Subo Qian, M.D., resident urologist, graduated from Shanghai Jiao Tong University School of Medicine, and also had the bachelor’s degree in pharmacy. He is doing postdoctoral research in Vanderbilt University Medical Center, specializing in kidney injury, as well as basic and clinical researches on genitourinary cancer. He has got more than 10 papers published in some authoritative journals in recent 5 years.
Xujun Sheng
Xujun Sheng, M.D., associate chief urologist, graduated from Shanghai Jiao Tong University School of Medicine, specializing in andrology and prostate disease.
Ding Xu
Ding Xu, M.D., resident urologist, graduated from Shanghai Jiao Tong University School of Medicine. He is an experienced surgeon in prostate disease, especially in benign prostatic hyperplasia.
Haibo Shen
Haibo Shen, M.D., Ph.D., associate chief urologist, master's supervisor, graduated from Shanghai Jiao Tong University School of Medicine. He is an experienced surgeon in urinary system tumors, and conducting some basic and clinical researches in this field.
Jun Qi
Jun Qi, M.D., Ph.D., chief urologist, director of the department of urology, doctoral supervisor. He is specializing in renal transplant, open and laparoscopic surgery on genitourinary cancers. He has more than 20 high-quality papers published in some authoritative journals in recent 5 years.
Yu Wu
Yu Wu, M.D., Ph.D., attending urologist, specializing in andrology, especially for benign prostatic hyperplasia and buried penis. He is now members of some professional associations in Urology and Andrology.
References
- Berry SJ, Coffey DS, Walsh PC. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479.
- Bardoli AD, Taylor WSJ, Mahmalji W. Can the UroLift prostatic implant device treat the symptoms of benign prostatic hypertrophy, avoid sexual dysfunction and reduce hospital TURP waiting times? A single centre, single surgeon experience and review of the literature. Aging Male. 2017;20:192–197.
- Erkoc M, Otunctemur A, Besiroglu H, et al. Evaluation of quality of life in patients undergoing surgery for benign prostatic hyperplasia. Aging Male. 2018;1–5. DOI:10.1080/13685538.2018.1433654.
- Lee C, Kozlowski JM, Grayhack JT. Etiology of benign prostatic hyperplasia. Urol Clin North Am. 1995;22:237–246.
- Holtgrewe HL. Economic issues and the management of benign prostatic hyperplasia. Urology. 1995;46:23–25.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Besiroglu H, Dursun M, Otunctemur A, et al. The association between triglyceride high density lipoprotein cholesterol ratio and benign prostate hyperplasia in non-diabetic patients:a cross-sectional study. Aging Male. 2017;20:1–204.
- Liao CH, Li HY, Chung SD, et al. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40-79 years. Aging Male. 2012;15:28–33.
- Coban S, Doluoglu OG, Keles I, et al. Age and total and free prostate-specific antigen levels for predicting prostate volume in patients with benign prostatic hyperplasia. Aging Male. 2016;19:124–127.
- Kwon JK, Han JH, Choi HC, et al. Clinical significance of peripheral zone thickness in men with lower urinary tract symptoms/benign prostatic hyperplasia. BJU Int. 2016;117:316–322.
- Foo KT. Pathophysiology of clinical benign prostatic hyperplasia. Asian J Urol. 2017;4:152–157.
- Pan JG, Jiang C, Luo R, et al. Association of metabolic syndrome and benign prostatic hyperplasia in Chinese patients of different age decades. Urol Int. 2014;93:10–16.
- Ferreira FT, Daltoe L, Succi G, et al. Relation between glycemic levels and low tract urinary symptoms in elderly. Aging Male. 2015;18:34–37.
- Mathur RP, Nayak S, Sivaramakrishnan R, et al. Role of alpha blockers in hypertension with benign prostatic hyperplasia. J Assoc Physicians India. 2014;62:40–44.
- Hwang EC, Kim SO, Nam DH, et al. Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. Low Urin Tract Symptoms. 2015;7:32–36.
- Yuen JS, Ngiap JT, Cheng CW, et al. Effects of bladder volume on transabdominal ultrasound measurements of intravesical prostatic protrusion and volume. Int J Urol. 2002;9:225–229.
- Tan YH, Foo KT. Intravesical prostatic protrusion predicts the outcome of a trial without catheter following acute urine retention. J Urol. 2003;170:2339–2341.
- Xu D, Yu Y, Zhu Y, et al. A new model consists of intravesical prostatic protrusion, prostate volume and serum prostatic-specific antigen in the evaluation of prostate cancer. Pathol Oncol Res. 2014;20:439–443.
- Kaplan SA, Te AE, Pressler LB, et al. Transition zone index as a method of assessing benign prostatic hyperplasia: correlation with symptoms, urine flow and detrusor pressure. J Urol. 1995;154:1764–1769.
- Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991;145:984–987.
- Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151–158.
- Hammarsten J, Högstedt B. Calculated fast-growing benign prostatic hyperplasia–a risk factor for developing clinical prostate cancer. Scand J Urol Nephrol. 2002;36:330–338.
- Hammarsten J, Högstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55.
- Gu X, Strom K, Spaliviero M, et al. Does age affect the efficacy and safety of GreenLight HPS laser photoselective vaporization prostatectomy?. Aging Male. 2012;15:63–67.
- Lin KH, Lin YW, Wen YC, et al. Efficacy and safety of orally disintegrating tamsulosin tablets in Taiwanese patients with benign prostatic hyperplasia. Aging Male. 2012;15:246–252.
- Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–199.
- Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41.
- Partin AW, Oesterling JE, Epstein JI, et al. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405–409.
- Xu D, Wu Y, Shen H, et al. High serum concentration of estradiol may be a risk factor of prostate enlargement in aging male in China. Aging Male. 2018;1–6. DOI:10.1080/13685538.2018.1481027.
- Asiedu B, Anang Y, Nyarko A, et al. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male. 2017;20:17–22.
- Urbanski HF, Sorwell KG, Garyfallou VT, et al. Androgen supplementation during aging: development of a physiologically appropriate protocol. Rejuvenation Res. 2014;17:150–153.
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14:53–58.
- Singam P, Hong GE, Ho C, et al. Nocturia in patients with benign prostatic hyperplasia: evaluating the significance of ageing, co-morbid illnesses, lifestyle and medical therapy in treatment outcome in real life practice. Aging Male. 2015;18:112–117.
- Kaplan SA, O’Neill E, Lowe R, et al. Prevalence of low testosterone in aging men with benign prostatic hyperplasia: data from the Proscar Long-term Efficacy and Safety Study (PLESS). Aging Male. 2013;16:48–51.
- McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345.
- Priest R, Garzotto M, Kaufman J. Benign prostatic hyperplasia: a brief overview of pathogenesis, diagnosis, and therapy. Tech Vasc Interv Radiol. 2012;15:261–264.
- Lee C, Kozlowski JM, Grayhack JT. Intrinsic and extrinsic factors controlling benign prostatic growth. Prostate. 1997;31:131–138.
- Choi JD, Kim JH, Ahn SH. Transitional zone index as a predictor of the efficacy of alpha-blocker and 5alpha-reductase inhibitor combination therapy in Korean patients with benign prostatic hyperplasia. Urol Int. 2016;96:406–412.
- Kurita Y, Ushiyama T, Suzuki K, et al. Transition zone ratio and prostate-specific antigen density: the index of response of benign prostatic hypertrophy to an alpha blocker. Int J Urol. 1996;3:361–366.
- Cao N, Lu Q, Si J, et al. The characteristics of the transitional zone in prostate growth with age. Urology. 2017;105:136–140.
- Post JM, Beebe-Dimmer JL, Morgenstern H, et al. The metabolic syndrome and biochemical recurrence following radical prostatectomy. Prostate Cancer. 2011;2011:245642.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752.
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421.
- Nandeesha H, Koner BC, Dorairajan LN, et al. Hyperinsulinemia and dyslipidemia in non-diabetic benign prostatic hyperplasia. Clin Chim Acta. 2006;370:89–93.
- Lee S, Min HG, Choi SH, et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity (Silver Spring). 2006;14:172–179.
- Laven BA, Orsini N, Andersson SO, et al. Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol. 2008;179:1891–1895.
- Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012;110:540–545.
- Aktas BK, Gokkaya CS, Bulut S, et al. Impact of metabolic syndrome on erectile dysfunction and lower urinary tract symptoms in benign prostatic hyperplasia patients. Aging Male. 2011;14:48–52.
- Platz EA, Smit E, Curhan GC, et al. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology. 2002;59:877–883.
