Abstract
This study investigated what sort of effects would occur in terms of sexual function in Japanese patients with benign prostatic hyperplasia (BPH), upon switching from combination therapy with an α1 blocker (AB) and dutasteride (DUT) to combination therapy with tadalafil (TAD) and DUT. The baseline and the 15-item International Index of Erectile Function (IIEF-15), Erection Hardness Score (EHS), International Prostate Symptom Score (IPSS), and Overactive Bladder Symptom Score (OABSS) 3 months after switching to the daily administration of TAD 5 mg/DUT 0.5 mg combination therapy, along with the age, prostatic volume, body mass index (BMI), and past medical history of 49 patients who were treated with AB/DUT as pretreatment, were retrospectively investigated. TAD/DUT combination therapy significantly improved the total score of IIEF-15 (from 17.8 ± 11.6 to 21.4 ± 13.9, p = .0047), erectile function domain (from 5.8 ± 5.8 to 7.6 ± 7.1, p = .0186), and EHS (from 1.9 ± 1.3 to 2.6 ± 1.2, p < .0001). Although IPSS and QOL index were significantly improved, no significant differences were observed for OABSS. Switching from AB/DUT combination therapy to TAD/DUT combination therapy brought about improvement in erectile function while leaving room to improve urinary status in Japanese patients.
Introduction
For males from middle age to old age, lower urinary tract symptoms (LUTS) such as dysuria associated with benign prostatic hyperplasia (BPH), along with an overactive bladder, are very common clinical symptoms, directly linked to a decline in quality of life (QOL) [Citation1]. The phosphodiesterase type 5 inhibitor (PDE5-I) is a recommended remedy for LUTS associated with BPH (LUTS/BPH), like 5α reductase inhibitors (5ARI) and α1 blockers (AB), in the guidelines of many countries [Citation2–4]. While tadalafil (TAD), one PDE 5-I, had originally been used as an erectile function-improving drug in Japan, it was recommended as a first-line drug against LUTS/BPH according to the Japanese guidelines in 2017 as well. While AB and 5ARI, which have been administered for BPH to date, have been found to have beneficial effects on LUTS, sexual dysfunction, including erectile dysfunction (ED), ejaculation disorder and decreased libido, are widely recognized as adverse effects (AE) of these drugs [Citation5]. It has also been reported that the incidence rate of sexual AE increased, not only upon single treatment of AB and 5ARI, but also upon using them in combination [Citation5–7]. Management of sexual AE is an important element of care, not only for men who are sexually active, but also in terms of the dignity and QOL of all men of the age for undergoing LUTS/BPH treatment.
TAD comprehensively improves LUTS via the activation of the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) signaling pathway and also due to its anti-inflammatory action [Citation8]. A recent study has suggested that the daily administration of 5 mg TAD resulted in an improvement of the vascular endothelial function by decreasing the brachial-ankle pulse wave velocity [Citation9]. It has been pointed out that LUTS and ED have common backgrounds including a decrease in the smooth muscle relaxant effect as well as ischemia and hypoxia through impairment of vascular endothelial function [Citation10], with several RCTs reporting that TAD combined with 5ARI as a treatment for LUTS incidentally improves sexual dysfunction [Citation11,Citation12].
Therefore, the purpose of this study was to retrospectively investigate what sort of effects on sexual function were observed prior to and following treatment, after 3 months of treatment upon switching to TAD/DUT combination therapy in Japanese BPH patients, in addition to investigating if there were any related factors that could have predicted the effect thereof prior to switching.
Methods
Study cohort
Patients simultaneously prescribed 5 mg Zaltia® and 0.5 mg Avolve® capsules at our institution were selected from the database of the electronic medical records system, using the drug code, from April 2017 to May 2018. Among a total of 74 selected cases, cases not treated with AB/DUT as a pretreatment for TAD/DUT combination therapy (10 cases), cases combined with anticholinergic drugs (5 cases), patients who underwent treatment for urinary tract infection during TAD/DUT combination therapy (3 cases), cases in which the 15-item International Index of Erectile Function (IIEF-15) [Citation13], Erection Hardness Score (EHS) [Citation14], International Prostate Symptom Score (IPSS) [Citation15], and Overactive Bladder Symptom Score (OABSS) [Citation16] that were routinely performed at the time that treatment was started and 3 months after the start of examinations, but were not confirmed on the electronic medical chart, or cases in which entry was missed (7 cases), were excluded from our cohorts. Regarding the cutoff value of each parameter, we used 65 for age, 40 mL as the median for prostate volume, and 25 kg/m2 for body mass index (BMI) as the Asian obesity cutoff value according to the WHO classification [Citation17]. In this study, sexual status before and 3 months after the TAD/DUT combination therapy were evaluated by IIEF-15, each domain of IIEF-15 and EHS, while the urinary status was evaluated by IPSS and OABSS. The erectile function (EF) domain score (range 1–30) calculated using the sum of the values of questions 1, 2, 3, 4, 5, and 15 of IIEF-15, were determined as the ED severity classification: 1–6 as severe; 7–12 as moderate; 13–18 as mild-moderate; 19–24 as mild; and 25–30 as normal. The protocol and all methods for the retrospective research were approved by the Ethics Committee of Koto Hospital.
Statistical analysis
The Wilcoxon signed-rank test was used for comparing continuous variables between two groups with correspondence, while a χ2 test was used for comparing categorical variables between two groups with no correspondence. p values less than .05 were considered to indicate statistical significance. All statistical analyses were performed using JMP® 14 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics at baseline before switching to TAD/DUT combination therapy
The patient characteristics are shown in . The mean age value ± SD for a total of 49 patients was 66.7 ± 6.9 years old, with a range of 50 to 78 years old. The median prostate volume was 40 mL. The mean value ± SD of the period when the AB/DUT combination therapy was performed prior to TAD/DUT combination therapy was 6.1 ± 2.2 months. Among 49 patients, 10 (20.4%) had diabetes, 14 (28.6%) had controlled hypertension, and 16 (32.7%) had dyslipidemia. Furthermore, 22 (44.9%) patients had BMI ≥25 kg/m2, which is the Asian obesity cutoff value according to the WHO classification [Citation15,Citation17], and 7 (14.3%) were smokers. The mean value ± SD of IIEF-15, EHS, IPSS, QOL index, and OABSS as a baseline prior to TAD/DUT combination therapy were respectively, 17.8 ± 11.6, 1.9 ± 1.3, 14.2 ± 8.6, 4.0 ± 1.4, and 5.0 ± 3.3 ().
Table 1. Baseline characteristics.
Table 2. Changes in IIEF-15, EHS, IPSS, and OABSS with the TAD/DUT combination therapy.
Changes in sexual status after switching to TAD/DUT combination therapy
The mean value ± SD of total IIEF-15, EF domain, Intercourse Satisfaction domain, Overall Satisfaction domain, and EHS 3 months after switching to TAD/DUT combination therapy, were respectively 21.4 ± 13.9, 7.6 ± 7.1, 1.4 ± 2.6, 5.3 ± 1.5, and 2.6 ± 1.2. They were significantly higher than the original baseline values (17.8 ± 11.6, 5.8 ± 5.8, 0.7 ± 2.0, 4.6 ± 1.7, 1.9 ± 1.3, respectively) (). While IPSS 3 months after switching and the mean values ± SD of the QOL index were 11.5 ± 7.6 and 3.3 ± 1.2, respectively, which were significantly lower than the baseline values (14.2 ± 8.6 and 4.0 ± 1.4, respectively), no significant differences were found for OABSS ().
Factors related to improving ED severity
Each ratio of ED severity classification before switching and 3 months after switching to TAD/DUT combination therapy is shown in . The baseline severity among 49 patients exhibited: 35 (71.4%) severe; 7 (14.3%) moderate; 3 (6.1%) mild-moderate; and 4 (8.2%) mild. The severity 3 months after switching to TAD/DUT combination therapy exhibited: 31 (63.3%) severe; 8 (16.3%) moderate; 5 (10.2%) mild-moderate; 3 (6.1%) mild; and 2 (4.1%) normal. Those in which the severity classification improved by one grade or more were defined as having an improved ED severity. In the comparative test between the ED severity improvement group and the unimproved group, being under age 65, prostate volume of less than 40 mL, BMI less than 25 kg/m2, and no hypertension were the factors related to the improved ED severity (p = .0048, p = .0278, p = .0129, and p = .025, respectively), while diabetes, dyslipidemia, and smoking history were not related factors ().
Figure 1. Changes in the patient ratio of ED severity, before and 3 months after switching to tadalafil/dutasteride combination therapy.
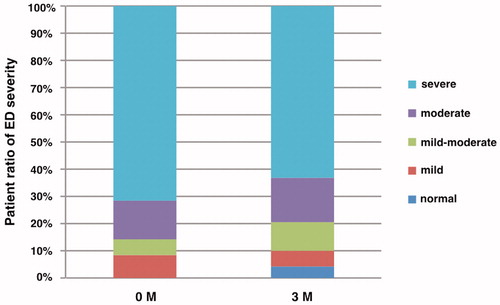
Table 3. Comparison of clinical characteristics between the improved ED group and unimproved ED group after 3 months of the TAD/DUT combination therapy.
Discussion
With respect to AB and 5ARI, which are therapeutic drugs for BPH, many reports on the expression rate of sexual AE have been acknowledged [Citation5,Citation7,Citation18]. Reduction of the smooth muscle relaxation effect, reduction of vascular endothelial function, and reduction of arterial blood flow rate causing tissue damage and hypoxic condition, which are cited as factors causing LUTS/BPH, are also originally included in the background of ED [Citation10], with the importance of sexual function assessment and monitoring of sexual AE associated with therapeutic agents having been emphasized for patients with LUTS/BPH [Citation19]. Three randomized, double-blinded trials involving the administration of 0.5 mg DUT once daily indicated, compared to the placebo control, that the incidence was significantly higher with respect to ED (7.3% vs. 4%), ejaculatory function decline (2.3% vs. 0.8%), and decreased libido (4.2% vs. 2.1%) [Citation20]. From the results of the 4-year CombAT study, it was found that when 0.4 mg Tamsulosin was used in combination with 0.5 mg DUT, a significantly increased expression rate was observed, more than with individual treatments, with ED, retrograde ejaculation, decreased libido, ejaculation inability, semen volume reduction, and libido disappearance [Citation6]. In our study, 71.4% of Japanese patients with BPH, who had already been treated with AB/DUT, had severe ED, resulting in no patients with normal erectile function. Some epidemiological studies have reported that age is the most important risk factor for ED and that the ED rate increase with age is higher for Japanese than Americans [Citation21]. Although reports on the relation between obesity and ED in Japanese people are few and clear conclusions have not been obtained [Citation22], in Europe and the United States, it is known that obesity and ED defined by an increase in BMI are also related [Citation23,Citation24]. It is considered that, based on our study, the fact that elderly patients over 65 years old accounted for 67% of all cohorts and obesity patients with a BMI of 25 kg/m2 or more also accounted for about half of the cohorts, might have greatly influenced the result that the ED ratio at baseline accounted for the majority.
TAD is a drug that improves LUTS and ED, with its main mechanism being the enhancement of the cGMP action produced by NO [Citation8]. According to a report on a review of four RCTs, which evaluated the therapeutic effects and safety upon daily administration of 5 mg TAD, there were no significant differences in the improvement of the total IPSS, in a comparison between the group made up of individuals aged 65 or younger and the group of those 66 or older, as well as between the group in which AB was administered for 12 months prior to the start of treatment and the group in which AB was not administered [Citation25]. This suggests that TAD may possibly demonstrate a positive effect on elderly ED patients aged 65 or older with low NO secretion regardless of whether or not the patients received AB intervention as a pretreatment. While patients aged 65 or older accounted for 67% in our study cohort, switching AB/DUT combination therapy to TAD/DUT combination therapy resulted in a significant improvement in the total score of IIEF-15, EF domain, and EHS. Our study ended up supporting the RCT of the United States in which the combination administration of TAD and FIN significantly improved the IIEF score of LUTS/BPH patients, over the placebo and FIN combination administration [Citation11], and also supported the Korean RCT, improved IIEF and sexual life satisfaction were obtained in the TAD/DUT combination group, compared with the combination group of Tamsulosin and DUT [Citation12]. Furthermore, focusing on improving the ED severity classified by EF domain, this study indicated that being 64 or younger, having a BMI of less than 25 kg/m2, having a prostatic volume of less than 40 mL, and an absence of hypertension could be factors enabling the prediction of EF improvement, in the case of switching from AB/DUT combination therapy to TAD/DUT combination therapy. In the report showing the relationship between the baseline characteristics and sexual function of 2916 males in the MTOPS study cohort, aging, especially an age of 70 years or more, and obesity defined by BMI of 30 kg/m2 or more are significantly associated with all declines in erectile function, ejaculation function, and sexual satisfaction [Citation24]. The report also indicates that patients having a baseline prostate volume of 30 mL or more have a significantly poorer erectile function and ejaculatory function than those with a baseline prostate volume of less than 30 mL [Citation24]. These findings suggest the importance of assessing age, BMI, and prostate volume, in predicting the presence or absence of sexual dysfunction, as a baseline before treatment in clinical practice. Switching to TAD/DUT combination therapy in consideration of age, BMI and prostatic volume can be a good option for patients who have already experienced ED during AB/DUT combination therapy or who are concerned about sexual AE expression due to treatment.
There is still no consensus regarding the association between decreases in erectile function and treatment with either AB and/or 5ARI; however, previous reports have suggested that the serum levels of testosterone and vitamin D and highly sensitive C-reactive protein play an important role in improving the erectile function [Citation26–29]. The most important limitation of this study is lack of information regarding these serum levels before and 3 months after TAD/DUT combination therapy. Therefore, the hormonal and metabolic mechanisms associated with an improvement in erectile function by switching to TAD/DUT combination therapy remain vague and unclear. Mondaini et al. randomly assigned patients with BPH who underwent administration of FIN to the group provided with information on sexual AE and the group not provided with the information, in order to conduct a blinded prospective comparison test, and reported that the group provided with information exhibited a significantly higher incidence of ED, decreased libido and ejaculation disorder [Citation30]. Although each physician explains sexual AE when prescribing DUT at our facility, when switching AB to TAD, we do not re-explain this with regard to AE of concurrent DUT. The period from first receiving an explanation on sexual AE to switching to TAD depends on the patient, with a possibility that the nosebo effect is not considered, in terms of whether or not the long-term memory of sexual AE of each patient is maintained. Furthermore, the period of AB/DUT combination therapy prior to TAD/DUT combination therapy was not consistent. This study is a retrospective study only for a small number of Japanese cases, so this limitation refers to the inability of examinations, upon excluding possible confounding factors that affect sexual functions, such as cardiovascular disease, diabetes, smoking history, sleep apnea syndrome, and mental illness.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012;367:248–257.
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–1109.
- Chua ME, Mendoza J, See M, IV, et al. A critical review of recent clinical practice guidelines on the diagnosis and treatment of non-neurogenic male lower urinary tract symptoms. Can Urol Assoc J. 2015;9:463–470.
- Lin KH, Lin YW, Wen YC, et al. Efficacy and safety of orally disintegrating tamsulosin tablets in Taiwanese patients with benign prostatic hyperplasia. Aging Male. 2012;15:246–252.
- Favilla V, Russo GI, Privitera S, et al. Impact of combination therapy 5-alpha reductase inhibitors (5-ARI) plus alpha-blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: a systematic review with meta-analysis. Aging Male. 2016;19:175–181.
- Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–131.
- Hagberg KW, Divan HA, Persson R, et al. Risk of erectile dysfunction associated with use of 5-α reductase inhibitors for benign prostatic hyperplasia or alopecia: population based studies using the Clinical Practice Research Datalink. Br Med J. 2016;354:i4823.
- Gacci M, Andersson KE, Chapple C, et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2016;70:124–133.
- Amano T, Earle C, Imao T, et al. Administration of daily 5 mg tadalafil improves endothelial function in patients with benign prostatic hyperplasia. Aging Male. 2018;21:77–82.
- Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2011;60:809–825.
- Casabe A, Roehrborn CG, Da Pozzo LF, et al. Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol. 2014;191:727–733.
- Park HJ, Park NC. Combination therapy with dutasteride and tadalafil in men with moderate-to-severe benign prostatic hyperplasia. Eur Urol Suppl. 2013;12:e1092.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830.
- Mulhall JP, Goldstein I, Bushmakin AG, et al. Validation of the erection hardness score. J Sex Med. 2007;4:1626–1634.
- Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557.
- Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology 2006;68:318–323.
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163.
- La Torre A, Giupponi G, Duffy D, et al. Sexual dysfunction related to drugs: a critical review. Part V: α-blocker and 5-ARI drugs. Pharmacopsychiatry 2016;49:3–13.
- Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol. 2005;47:824–837.
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002;60:434–441.
- Masumori N, Tsukamoto T, Kumamoto Y, et al. Decline of sexual function with age in Japanese men compared with American men-results of two community-based studies. Urology 1999;54:335–344.
- Terai A, Ichioka K, Matsui Y, et al. Association of lower urinary tract symptoms with erectile dysfunction in Japanese men. Urology. 2004;64:132–136.
- Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302–306.
- Fwu CW, Kirkali Z, McVary KT, et al. Cross-sectional and longitudinal associations of sexual function with lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol. 2015;193:231–238.
- Porst H, Oelke M, Goldfischer ER, et al. Efficacy and safety of tadalafil 5 mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analyses of pooled data from 4 multinational, randomized, placebo-controlled clinical studies. Urology. 2013;82:667–673.
- Maeda T, Kikuchi E, Hasegawa M, et al. A prospective longitudinal survey of erectile function status in symptomatic benign prostatic hyperplasia patients treated with dutasteride. Aging Male. 2016;19:111–116.
- Canguven O, Talib RA, El Ansari W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017;20:9–16.
- Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. Aging Male. 2016;19:239–243.
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–246.
- Mondaini N, Gontero P, Giubilei G, et al. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med. 2007;4:1708–1712.
