Abstract
Background and aim
We examined the relationship among vit.D3, AMH, FT3, FT4, and TSH, in addition to the serum levels of reproductive hormones (FSH, LH, prolactin, and free testosterone), in oligoasthenoteratozoospermia and azoospermia patients in a cohort of infertile men from Egypt to establish a clinical marker/cause-effect relationship.
Methods
This cross-sectional cohort study was carried out on 301 men (105 males with oligoasthenoteratozoospermia and 96 males with azoospermia), in addition to 100 controls. Measurements of serum vit.D3, AMH, FT3, FT4, and TSH levels, in addition to reproductive hormone assays, were performed on all included subjects, using ELISA kits.
Results
Overall, results showed significantly lower serum levels of vit.D3 in infertile men than in the controls, with a greater decrease observed in men with azoospermia than in oligoasthenoteratozoospermia patients, (p < .05 for all). Significantly higher serum TSH and FSH levels and significantly lower serum free testosterone levels were observed in males with azoospermia than in males with oligoasthenoteratozoospermia and the controls (p < .05 for both). There were no significant differences between the studied groups in terms of AMH, FT3 or FT4 levels. LH levels were negatively correlated with TSH levels and positively correlated with AMH levels among men with oligoasthenoteratozoospermia, while among men with azoospermia, LH levels were positively correlated with vit.D3 levels (p < .05 for all).
Conclusion
Decreased Vit.D3 could play a role in male infertility, in addition to abnormal thyroid function, which needs further investigation.
Introduction
Human infertility was defined as the inability to achieve a clinical pregnancy after 12 months of regular, contraceptive-free sexual intercourse [Citation1].
The steroid hormone 25-hydroxy cholecalciferol [vit.D3, 25(OH)D] plays an important role in regulation of the reproductive physiology. There is little data available regarding the vit.D3 status of male partners in couples seeking pregnancy [Citation2]. In humans, vit.D3 receptors (VDRs) and vit.D3-metabolizing enzymes are co-expressed during the late stages of spermatogenesis in the necks of spermatozoa [Citation3]. Decreased serum levels of vit.D3, in men with erectile dysfunction or hypogonadism, has been demonstrated by many studies and significant positive correlation between serum vit.D3 and the severity of erectile dysfunction was reported [Citation4–7]. Additionally, Ref. [Citation8] suggested that older adults with low vit.D3 were more liable to be frail than those having sufficient vit.D3 levels.
Anti-Müllerian hormone (AMH) is produced by immature Sertoli cells (SCs) and is responsible for Müllerian duct regression in male fetuses as a part of sexual differentiation; additionally, this hormone is involved in the development and function of testes [Citation9,Citation10]. The decrease in AMH levels that occurs during puberty results from the gradual hypothalamo-pituitary-gonadal (HPG) axis activation with a subsequent increase in intratesticular testosterone levels, rather than from the interaction between spermatogenic cells and SCs [Citation11].
Alterations in thyroid function could result in reduced sexual activity with evident adverse effects on male fertility [Citation12–14], and could lead to a decreased testicular size, sperm motility, and ejaculate volume, establishing a link between thyroid hormones, testicular development, and spermatogenesis [Citation15].
Based on the literature, this is the first study to collectively assess the serum levels of vit.D3 and AMH and the thyroid profile as possible clinical markers of male factor infertility, in addition to routine reproductive hormone assays [follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin and free testosterone), among men with primary infertility and poor semen parameters (oligoasthenoteratozoospermia (OAT) and azoospermia]. Additionally we searched for correlations between these measured parameters and various semen analysis parameters and reproductive hormones.
Methods
Study design and participants
The present cross-sectional hospital based study, was conducted on 301 men who were classified into three groups: group A, which included 105 males with OAT, and group B, which included 96 males who had azoospermia, in addition, group C, consisting of 100, age-matched, fertile (based on both normal semen analysis parameters and proven fertility by history of positive pregnancies or live births), healthy males, selected as controls, was included. The subjects were recruited from the Andrology Outpatient Clinic, South Valley (Qena) and Aswan Universities, Egypt. Written informed consent was obtained from both patients and control subjects before enrolment in the study. The current study protocol conforms to the ethical guidelines of the Declaration of Helsinki and also received approval from the local Medical Ethics Committee. The study period was from April 2016 to May 2017.
The ages of the selected patients ranged from 30 to 45 years, exhibiting primary infertility for one year or more with a normal female factor. Those with cryptorchidism, maldescended testes or any urogenital anomalies, obstructive azoospermia, hypertension, autoimmune or inflammatory disorders, coronary artery disease, diabetes mellitus, or chronic renal failure or those receiving hormonal therapy or chemotherapy were excluded from the study.
Semen analysis
Semen samples were collected from both patients and controls, after 3–5 days of sexual abstinence, by masturbation. Macrosopic and microscopic semen analysis was performed according to WHO criteria [Citation16]. The macroscopic examination was performed by assessing the liquefaction, viscosity, appearance, and volume of the semen samples, while the microscopic examination involved analysis of the motility, concentration, and morphology of the spermatozoa [Citation17].
Blood samples and assays
After 10 h of fasting, 5 mL of venous blood sample was withdrawn from each included subject and evacuated into a serum separator gel tube, where the sample was allowed to clot for 30 min at 37 °C before centrifugation, and the separated sera after centrifugation were aliquoted into 1 mL cryotubes and stored at –20 °C until biochemical analysis of the following parameters, was performed using a microplate ELISA reader (EMR-500):
Solid-phase competitive ELISA kits were used for the measurement of vit.D3 (supplied by Chongqing Biospes Co., Ltd. (Chongqing, People’s Republic of China); with catalogue number BYEK1472) and free triiodothyronine (FT3), free tetraiodothyronine (FT4), and thyroid-stimulating hormone (TSH) (supplied by Calbiotech Inc., (USA); catalogue numbers were F3106T, F4107T, and TS227T, respectively). The reference values for these parameters were as mentioned in a previously published work [Citation18].
Other kits were also used for ELISA assays of AMH (supplied by Kamiya Biomedical Company; catalogue number KT-6945), free testosterone (supplied by supplied by ALPCO; catalogue number 11-FTSHU-E01), LH (supplied by Cayman Chemical; catalogue number 500720), FSH and prolactin (supplied by Calbiotech Inc.; catalogue numbers FS046F and PR234F, respectively).
Statistical analysis
Data were analysed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY) [Citation19]. The data were tested for normality using Kolmogrov-Smirnov and Shapiro-Wilk tests. Quantitative data were expressed as the means ± standard deviations. Student's t-test and analysis of variance (ANOVA) were used to compare continuous quantitative variables of parametric data. Correlation coefficients were assessed using the Pearson method. A two-tailed test was considered significant when p was ≤ .05%.
Results
The present study was carried out on 301 men (group A included 105 males with OAT and group B contained 96 males with azoospermia); the mean ages of the subjects were 38.83 ± 4.96, and 32.75 ± 3.59 years, respectively. In addition, 100 males (group C) served as controls, with a mean age of 36 ± 7.07 years. Non-significant differences between the three groups (p = .266) indicated age matching.
Regarding the semen parameters for both groups A and C, the mean ± SD values of sperm concentration (million/mL) were 6.17 ± 3.76 and 39.4 ± 5.59, respectively. The total sperm motility percentage was 22 ± 5 and 77 ± 12, respectively, while the mean percentage of abnormal sperm forms was 75 ± 15, and 12 ± 4 respectively (p < .05 for all).
The vit.D3 serum levels among infertile men (groups A and B had values of 19.87 ± 4.58 ng/mL and 11.85 ± 2.16 ng/mL, respectively) were significantly low, with lower values observed in men with azoospermia than in infertile men with OAT, in comparison to the controls (40.88 ± 4.53 ng/mL, p < .05 for all; and ).
Figure 1. 25-Hydroxy cholecalciferol and anti-Müllerian hormone levels (A), and thyroid profiles (B) and reproductive hormones (C) among men with azoospermia, men with oligoasthenoteratozoospermia, and the controls.
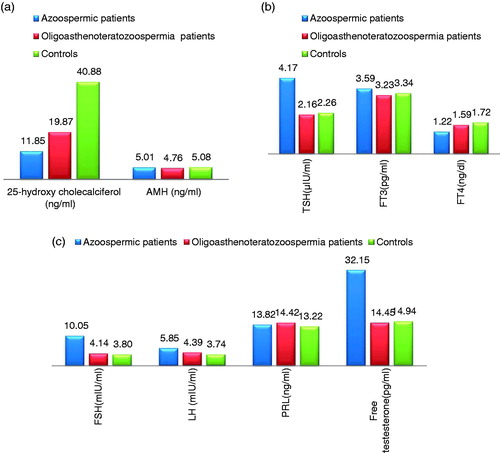
Table 1. Comparison between serum levels of the measured hormones (Vitamin D3, AMH, gonadotropins, prolactin, free testosterone, TSH, FT3, and FT4) among the studied groups.
Group B exhibited significantly lower free testosterone levels and significantly higher TSH and FSH serum levels (14.45 ± 0.54 pg/mL, 4.17 ± 0.38 µIU/mL, and 10.05±2.93 mIU/mL respectively) than group A (48.15 ± 7.45 pg/mL, 2.16 ± 0.64 µIU/mL, and 4.14 ± 0.36 mIU/mL respectively p < .05 for all) and C (52.94 ± 1.04 pg/mL, 2.26 ± 0.86 µIU/mL, and 3.74 ± 0.4 mIU/mL respectively, p < .05 for all) ( and ).
There were nonsignificant differences in the serum levels of AMH, LH, prolactin, FT3, and FT4 among the study groups (p > .05 for all; and ).
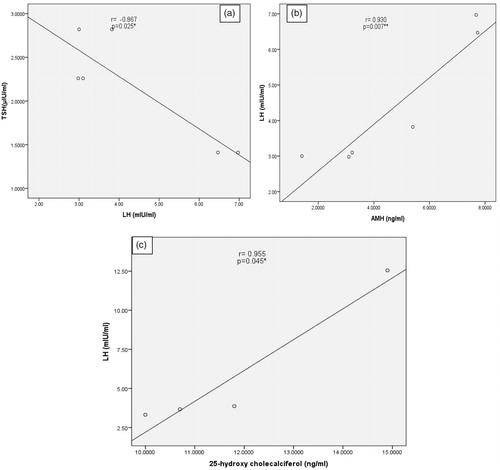
Among men with OAT, there was a significant negative correlation between serum TSH and LH levels (r = –.867, p = .025) and a significant positive correlation between the serum levels of AMH and LH (r = .930, p = .007). However, there was a significant positive correlation between serum levels of LH and vit.D3 among men with azoospermia (r = .955, p = .045) (). No other significant correlations were observed.
Figure 2. Negative correlation between thyroid-stimulating hormone and luteinizing hormone, positive correlation between anti-Müllerian hormone and luteinizing hormone among oligoasthenoteratozoospermia patients (A,B); positive correlation between luteinizing hormone and 25-hydroxyl cholecalciferol among men with azoospermia (C).
Discussion
Although the well-known role of vit.D3 in regulation of expression of genes related to calcium homeostasis, and to some extent cancer, autoimmune diseases, and infection-related genes [Citation20], and its deficiency has been demonstrated to be associated with metabolic syndrome, diabetic complications and prostate cancer [Citation21–24], but its role in the male reproductive system is still being debated [Citation25]. The importance of vit.D3 in male reproduction has been elucidated in rodents in many studies, demonstrating that deficiency of vit.D3 could reduce sperm counts and sperm motility, and decreased fertility rates were observed in females inseminated with semen from males with vit.D3 deficiency [Citation26,Citation27]. The findings of the present study revealed a significantly decreased serum levels of vit.D3 among infertile males compared with the controls. There was also a greater decrease in serum vit.D3 levels among those with azoospermia than OAT patients. These findings were consistent with a study by [Citation28], who reported that low serum vit.D3 levels could be a risk factor for poor semen quality in infertile men. Additionally, it has been reported by many researchers that fertile individuals have higher serum vit.D3 levels than infertile patients [Citation29–32].
In the present study, there were non-significant correlations between vit.D3 levels and semen parameters, reproductive hormones (FSH, LH, free testosterone) levels or prolactin levels, between patients with OAT and the controls. These findings were consistent with the results of several previous studies [Citation25,Citation33,Citation34]. The exact mechanisms by which vit.D3 affects male reproduction remain undetermined. Optimal sperm function may directly depend on vit.D3 and indirectly on calcium homeostasis, as evidenced by the partial restoration of fertility in animal models after normalization of serum calcium levels [Citation25,Citation36]. Calcium has been identified as a critical intracellular signal involved in spermatogenesis, sperm motility and hyper-activation of the acrosome reaction [Citation37]. Loss of the VDR-regulated calcium transporter, which is expressed in the epididymis, results in impaired cellular transport of calcium with changes in epididymal fluid concentration and subsequent impairment of sperm motility and infertility [Citation38]. Ref. [Citation39] reported improved serum testosterone levels following vitamin D therapy in middle-aged men. Additionally, Ref. [Citation40] reported significant association of vit.D3 with total testosterone in Malaysian men. In our study, serum vit.D3 levels exhibited a positive correlation with LH among men with azoospermia; thus, the effect of low serum vit.D3 levels on semen parameters could be partially mediated by the HPG axis in such patients, as LH regulates the production of testosterone. Ref. [Citation41] reported the absence of correlation between vit.D3 and testosterone as reported here; However, the study also observed a significant negative correlation between vit.D3 and LH among infertile men and these two contradictory findings suggest the complex role of vit.D3 in male infertility.
The serum level of AMH could be a useful marker for ovarian reserve, while in males, this hormone is secreted in both serum and seminal plasma and its measurement could be useful for clinical assessment of males with infertility [Citation42]. The findings of the present study revealed nonsignificant differences in the serum levels of AMH among infertile men compared with the controls and nonsignificant differences when comparing men with OAT and men with azoospermia. Additionally, AMH levels did not exhibit significant correlations with semen parameters (sperm count, sperm motility, and abnormal forms) or other measured hormones among infertile men or controls, except for a significant positive correlation with LH among males with OAT. These findings were consistent with those of [Citation43] and [Citation44], who reported nonsignificant differences in serum AMH levels between infertile men and healthy controls with a nonsignificant correlation of AMH with semen parameters. In contrast, Ref. [Citation45] reported decreased seminal AMH concentrations in cases of spermatogenesis impairment, and many investigators have reported higher seminal AMH concentrations in normospermic men than in oligospermic men [Citation46–48]; however, we measured the AMH levels in the serum in the present study, not in the seminal plasma. Refs. [Citation49] and [Citation50], reported that serum AMH levels could not predict the efficiency of testicular sperm retrieval in men with nonobstructive azoospermia or the presence of sperm in fine-needle aspiration. Additionally, [Citation42] concluded in their review article that AMH did not seem to be a satisfactory single clinical marker of spermatogenesis when evaluating infertile men, as the wide overlapping of values between controls and infertile males prevents this hormone from being a useful diagnostic marker. The positive correlation between serum AMH levels and LH levels among males with azoospermia observed in the present study requires further assessment and evaluation in larger-scale studies.
Regarding the evaluation of thyroid function among infertile men, the findings of the present study revealed nonsignificant differences in the serum levels of FT3 and FT4 between the studied groups, but the TSH serum levels were significantly higher among men with azoospermia than in men with OAT and the controls, although the values remained within the normal reference range, indicating the tendency for subclinical hypothyroidism in azoospermia patients. Moreover, there was a significant negative correlation between the serum levels of LH and TSH among OAT patients. This correlation has been established in infertile women [Citation51,Citation52]. A study performed by [Citation53] showed that alterations in thyroid status negatively affect semen quality, more so in hypothyroidism than in hyperthyroidism, with improvement following thyroid hormone replacement therapy. Decreased thyroid function results in decreased levels of most reproductive hormones including LH [Citation54]. It has been reported that FT4 has a protective effect against sperm DNA damage and associated with human semen parameters [Citation55,Citation56].
In conclusion, the present study indicates a role of vit.D3 and thyroid hormones in male infertility, with a nonsignificant role for AMH in infertile men with OAT or nonobstructive azoospermia. However, larger-scale studies, including studies of racial and ethnic groups, are required to confirm the findings of the present study. Additionally, this study indicates novel potential therapeutic benefits of vit.D3 with or without thyroid hormones in the management of male infertility, which needs further researches.
Disclosure statement
The authors report no conflicts of interest associated with this work.
References
- World Health Organization (WHO). Infertility. 2013. Available from: http://www.who.int/reproductivehealth/topics/infertility/definitions/en/
- Tartagni M, Matteo M, Baldini D, et al. Males with low serum levels of vitamin D have lower pregnancy rates when ovulation induction and timed intercourse are used as a treatment for infertile couples: results from a pilot study. Reprod Biol Endocrinol. 2015;13:127.
- Nangia AK, Hill O, Waterman MD, et al. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro. J Urol. 2007;178:1092–1096.
- La Vignera S, Condorelli RA, Cimino L, et al. Late-onset hypogonadism: the advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male 2016;19:34–39.
- Basat S, Sivritepe R, Ortaboz D, et al. The relationship between vitamin D level and erectile dysfunction in patients with type 2 diabetes mellitus. Aging Male 2018;21:111–115.
- Culha MG, Atalay HA, Canat HL, et al. The relationship between erectile dysfunction severity, mean platelet volume and vitamin D levels. Aging Male 2018;4:1–6.
- Park SG, Yeo JK, Cho DY, et al. Impact of metabolic status on the association of serum vitamin D with hypogonadism and lower urinary tract symptoms/benign prostatic hyperplasia. Aging Male 2018;21:55–59.
- Gutiérrez-Robledo LM, Ávila-Funes JA, Amieva H, et al. Association of low serum 25-hydroxyvitamin D levels with the frailty syndrome in Mexican community-dwelling elderly. Aging Male 2016;19:58–63.
- Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993;14:152–164.
- Rey RA, Grinspon RP. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract Res Clin Endocrinol Metab. 2011;25:221–238.
- Hero M, Tommiska J, Vaaralahti K, et al. Circulating antimullerian hormone levels in boys decline during early puberty and correlate with inhibin B. Fertil Steril. 2012;97:1242–1247.
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755.
- Krajewska-Kulak E, Sengupta P. Thyroid function in male infertility. Front Endocrinol. 2013;4:174.
- Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36:353.
- Rajender S, Monica MG, Walter L, et al. Thyroid, spermatogenesis, and male infertility. Front Biosci. 2011;E3:843–855.
- World Health Organization (WHO). WHO laboratory manual for the examination and processing of human semen. 5th ed. Cambridge (England): The Press Syndicate of University of Cambridge; 2010.
- Patricio A, Cruz DF, Silva JV, et al. Relation between seminal quality and oxidative balance in sperm cells. Acta Urol Port. 2016;33:6–15.
- Desoky T, Hassan MH, Fayed HM, et al. Biochemical assessments of thyroid profile, serum 25-hydroxycholecalciferol and cluster of differentiation 5 expression levels among children with autism. Neuropsychiatric Dis Treat. 2017;13:2397–2403.
- Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont (CA): Wadsworth, Cengage Learning; 2013.
- Canguven O, El Ansari W, Yassin A. 2018. Vitamin D supplementation as a potential therapeutic mediator in asthma: does dose really matter? A critical review of the literature. Aging Male 2018;29:1–8.
- Filus A, Trzmiel A, Kuliczkowska-Płaksej J, et al. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. Aging Male 2008;11:134–139.
- Sivritepe R, Basat S, Ortaboz D. Association of vitamin D status and the risk of cardiovascular disease as assessed by various cardiovascular risk scoring systems in patients with type 2 diabetes mellitus. Aging Male 2018;7:1–7.
- Toprak B, Colak A, Yalcin H, et al. No association of serum PSA with vitamin D or total oxidant-antioxidant capacity in healthy men. Aging Male 2018;7:1–4.
- Ucak S, Sevim E, Ersoy D, et al. Evaluation of the relationship between microalbuminuria and 25-(OH) vitamin D levels in patients with type 2 diabetes mellitus. Aging Male 2018;26:1–5.
- Abbasihormozi S, Kouhkan A, Alizadeh AR, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology 2017;5:113–118.
- Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989;119:741–744.
- Sun W, Chen L, Zhang W, et al. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015;308:E51–E62.
- Zhu CL, Xu QF, Li SX, et al. Investigation of serum vitamin D levels in Chinese infertile men. Andrologia 2016;48:1261–1266.
- Rahnavard Z, Eybpoosh S, Homami MR, et al. Vitamin d deficiency in healthy male population: results of the Iranian multi- center osteoporosis study. Iran J Public Health 2010;39:45–52.
- Karras S, Anagnostis P, Kotsa K, et al. Vitamin D and gonadal function in men: a potential inverse U-shaped association? Andrology 2016;4:542–544.
- Akhavizadegan H, Karbakhsh M. Comparison of serum vitamin D between fertile and infertile men in a vitamin D deficient endemic area: a case-control study. Urologia 2017;84:218–220.
- Albaldawy MT, Alsalami AS. Study of association among Vitamin D, testosterone and semen quality in fertile and Iraqi infertile men. J Pharm Sci Res. 2017;9:1067–1071.
- Ramlau-Hansen CH, Moeller UK, Bonde JP, et al. Are serum levels of vitamin D associated with semen quality? Fertil Steril. 2011;95:1000–1004.
- Blomberg JM, Dissing S. Non-genomic effects of vitamin D in human spermatozoa. Steroids 2012;77:903–909.
- Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992;122:1338–1344.
- Menegaz D, Rosso A, Royer C, et al. Role of 1alpha,25(OH)2 vitamin D3 on alpha-[1-(14)C]MeAIB accumulation in immature rat testis. Steroids 2009;74:264–269.
- Yoshida M, Kawano N, Yoshida K. Control of sperm motility and fertility: diverse factors and common mechanisms. Cell Mol Life Sci. 2008;65:3446–3457.
- Weissgerber P, Kriebs U, Tsvilovskyy V, et al. Male fertility depends on Ca+2 absorption by TRPV6 in epididymal epithelia. Sci Signal. 2011;4:27.
- Canguven O, Talib RA, El Ansari W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male 2017;20:9–16.
- Chin KY, Ima-Nirwana S, Wan Ngah WZ. Vitamin D is significantly associated with total testosterone and sex hormone-binding globulin in Malaysian men. Aging Male 2015;18:175–179.
- Knopf JK, Scosyrev E, O'Brien J. Correlation of vitamin D and LH levels. Fertil Steril. 2012;98:S143–S144.
- La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113–130.
- Tuttelmann F, Dykstra N, Themmen AP, et al. Anti-Mullerian hormone in men with normal and reduced sperm concentration and men with maldescended testes. Fertil Steril. 2009;91:1812–1819.
- Kucera R, Ulcova-Gallova Z, Windrichova J, et al. Anti-Müllerian hormone in serum and seminal plasma in comparison with other male fertility parameters. Syst Biol Reprod Med. 2016;62:223–226.
- Muttukrishna S, Yussoff H, Naidu M, et al. Serum anti-Mullerian hormone and inhibin B in disorders of spermatogenesis. Fertil Steril. 2007;88:516–518.
- Fujisawa M, Yamasaki T, Okada H, et al. The significance of anti-Mullerian hormone concentration in seminal plasma for spermatogenesis. Hum Reprod. 2002;17:968–970.
- Mostafa T, Amer MK, Abdel-Malak G, et al. Seminal plasma anti-Mullerian hormone level correlates with semen parameters but does not predict success of testicular sperm extraction (TESE). Asian J Androl. 2007;9:265–270.
- Duvilla E, Lejeune H, Trombert-Paviot B, et al. Significance of inhibin B and anti-Mullerian hormone in seminal plasma: a preliminary study. Fertil Steril. 2008;89:444–448.
- Isikoglu M, Ozgur K, Oehninger S, et al. Serum anti-Mullerian hormone levels do not predict the efficiency of testicular sperm retrieval in men with non-obstructive azoospermia. Gynecol Endocrinol. 2006;22:256–260.
- Goulis DG, Tsametis C, Iliadou PK, et al. Serum inhibin B and anti-Mullerian hormone are not superior to follicle-stimulatinghormone as predictors of the presence of sperm in testicular fine-needle aspiration in men with azoospermia. Fertil Steril. 2009;91:1279–1284.
- Fupare S, Gadhiya BM, Jambhulkar RK, et al. Correlation of thyroid hormones with FSH, LH and Prolactin in infertility in the reproductive age group women. Int J Clin Biol Res. 2015;2:216–222.
- Lal RZ, Biyani S, Lodha R. Correlation of thyroid hormones with FSH, LH and prolactin in infertility in the reproductive age group women. IAIM 2016;3:146–150.
- Buitrago JMG, Diez LCG. Serum hormones and seminal parameters in males with thyroid disturbance. Andrologia 2009;19:37–41.
- Krassas GE, Perros P. Thyroid disease and male reproductive function. J Endocrinol Invest. 2003;26:372–380.
- Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertilityclinic. J Androl. 2006;28:397–406.
- Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. J Androl. 2008;29:379–388.
